Obstructive sleep apnea is associated with severity and long-term prognosis of acute coronary syndrome
2018-05-23ShuoJIAYuJieZHOUYiYUSiJingWUYanSUNZhiJianWANGXiaoLiLIUBrightEricKingYingXinZHAODongMeiSHIYuYangLIUZhiMingZHOU
Shuo JIA, Yu-Jie ZHOU, Yi YU, Si-Jing WU, Yan SUN, Zhi-Jian WANG, Xiao-Li LIU, Bright Eric King,Ying-Xin ZHAO, Dong-Mei SHI, Yu-Yang LIU, Zhi-Ming ZHOU
Bejing Anzhen Hospital, Capital Medical University, Beijing Institute of Heart Lung and Blood Vessel Disease, Beijing, China
1 Introduction
Obstructive sleep apnea (OSA) is a prevalent sleep disorder that affects 3%-7% of the general population,[1]and 30%-60% of patients with known cardiovascular disease.[2]It is caused by repeated episodes of complete or partial upper airway obstruction during sleep and leads to transient hypoxemia, arousal from sleep, tachycardia, and a surge in systemic blood pressure (BP).
The close relationship between sleep apnea and coronary artery disease (CAD) has been supported by many studies.Growing evidence has shown that OSA is pathologically associated with cardiovascular disease.[3–5]Indeed, previous studies had shown that continuous positive airway pressure(CPAP) treatment was effective in reducing the incidence of hypertension and cardiovascular events.[6]In contrast, a recent report from The Sleep Apnea cardiovascular Endpoints(SAVE) study reveals that CPAP therapy plus conventional care, as compared with conventional care alone, did not prevent cardiovascular events in patients with moderate-tosevere obstructive sleep apnea and established cardiovascular disease.[7]Furthermore, a new hypothesis indicates that in the context of acute myocardial infarction (AMI), OSA plays a cardio-protective role via ischemic preconditioning,which results in activation of adaptive mechanisms, such as the increased recruitment of proliferative and angiogenic endothelial progenitor cells.[8]In line with this hypothesis,some studies demonstrated that patients with OSA reportedly exhibit less severe cardiac injury during an acute non-fatal myocardial infarction compared with patients without OSA.[9,10]
Therefore, it is still controversial on whether sleep apnea worsening or ameliorate the outcomes of patients with acute coronary syndrome (ACS). However, none of these studies addressed the impact of sleep apnea on the long-term prognosis in ACS patients after coronary revascularization therapy with drug-eluting stents (DES). Therefore, we performed this study to evaluate the impact of OSA on the severity and prognosis of patients with ACS after receiving percutaneous coronary intervention (PCI). In this study, we correlate the severity of OSA with cardiovascular profiles such as ejection fraction, number of diseased vessels, SYNTAX scores,length of hospitalization and major adverse cardiovascular events (MACE) of a cohort of ACS patients.
2 Methods
2.1 Study design and patient population
In this single-center prospective cohort study, we recruited consecutive patients who were hospitalized in the Department of Cardiology at Beijing Anzhen Hospital, Capital Medical University from December 2012 to December 2015. Inclusion criteria were: patients who hospitalized with ACS for coronary angiography and intervention, age > 18 years.
The exclusion criteria were: any medical, social or geographical factor that could jeopardize patient compliance,previous treatment with CPAP (or treatment after discharge),any previously diagnosed chronic obstructive pulmonary disease (COPD), patients in cardiogenic shock, any process that reduces life expectancy to < 1 year.
2.2 Angiography/PCI and treatment for ACS
ACS was defined as the acute presentation of coronary disease with or without ST elevation infarction (NSTEMI),unstable angina (UA), or type 1 myocardial infarction(MI).[11]Multi-vessel lesion was defined as ≥ 2 vessels (diameter > 2 mm) with significant stenosis (≥ 70% lumen area). SYNTAX score was used to assess the complexity and severity of coronary artery disease.[12]All of the recruited patients were treated according to standard clinical practice,[10]and all stents were DES. Briefly, all patients received dual-antiplatelet (aspirin plus clopidogrel/ticagrelor), statins and antihypertensive/antidiabetic drugs if necessary.
2.3 Polysomnography (PSG)
In all recruited patients, Standard PSG parameters (Embletta, JET FLOW) were measured including electroencephalogram (EEG), nasal airflow (nasal cannula), thoracoabdominal movements (inductive respiratory bands), arterial oxygen saturation, snoring episodes derived from the integrated pressure transducer, limb movement, electrocardiogram and body position during the first 48–72 h after admission. Apnea was defined as an absence of airflow lasting ≥ 10 s. Hypopnea was defined as a reduction in airflow lasting ≥ 10 s associated with oxygen desaturation.Oxygen desaturation was defined as a decrease in arterial oxygen saturation > 4%. Apnea-hypopnea index (AHI) was defined as the number of episodes of apnea and hypopnea per hour of recording.[1]Based on previous studies and consensus, we decided that patients with AHI > 15 events/h were defined as moderate to severe OSA group,[13]and those with an AHI ≤ 15 events/h were defined as controls. The Epworth Sleepiness Scale (ESS) was administered to the patients to determine their degree of excessive daytime sleepiness after PCI.[14]
2.4 Baseline characteristics and follow-up for cardiovascular events
At baseline, patients’ demographic and anthropometric data were collected: age, sex, body mass index (BMI),blood pressure, heart rate and rhythm, blood biochemistry(for the first time after admission), cardiovascular risk factors (e.g., diabetes mellitus, hypertension, hyperlipidemia,and smoking), PSG and angiographic parameters. After hospital discharge, the cardiovascular outcomes were prospectively collected every six months by clinic visits or telephone calls. All patients were recommended to improve their lifestyle. Major adverse cardiovascular events (MACE)were defined as cardiac death, myocardial infarction, unplanned revascularization, or heart failure requiring hospitalization. To verify the events, source documents of the patients who had experienced adverse cardiovascular events were reviewed by the investigators. This study was approved by the institutional review board and all patients provided written informed consent prior to participation.
2.5 Data analysis
The frequencies (%) were computed to evaluate the differences between the control and moderate to severe OSA patients with respect to anthropometric and clinical variables and ACS related risk factors, assessing the significance with Mann-Whitney or Chi-squared tests, respectively.The mean ± SD were computed for continuous covariates such as age and BMI between groups and compared by using an independent samplettest.
The Kaplan-Meier cumulative incidence curves were plotted and the difference in cumulative incidence of adverse event rates between groups was compared by using the log-rank test. The Cox regression was then applied to adjust for baseline confounders. All of the statistical analyses were carried out with SPSS 20.0. Statistical significance was defined asP< 0.05 for all comparisons.
3 Results
3.1 Baseline characteristics
Form December 2012 to December 2015, we assessed 892 eligible patients and finally included 529 patients for analysis (Figure 1). There were no difference in proportion of congestive heart failure patients between the control group and moderate to severe OSA group (1.3%vs. 4.6%,P= 0.065) and none of them accompany with obvious symptoms. The demographic and baseline characteristics of the included patients are shown in Table 1. Compared with the control group, the moderate to severe OSA group had higher proportion of males (79.9%vs. 69.2%,P= 0.008),hypertension (75.3%vs. 64.1%,P= 0.009) and higher mean BMI. There were no statistical differences in proportion of diabetes mellitus, dyslipidemia, current or former smoker and alcohol abuse between the two groups. There were also no statistical differences between the two groups with respect to age years, heart rate, systolic pressure, low density lipoprotein (LDL) cholesterol, hypersensitive C reactive protein (HSCRP), B-type natriuretic peptide (BNP) and troponin I (TNI). Not surprisingly, moderate to severe OSA group had higher mean apnea–hypopnea index events/h and Epworth sleepiness scale (AllP< 0.001).
3.2 Severity of ACS and coronary lesions
With respect to ACS severity, there were no difference in respects of proportion of patients with ST elevated MI(STEMI), NSTEMI and UA. However, moderate to severe OSA group had higher proportion of patients with multivessel lesions, higher mean of SYNTAX score, and length of hospitalization days (P< 0.001 andP= 0.007, respectively) and lower ejection fraction (P< 0.001) (Table 2).Baseline characteristics of coronary angiography are shown in Table 3. Moderate to severe OSA group had significantly more culprit lesion vessels and higher proportion of culprit lesion in left anterior descending branch (LAD), left circumflex branch (LCX) and right coronary artery (P<0.001).
3.3 Adverse cardiovascular events
The median follow-up duration was 28 ± 4.6 months.Among 156 patients in the control group, five patients developed five adverse cardiovascular events, including three myocardial infarctions and two unplanned revascularizations. Among 373 patients in the moderate to severe OSA group, 32 patients developed 32 adverse cardiovascular events, including 10 cardiac deaths, 4 myocardial infarctions and 18 unplanned revascularizations. Clinical characteristics between MACE group and Non-MACE group are shown in Table 4. The Kaplan-Meier cumulative incidence of adverse cardiovascular events is shown in Figure 2. The event rate at 30 months was significantly higher by log rank test in the moderate to severe OSA than the control group (8.6%vs.3.2%,P= 0.028).
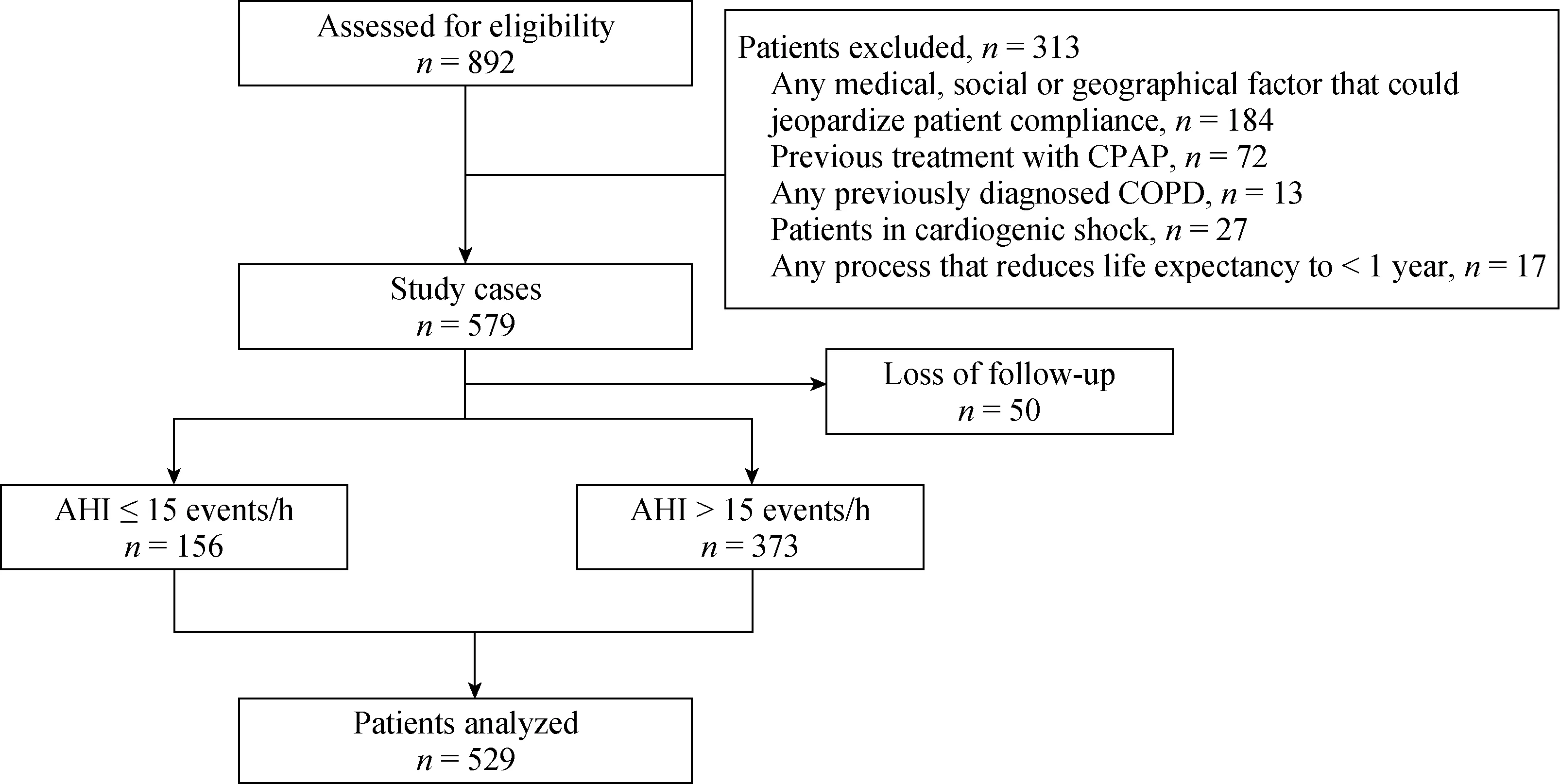
Figure 1. Flow chart. AHI: apnea-hypopnea index; CAD: coronary artery disease; COPD: chronic obstructive pulmonary disease; CPAP:continuous positive airway pressure.

Table 1. The basic characteristics of the participants.

Table 2. Variables related to acute coronary syndrome severity.
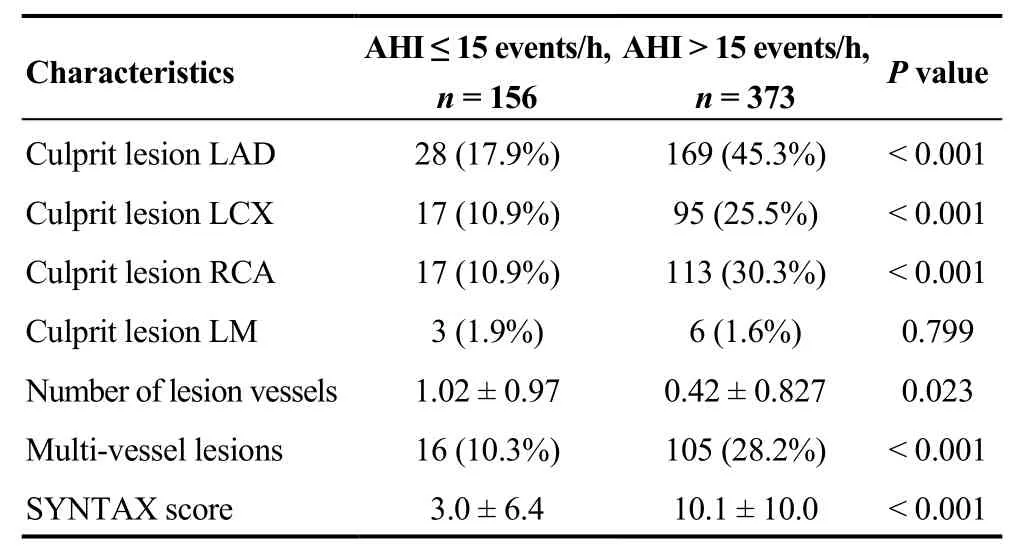
Table 3. Baseline characteristics of coronary angiography.
Potential confounding factors such as age, sex, BMI, TNI,hypertension, diabetes mellitus, dyslipidemia and SYNTAX score was screened by univariate analysis. Confounders including BMI, cardiac troponin I, hypertension, dyslipidemia did not achieve statistical significance were excluded from final Multivariate Cox Regression Analysis. The multivariable Cox regression analysis of predictors of MACE showed increased hazard of adverse event with moderate to severe OSA (P= 0.047, HR = 1.618, 95% CI: 1.069–3.869)with SYNTAX score (P= 0.037, HR = 1.842, 95% CI:1.055– 4.788) (Table 5).
4 Discussion
In this prospective single-center cohort study of over 500 patients, we found that patients with moderate to severe OSA also had more severe CAD, in term of higher SYNTAX score and lower ejection fraction, as well as longer length of hospitalization days. More importantly, we found that moderate to severe OSA was a risk factor for long-term adverse cardiovascular events after angiography/PCI.
Our findings that OSA was associated with severer ACS are in line with previous reports that sleep-disordered breathing, especially when it is severe, is associated with both all-cause and coronary artery disease mortality.[15,16]Yumino,et al.[17]performed a sleep study in 89 patients with ACS who were successfully treated with PCI, mean period of follow-up 227 days. The incidence of major adverse cardiac events was significantly higher in patients with OSA (23.5%vs. 5.3%,P= 0.022) and multivariate analysis showed presence of OSA was an independent predictor for MACE. Mazaki,et al.[18]studied 241 patients withACS treated with primary PCI and results showed that incidence of major adverse cardiocerebrovascular events was significantly higher in patients with sleep-disordered breathing (SDB) than in those without SDB (21.4%vs.7.8%,P= 0.006). Lee,et al.[19]reported that in total of 120 patients with STEMI, severe OSA group was associated with a lower event-free survival rate at 18-month follow-up.A recent published meta-analysis demonstrated that the presence of OSA can increase the risk of cardiovascular events in patients who underwent PCI.[20]But almost all patients in these study mentioned above were treated with bare metal stents and the sample size were relatively low.[17–23]Our study proved that compare to non-OSA group,OSA is related to more adverse events in DES implanted patients. This is similar to previous studies in which bare metal stents were used. In DES era, the use of DES has been proven to be associated with a lower risk of revascularization rate and stent-related adverse events.[23]This proves that the effect of OSA on patients’ prognosis is independent to the benefit of the stent itself. The essential features of OSA were intermittent episodes of hypoxia, increase in sympathetic activity, sudden changes in systemic blood pressure and inflammatory reaction.[24,25]These pathophysiological changes are closely related to the coronary artery
disease and abnormalities in cardiac autonomic and electrophysiological factors, such as heart rate variability and the duration of the QT interval.[26,27]Altogether, the sleep-disordered breathing may worsen the coronary artery disease by increasing the plaque burden, deteriorating infarction expansion and impairing healing of myocardial tissues.[28,29]
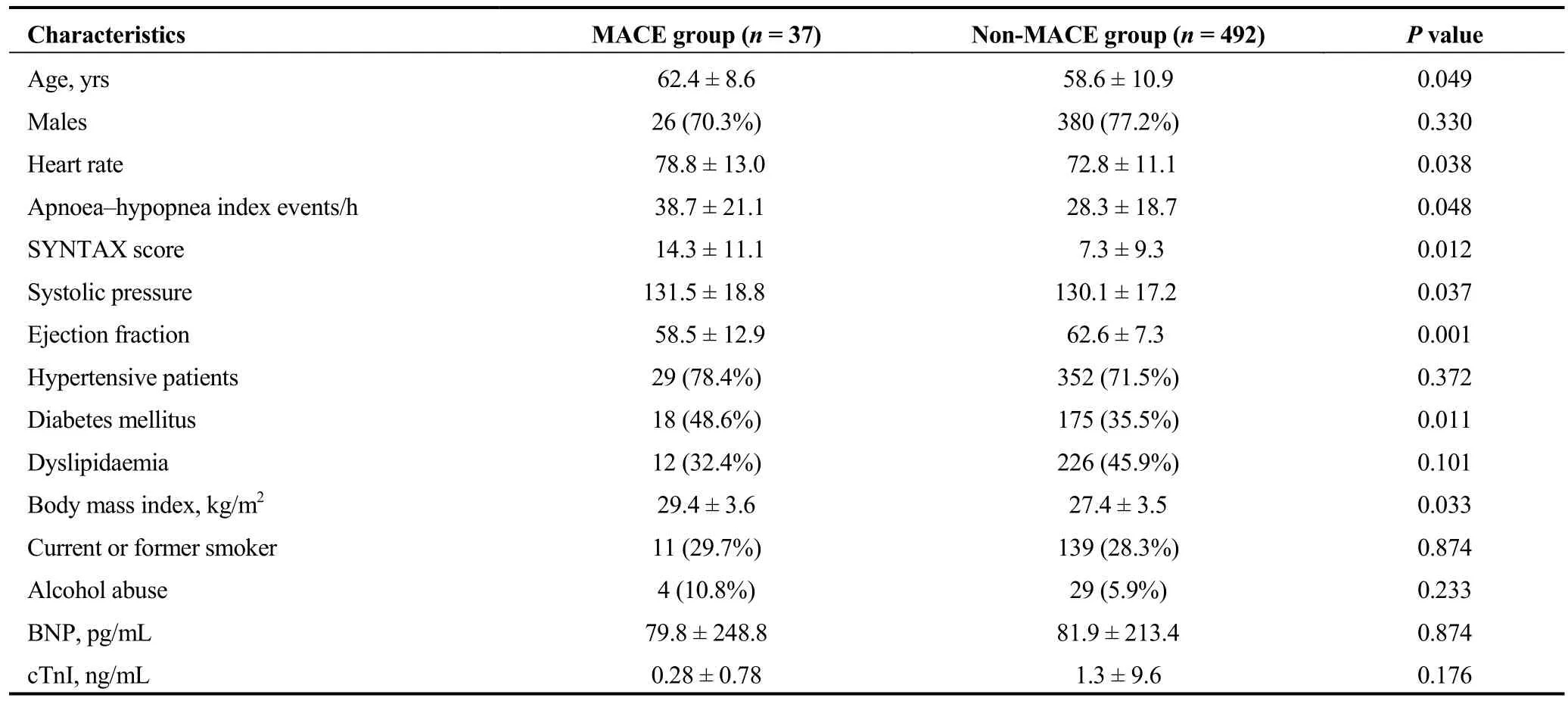
Table 4. Clinical characteristics between MACE group and Non-MACE group.

Table 5. Multivariable Cox Regression analysis of predictors of mace.
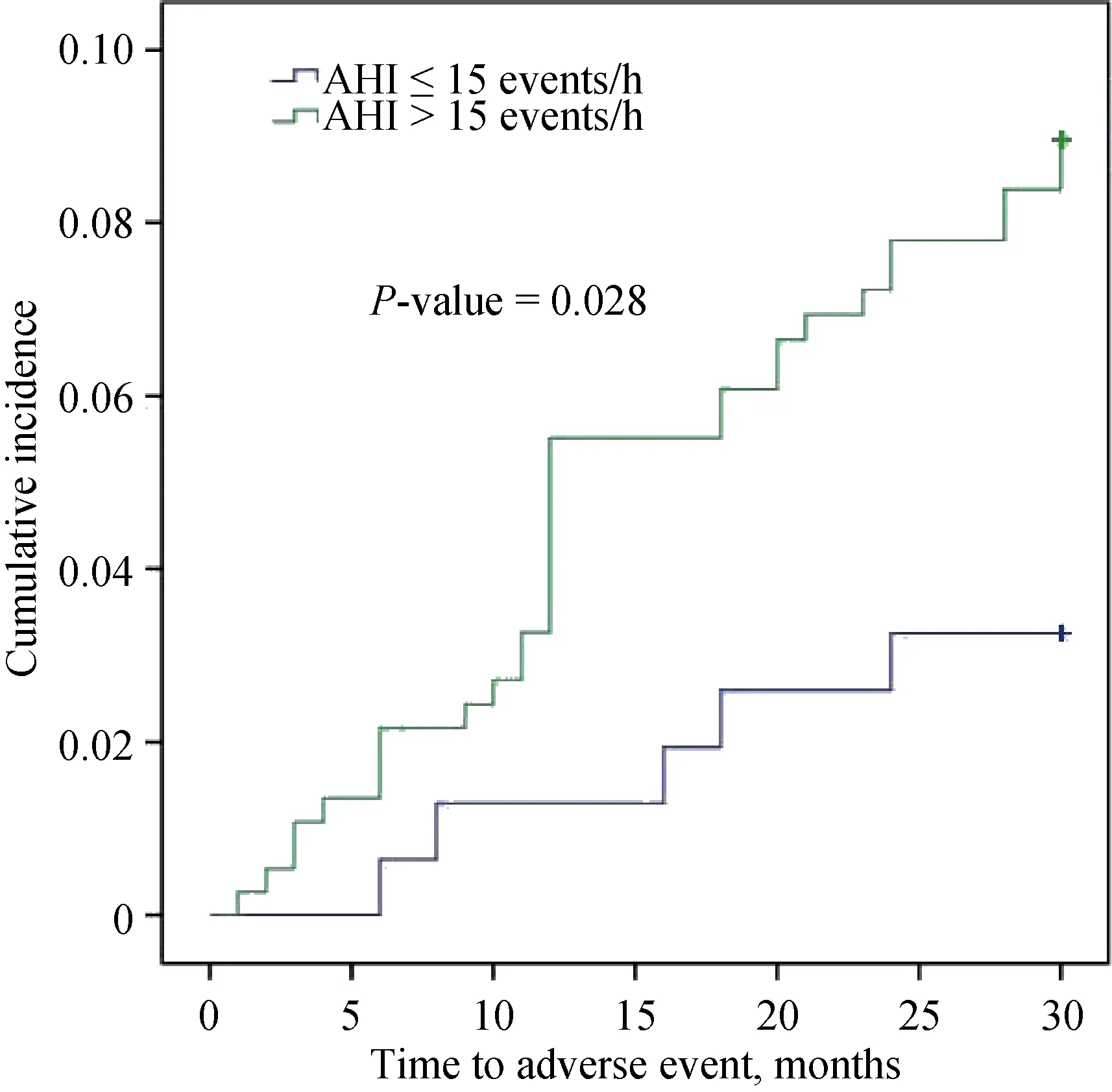
Figure 2. Kaplan-Meier cumulative incidence curves according to presence of moderate to severe OSA. AHI: apnea-hypopnea index; OSA: obstructive sleep apnea.
We also noticed that some investigators reported completely different results. For example, Lee,et al.[30]reported that OSA did not worsen the microvascular perfusion in patients of acute myocardial infarction either at baseline or after PCI. Furthermore, Shah,et al.[9]reported that patients with OSA have even less severe cardiac injury than patients without OSA during an acute non-fatal MI. However, the former study included relatively small number of patients and high excluded risk CAD patients,[30]and the latter study assessed OSA in the acute setting of MI which may exaggerate OSA symptoms,[9]implying that these confounding factors may bias the association between OSA status and cardiac injury. In contrast, our study showed that OSA was associated with severer coronary artery atherosclerosis and the association still existed even after controlling confounding factors such as BMI, age, hypertension, diabetes and dyslipidemia.
The novelty of our study is the finding that moderate-tosevere OSA was associated with long-term adverse cardiac events after angiography or PCI. All patients were treated with drug-eluting stents which most previous studies were not used,[17–23]thus it is important to confirm whether the use of drug-eluting stents could have different results in prognosis of OSA and ACS population. Most studies reported patients with or without OSA had same outcomes at 30 days or at 1 year follow up.[5,10]Meng,et al.[31]also reported that ACS patients with or without OSA had similar rates of MACEs, although OSA was associated with increased CAD severity and blood coagulability, as well as interventricular septum thickness after successful PCI.However, considering their study recruited relatively small number of patients and included patients with less severe OSA (AHI ≥ 5), the correlation between OSA and MACE may be minimized.
The strengths of our study are reflected by the relatively large number of patients and long follow-up time. However,several issues need to take into consideration in interpreting the present study. CAD are quite complex, we cannot exclude all the undetermined confounders. First, this study included predominantly male patients and diagnosed with UA. Therefore, we cannot rule out the possibility that patients with OSA or with strong risk factors for OSA such as obesity, were more likely to be symptomatic and seek medical service when they experienced even a mild coronary ischemic event. Second, some information on cardiovascular outcomes may be inaccurate since they were based on the self-report of the patients; but we believe the possibility was very low to miss the major events such as MI,hospitalization for heart failure or death. Third, we were unable to determine the duration of OSA prior to the index ACS and therefore, we could not measure the effect of exposure duration to hypoxia. Fourth, repeat angiography was not performed during follow up, so we could not capture data on restenosis rates unless the patient is re-hospitalized for MACE. And single-center study may have introduced bias.
In conclusion, this prospective study in a big Chinese cohort with ACS demonstrated that moderate or severe OSA is correlated with more serious ACS and worse long-term prognosis after PCI in spite of DES implantation.It is suggested that moderate-to-severe OSA facilitates plaque vulnerability and the progression of coronary atherosclerosis. This study provides more evidence for the causal relationship between OSA and coronary artery disease and raising the possibility that early diagnose and interventions to OSA could improve long-term outcomes in ACS patients.Moreover, studies to evaluate effective treatment for OSA and ACS are still warranted.
Acknowledgements
This work were supported by the grant from National Key Research and Development Program of China (2017 YFC0908800), the Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support (code: ZYLX201303), the National Key Clinical speciality Construction Project (2013–2014), and the“Beijing Municipal Administration of Hospitals” Ascent Plan (code: DFL20150601). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.Informed consent was obtained from all individual participants included in the study. Additional informed consent was obtained from all individual participants for whom identifying information is included in this article.
References
1 Peppard PE, Young T, Barnet JH,et al. Increased prevalence of sleep-disordered breathing in adults.Am J Epidemiol2013;177: 1006–1014.
2 Mooe T, Franklin KA, Holmstrim K,et al. Sleep-disordered breathing and coronary artery disease: long-term prognosis.Am J Respir Crit Care Med2001; 164: 1910–1913.
3 Marin JM, Carrizo SJ, Vicente E,et al. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study.Lancet2005; 365:1046–1053.
4 Schiza SE, Simantirakis E, Bouloukaki I,et al. Sleep disordered breathing in patients with acute coronary syndromes.J Clin Sleep Med2012; 8: 21–26.
5 Shahar E, Whitney CW, Redline S,et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study.Am J Respir Crit Care Med2001; 163: 19–25.
6 Barbé F, Durán-Cantolla J, Sánchez-de-la-Torre M,et al.Effect of continuous positive airway pressure on the incidence of hypertension and cardiovascular events in nonsleepy patients with obstructive sleep apnea: a randomized controlled trial.JAMA2012; 307: 2161–2168.
7 McEvoy RD, Antic NA, Heeley E,et al. CPAP for Prevention of Cardiovascular Events in Obstructive Sleep Apnea.N Engl J Med2016; 375: 919–931.
8 Berger S, Aronson D, Lavie P,et al. Endothelial progenitor cells in acute myocardial infarction and sleep-disordered breathing.Am J Respir Crit Care Med2013; 187: 90–98.
9 Shah N, Redline S, Yaggi HK,et al. Obstructive sleep apnea and acute myocardial infarction severity: ischemic preconditioning.Sleep Breath2013; 17: 819–826.
10 Barbé F, Sánchez-de-la-Torre A, Abad J,et al. Effect of obstructive sleep apnoea on severity and short-term prognosis of acute coronary syndrome.Eur Respir J2015; 45: 419–427.
11 Deckers JW. Classification of myocardial infarction and unstable angina: a re-assessment.Int J Cardiol2013; 167:2387–2390.
12 Sianos G, Morel MA, Kappetein AP,et al. The SYNTAX Score: an angiographic tool grading the complexity of coronary artery disease.EuroIntervention2005; 1: 219–227.
13 Epstein LJ, Kristo D, Strollo PJ,et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults.J Clin Sleep Med2009; 5: 263–276.
14 Johns MW. A new method for measuring daytime sleepiness:the Epworth sleepiness scale.Sleep1991; 14: 540–545.
15 Gami AS, Howard DE, Olson EJ,et al. Day-night pattern of sudden death in obstructive sleep apnea.N Engl J Med2005;352: 1206–1214.
16 Punjabi NM, Caffo BS, Goodwin JL,et al. Sleep-disordered breathing and mortality: a prospective cohort study.PLoS Med2009; 6: e1000132.
17 Yumino D, Tsurumi Y, Takagi A,et al. Impact of obstructive sleep apnea on clinical and angiographic outcomes following percutaneous coronary intervention in patients with acute coronary syndrome.Am J Cardiol2007; 99: 26–30.
18 Mazaki T, Kasai T, Yokoi H, et al. Impact of sleep-disordered breathing on long-term outcomes in patients with acute coronary syndrome who have undergone primary percutaneous coronary intervention.J Am Heart Assoc2016; 5: e003270.
19 Lee CH, Khoo SM, Chan MY,et al. Severe obstructive sleep apnea and outcomes following myocardial infarction.J Clin Sleep Med2011; 7: 616–621.
20 Zhao Y, Yu BY, Liu Y,et al. Meta-analysis of the effect of obstructive sleep apnea on cardiovascular events after percutaneous coronary intervention.Am J Cardiol2017; 120:1026–1030.
21 Belaidi E, Joyeux-Faure M, Ribuot C,et al. Major role for hypoxia inducible factor-1 and the endothelin system in promoting myocardial infarction and hypertension in an animal model of obstructive sleep apnea.J Am Coll Cardiol2009; 53:1309–1317.
22 Nakashima H, Kurobe M, Minami K,et al. Effects of moderate-to-severe obstructive sleep apnea on the clinical manifestations of plaque vulnerability and the progression of coronary atherosclerosis in patients with acute coronary syndrome.Eur Heart J Acute Cardiovasc Care2015; 4: 75–84.
23 Stefanini GG, Holmes DR. Drug-eluting coronary-artery stents.N Engl J Med2013; 368: 254–265.
24 Kohler M, Stradling JR. Mechanisms of vascular damage in obstructive sleep apnea.Nat Rev Cardiol2010; 7: 677–685.
25 Somers VK, Dyken ME, Clary MP,et al. Sympathetic neural mechanisms in obstructive sleep apnea.J Clin Invest1995; 96:1897–1904.
26 Dumitrascu R, Heitmann J, Seeger W,et al. Obstructive sleep apnea, oxidative stress and cardiovascular disease: lessons from animal studies.Oxid Med Cell Longev2013; 2013:234631.
27 Drager LF, Yao Q, Hernandez KL,et al. Chronic intermittent hypoxia induces atherosclerosis via activation of adipose angiopoietin-like 4.Am J Respir Crit Care Med2013; 188:240–248.
28 Buchner S, Satzl A, Debl K,et al. Impact of sleep-disordered breathing on myocardial salvage and infarct size in patients with acute myocardial infarction.Eur Heart J2014; 35:192–199.
29 Kent BD, Garvey JF, Ryan S,et al. Severity of obstructive sleep apnoea predicts coronary artery plaque burden: a coronary computed tomographic angiography study.Eur Respir J2013; 42: 1263–1270.
30 Lee CH, Khoo SM, Tai BC,et al. Obstructive sleep apnea in patients admitted for acute myocardial infarction. Prevalence,predictors, and effect on microvascular perfusion.Chest2009;135: 1488–1495.
31 Meng S, Fang L, Wang CQ,et al. Impact of obstructive sleep apnoea on clinical characteristics and outcomes in patients with acute coronary syndrome following percutaneous coronary intervention.J Int Med Res2009; 37: 1343–1353.
杂志排行
Journal of Geriatric Cardiology的其它文章
- Comparison of the safety and efficacy of two types of drug-eluting balloons(RESTORE DEB and SeQuent® Please) in the treatment of coronary in-stent restenosis: study protocol for a randomized controlled trial (RESTORE ISR China)
- Comparison of in-hospital outcomes between octogenarians and nonagenarians undergoing transcatheter aortic valve replacement: a propensity matched analysis
- Patterns of in-hospital mortality and bleeding complications following PCI for very elderly patients: insights from the Dartmouth Dynamic Registry
- New predictors of in-stent restenosis in patients with diabetes mellitus undergoing percutaneous coronary intervention with drug-eluting stent
- Adherence to pharmacological and non-pharmacological treatment of frail hypertensive patients
- Long term outcomes of drug-eluting stent versus coronary artery bypass grafting for left main coronary artery disease: a meta-analysis
