A study on JA-and BTH-induced resistance of Rosa rugosa‘Plena’to powdery mildew(Sphaerotheca pannosa)
2018-05-19JunxinYanYananDengJiaYuYongqiangZhangDefuChi
Junxin Yan•Yanan Deng•Jia Yu•Yongqiang Zhang•Defu Chi
Introduction
Roses,one of the most important commercial crops,have a long history in the service of humankind(Rout et al.1999;Uggla and Carlson-Nilsson 2005;Pati et al.2006).Rosa rugosa‘Plena’,belonging to the genusRosa,is a perennial shrub with multiple values such as for ornamentals,and sources of food and medicines(Zhang et al.2014).In recent years,the exploitation and industrial production ofRosa-based products have been growing rapidly.However,with the extensive cultivation and application ofR.rugose‘Plena’,it has been reported to be affected by severe pests and diseases(Yan et al.2017).Powdery mildew,Sphaerotheca pannosa(Wallr.),is one of the most common and important plant diseases and has a signi ficant negative impact on plant development,even leading to death of the plant(Pasini et al.1997;Mortensen and Gislerød 2005).Traditional control by pesticides on the pathogenic bacteria pollutes environment and damages its pharmacological ef ficacy and edible value(Zhao et al.2017).Studies on the induction of plant disease resistance provide new ideas for exploring environmentally friendly methods of disease control(Zhao et al.2003;Wang 1994;Chen et al.2007;Terry and Joyce 2004;Walters et al.2005).
Peroxidase(POD)is an important active oxygen scavenger in plant cells.It catalyzes the oxidation reaction of phenolic substances and synthesis of coniferyl alcohol,a precursor of lignin.It participates in a polymerization reaction at the final step of lignin synthesis,so it has a close correlation with plantdisease resistance (He 2001;Shivakumar et al.2003;Yanti 2015).Phenylalanine ammonia-lyase(PAL)is another defensive enzyme and involved in the generation and sediment of lignin in cells.Polyphenol oxidase(PPO)can catalyze the oxidation of phenolic substances to quinones which have a higher toxicity to pathogenic bacteria as compared with phenolic substances.The PAL and PPO both promote the ligni fication of cell walls to defend against various pathogenic bacteria(Wang et al.2005;Babu et al.2015).A previous study showed that increasing PAL,PPO and POD activities could re flect the dynamics of plant disease resistance and be considered as a biochemical index(Ran et al.2004;Duzan et al.2005).
Phenolic substances can accumulate in plants that have been infected by pathogenic bacteria or were treated by inductors.These phenolic substances could inhibit the growth and spore production of pathogenic bacteria and in addition,they would induce the generation of flavonoids such as pisatin and phaseolin to prevent secondary infection by some pathogenic bacteria(Chen et al.2010a,b;Aires et al.2011).When infected by pathogenic bacteria,treatment by a biological agent or induction by physical and chemical factors increases the lignin content signi ficantly,leading to the thickening of cell walls which is bene ficial to prevent the spread of infection of pathogenic bacteria(Ren et al.2007).Plant secondary metabolites play important roles in improvement of disease resistance.
Jasmonic acid(JA)is a key hormone in plant resistance to pathogenic bacteria and pests.It is a signal molecule inducing the expression of resistance genes(Niu et al.2011;Liu et al.2006;Gaige et al.2010).When plants are infected by fungi,jasmonic acid can induce physiological changes and form a defensive structure(Lorenzo et al.2004).Benzothiadiazole(BTH)is an arti ficially synthesized inductor,the most commonly used chemical systemic-acquired resistance(SAR)inducer(Perazzolli et al.2008).It does not display any noticeable direct activity against pathogens,but does increase crop resistance to diseases caused by viruses,bacteria and fungi by activating SAR-signaling pathways(Bovie et al.2004)and plant priming(Kohler et al.2002).Recent studies have shown that BTH has signi ficant induced-resistance to some fungal diseases of crops such as:downy mildew in cucumber,powdery mildew in melon,Sclerotinia sclerotiorumin cauli flower,Alternariasolanin potato,Peronospora hyoscyamiin tobacco(Bokshi et al.2003;Perez et al.2003;Cheng et al.2006;Chen et al.2011;Sun et al.2012;Sillero et al.2012).However,it is still not well known if inducedresistance to powdery mildew ofR.rugosa‘Plena’could be induced by applying JA or BTH,and how their resistancephysiological index might be changed.
In this study,we investigated the effects of JA and BTH onR.rugosa‘Plena’resistance.The results obtained will provide a reference to the study of disease resistance inducing ofR.rugosa‘Plena’and might provide new ideas for exploring environmentally friendly methods of plant disease control.
Materials and methods
Materials
2-year-old healthyR.rugosa‘Plena’seedlings with similar growth vigor were used.
Induction and inoculation methods
Based on preliminary experimental results,three concentrations of JA and three of BTH were used in this experiment.JA and BTH at concentrations of 0.1,0.5 and 1.0 mmol/L were sprayed on the leaves ofR.rugosa‘-Plena’.The inductive agents were evenly sprayed on the leaves at a dosage of 20 ml per seedling.Each concentration treated 40 seedlings.S.pannosainoculation was carried out by leaf spraying 2 days(48 h)after spraying with JA or BTH.Spores ofS.pannosawere obtained from powdery mildew-infected leaves on outdoor cultivatedR.rugosa‘Plena’and were diluted with distilled water to a suspension liquid with a concentration of 3×104spores/ml for inoculation.Distilled water spraying alone was used as the control 1(CK1),and distilled water+spores inoculation was control 2(CK2).Three replications were conducted for each treatment.
Leaf samples were collected on the 1st,3rd,5th,7th,9th,11th and 14th day the second day after inductive agents were sprayed for 24 h,as well as on the 1st,3rd,5th,7th,9th and 12th day afterS.pannosawas inoculated.Samples were immediately sealed into plastic bags,stored in a freezer at-80°C for measurement.
Index measured and methods
Statistics on disease injury
The third day afterS.pannosainoculation,morbidity was investigated at intervals of 2 days,including the number of leaves at different infection classi fications,as well as morbidity ratios,disease index,and inductive effects.Diseased leaf classi fication index was based on ‘‘Plant Disease Research Methods’’(Yan et al.2013a,b).In this study,disease degree was classi fied into five classes(Table 1).
Physiological parameters
PAL was measured according to the method described by Hu et al.(2009)and Yan et al.(2013a,b).A 0.2 g leaf sample was weighed and ground in a mortar with liquid nitrogen,and 5-ml of an ice-cold borate buffer(5 mmol/L,pH 8.8)was added.The homogenates were centrifuged at 6000rmin-1for 20 min at 4°C.The supernatant was used as a crude extract for PAL activity assay.A 0.05 ml supernatant was mixed with 1.0 ml of 0.02 mol/L phenylalanine and 2.95 ml distilled water to produce a 4-ml reaction system.The mixtures were shaken well and transferred to a quartz cuvette and measured at 290 nm.The mixtures were placed in a thermostatic water bath at 30°C for 30 min,and measured again at 290 nm.The reaction system without substrate had 1.0 ml distilled water added and was taken as the control.The results were expressed as U min-1g-1Fw.
PPO measurement is based on Yan et al.(2013a,b).A 0.5 g leaf sample was weighed and ground under liquid nitrogen,and 5-ml of 0.05 mol/L phosphate buffer(pH 7.0)added.Thehomogenatewascentrifugedat4000rmin-1for 15 minat4°C.A0.1 mlsupernatantwasmixedwith1.5 ml of 0.02 mol/L pyrocatechol and 1.5 ml phosphate buffer.Thereactionsystemwasleftstandingat30°Cfor2 minand then measured at 398 nm.One unit of enzyme activity was de fined as an increase in absorbance of 0.01/min.
Peroxidase(POD)was measured as described by Yan et al.(2013a,b)and Li et al.(2016).A 0.3 g leaf sample was weighed and placed into a pre-cooled mortar for grinding and 5 ml of 0.2 mol/L sodium phosphate buffer(pH 7.4)was added.The homogenate was centrifuged at 3000rmin-1at 4°C for 10 min.The supernatant was used as a crude enzyme solution and 0.1 ml of the supernatant and 2 μl guaiacol were transferred to a quartz cuvette.Optical density was record at 470 nm immediately within 2 min after adding 0.6%H2O2.The control mixture contained no hydrogen peroxide.Enzyme activity was measured at an absorbance of OD470,one unit of enzyme activity was de fined as an increase in absorbance of 0.01/min.Enzyme activity was expressed as U min-1g-1Fw.
Total phenolics and flavonoids were measured according to the measurement of Lin et al.(1995)with a minor modification.A 0.2 g leaf sample was ground in a pre-cooled mortarwith 5 ml ofa1%HCl-methanol solution.Theliquid was centrifuged at 6000rmin-1at 4°C for 20 min,and then measured at 280 nm after standing at 4°C for 24 h.Totalphenoliccontent wascalculatedonthebasisofagallic acidstandardcurve(y=0.0066x+0.0077,R2=0.9963),expressed by mg g-1.Flavonoid content was measured at 325 nm,expressed by mg g-1.
LignincontentwasmeasuredaccordingtoYuetal.(2013)and Wang et al.(2015)with a minor modi fication.A 0.2 g leafsamplewasgroundin95%ethylalcohol.Theliquidwas filtered and washed in 95%ethyl alcohol three times and subsequently three times in a mixture of ethanol and n-hexane(1:2,V/V).Thesedimentobtainedwasdissolvedin 25%acetyl bromide glacial acetic acid,and then placed in a waterbathof70°Cfor30 min.A0.9 mLof2 mol/LNaOH,3 mL glacial acetic acid and 0.1 mL 7.5 mol/L hydroxylaminehydrochloridewereaddedinsequencetothereaction systemandcentrifugedat4500rmin-1for10 min.A20 μL supernatantwasdilutedbyaddingthreemLofdistilledwater and measured at 280 nm.
Data analysis
The data were analyzed using SPSS 19.One-Way ANOVA and LSD testing was used for signi ficance analysis of difference.
Results and analysis
Effects of JA and BTH on leaf morbidity ratio
As shown in Table 2,the disease indices of leaves treated by different concentrations of JA and BTH were lower thanthose in CK2,indicating inhibitory effects of JA and BTH treatment on theS.pannosa.The inductive effect of 0.5 mmol/L BTH was optimal on the third day after inoculation,reaching 66.36%.After this point,it decreased with increasing time;on the 12th day it dropped to 36.36%.The 0.5 mmol/L JA had the optimal inductive rate of 54.49%on the third day,with an inductive effect of 27.37%on the 12th day.The disease indices of JA-and BTH-treated leaves on the 3rd to 9th day were signi ficant lower than that of CK2(p<0.01);although the inductive effects of 0.1 mmol/L JA and/or BTH both decreased significantly on the 12th day,the disease index of the treated groups was still lower than that of CK2(p<0.05).In summary,JA and BTH treatments had inhibitory impacts on powdery mildew onR.rugosa‘Plena’,among them,the inductive effect of 0.5 mmol/L BTH was better than the other treatments.
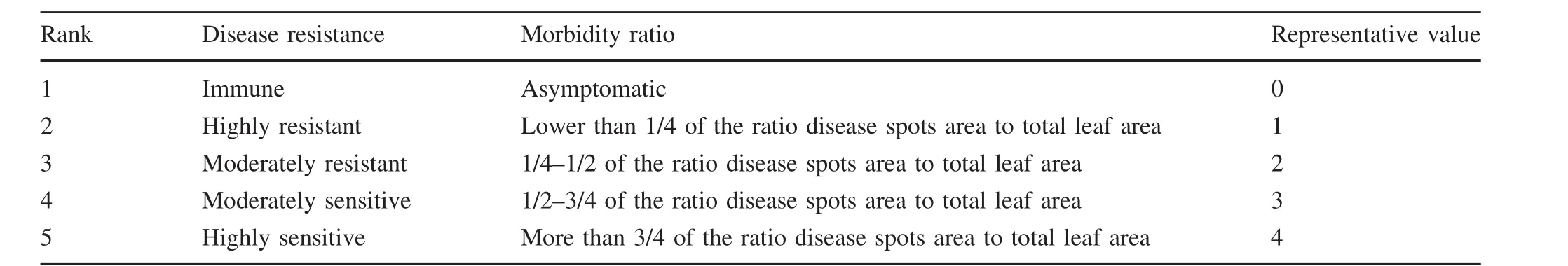
Table 1 Rank of S.pannosa infected leaves of Rosa rugosa‘Plena’
Effects on defensive enzymes of Rosa rugosa ‘Plena’induced by JA and BTH
PAL activity
Figure 1 shows that the activity of PAL in leaves treated with different concentrations of JA and BTH increased at first and then decreased subsequently.For the JA-treated leaves,PAL activity of 1.0 mmol/L JA was significantly higher than that of CK1 and CK2(p<0.05)at the same point in time.The maximum value of PAL activity occurred on the seventh day(the fifth day after inoculation withS.pannosa)after spraying with 1.0 mmol/L JA which was 1.58 times higher than that of CK1 and 1.29 times higher than that of CK2.Leaves treated with 0.5 mmol/L BTH showed higher PAL activity.The maximum value occurred on the seventh( fifth)day,1.72 times as high as that of CK1 and 1.41 times as high as CK2.PAL values of 0.5 mmol/L BTH treated in the other time periods were also significantly higher than that of CK1,and CK2(p<0.05).

Table 2 Disease index of S.pannosa on R.rugosa‘Plena’leaves treated with JA and BTH after inoculation
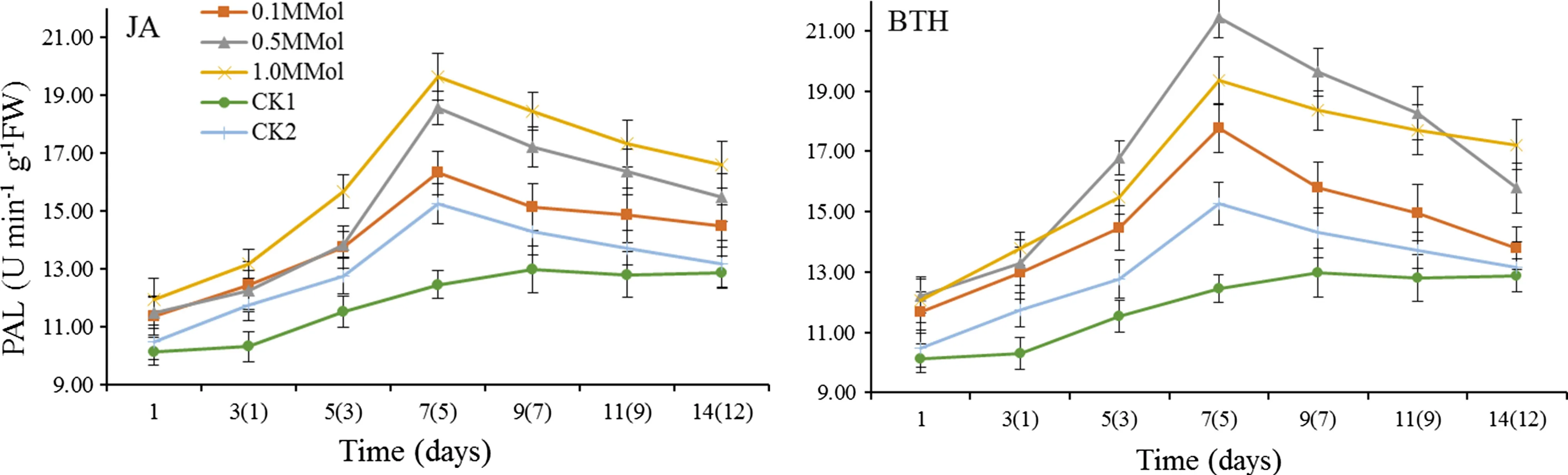
Fig.1 PAL activity of R.rugosa‘Plena’induced by JA and BTH(Parentheses indicated the numbers of days after S.pannosa was inoculated)
PPO activity
As shown in Fig.2,PPO activity in the leaves treated by different concentrations of JA and BTH took on a trend of increasing first and decreasing subsequently.The maximum activity of PPO was in leaves treated with 0.5 mmol/L JA and BTH.PPO activity was significantly higher than that of CK1 and CK2(p<0.05)from the first day after spraying,reaching the maximum value on the seventh( fifth)day.The maximum value treated with 0.5 mmol/L JA was 1.50 times as high as that of CK1 and 1.29 times as high as that of CK2.In the 0.5 mmol/L BTH treatment,it was 1.58 times as high as that of CK1 and 1.35 times as high as that of CK2.
POD activity
POD activity of the treated leaves also took on a trend of increasing first and decreasing subsequently(Fig.3).POD activity was highest in the 1.0 mmol/L JA and BTH treated groups,increasing from the first day after spraying,reaching the maximum value on the seventh( fifth)day,and then starting to decrease.During the whole sampling period,the POD values were higher than that of CK1 and CK2(p<0.05).ThemaximumPODvalueof1.0 mmol/LJAwas 1.89 times as high as that of CK1,and 1.43 times as high as CK2.The maximum POD value of 1.0 mmol/L BTH was 2.18 times as high as that of CK1,and 1.65 times as high as CK2.POD activity of the 0.5 mmol/L BTH treatment was highest on the seventh( fifth)day,being significantly higher than that of CK1 and CK2(p<0.05),and 1.75 and 1.33 times higher than CK1 and CK2,respectively.At the concentration of 0.5 mmol/L,the POD value of the BTH treatment was higher than that of the JA treatment during the sampling period but with no signi ficant difference.
Effects on total phenolic content of Rosa rugosa‘Plena’induced by JA and BTH
As shown in Fig.4,the total phenolic content ofR.rugosa‘Plena’treated with JA and BTH showed a trend of increasing first and decreasing subsequently;the optimal concentration for inducing was 0.5 mmol/L for both inductive agents.
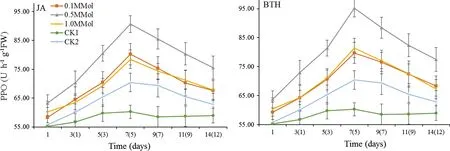
Fig.2 PPO activity of R.rugosa‘Plena’induced by JA and BTH
The total phenolic content of the 0.5 mmol/L JA treatment was higher than that of CK1 and CK2 at all time periods(p<0.05).The maximum value was on the seventh( fifth)day which was 1.68-times higher than CK1 and 1.47-fold higher than CK2.Total phenolic content of the 0.5 mmol/L BTH treatment showed a similar trend to that of the 0.5 mmol/L JA treatment,total phenolic content was 1.90-and 1.66-fold higher than CK1 and CK2(p<0.05),respectively.
Effects on flavonoid content of Rosa rugosa ‘Plena’induced by JA and BTH
Flavonoid content ofR.rugosa‘Plena’treated with JA and BTH increased first and decreased subsequently(Fig.5).During the sampling period,the flavonoid content in the 0.5 mmol/L JA treatment was significantly higher than in the CK1 and CK2 treatments(p<0.05),and reached a maximum value on the seventh( fifth)day.This was 2.5-and 1.42-fold higher than CK1 and CK2,respectively.After treatment with 1.0 mmol/L BTH,there was an induced rapid increase in flavonoid content which was significantly higher than CK1 and CK2(p<0.05).The maximum value occurred on the seventh( fifth)day which was 2.37 and 1.34 times higher than CK1 and CK2,respectively.In summary,the 0.5 mmol/L JA treatment produced the highest flavonoid contentofallthe treatments.
Effects on lignin content of Rosa rugosa ‘Plena’induced by JA and BTH
Lignin content in JA-and BTH-treated leaves increased with increasing time after treatment(Fig.6).The optimal inductive effects for the two agents was 0.5 mmol/L.Lignin content in JA treated leaves was 1.17-,1.29-,1.55-,1.76-,1.81-,1.85-,and 1.85-fold higher than CK1 on the 1st,3rd,5th,7th,9th,11th and 14th day,respectively,and was significantly higher than CK2(p<0.05).Similarly,lignin content in BTH treated leaves was significantly higher than in CK1 and CK2(p<0.05)leaves during the 1st–14th day after treatment,with the maximum value of 6.12 OD g-1FW.The optimal concentration for lignin induction by both JA and BTH was 0.5 mmol/L.

Fig.3 POD activity of R.rugosa‘Plena’induced by JA and BTH
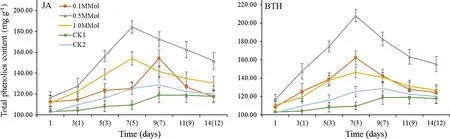
Fig.4 Total phenolic content of R.rugosa‘Plena’induced by JA and BTH
Discussion and conclusions
Co-actions of multi disease-tolerance factors are usually involved in induced tolerance of plants for protection from infection by pathogens but are not depend on a single disease-resistant factor.Plant disease resistance is commonly induced by enzymatic actions.The activity of protective enzymes is usually increased when plants are infected by pathogens or are treated by inductive agents,which is considered an important mechanism for plant disease resistance induction(Mao et al.2005).POD,PPO,and PAL are three vital protective enzymes in plant disease resistance reactions.Increasing their activities is considered a biochemical index of plant disease resistance(Chen et al.2010a,b).POD plays a role in plant disease resistance by catalyzing phenolics to form quinones(Li et al.2016).PPO and POD participate in lignin synthesis,leading to thickness of cell walls to protect plants from infection.(Shivakumar et al.2003;Chen et al.2010a,b;Yanti 2015).PAL is a key and rate-limiting enzyme in catalyzingL-phenylalanine to trans-cinnamic acid,it can improve the disease resistance of plants by promoting the synthesis of phenolic substances and lignin(Xue et al.1983;Zhang et al.1987).Total phenolics and flavonoids were higher in disease resistant cultivars than in disease-sensitive ones(Chen et al.2010a,b);for example,BTH treatment improved cucumber resistance to downy mildew by inducing synthesis of phenolics and lignin(Wang et al.2005).
In this study,different concentrations of JA and BTH increased activities of PAL,PPO,and POD inR.rugosa‘Plena’leaves.This is in agreement with that reported by Yu et al.(2013)and Chen et al.(2011).The optimal concentration for both exogenous BTH and JA was 0.5 mmol/L for inducingR.rugosa‘Plena’resistance toS.pannosa.After treatment with BTH and JA,the total content of phenols, flavonoids and lignin were higher than in the controls.The results in this study are consistent with melon resistance to powdery mildew(Chen et al.2010a,b)and cucumber resistance to downy mildew(Wang et al.2005),but with a difference in induction time possibly due to differences in the stress response ratio of different plants to inductive agents.The results suggest that BTH-and JA-induced increase in activities of the protective enzymes and contents of secondary metabolites is a possible mechanism of enhanced resistance ofR.rugosa‘Plena’toS.pannosa.

Fig.5 Flavonoid content in R.rugosa‘Plena’induced by JA and BTH
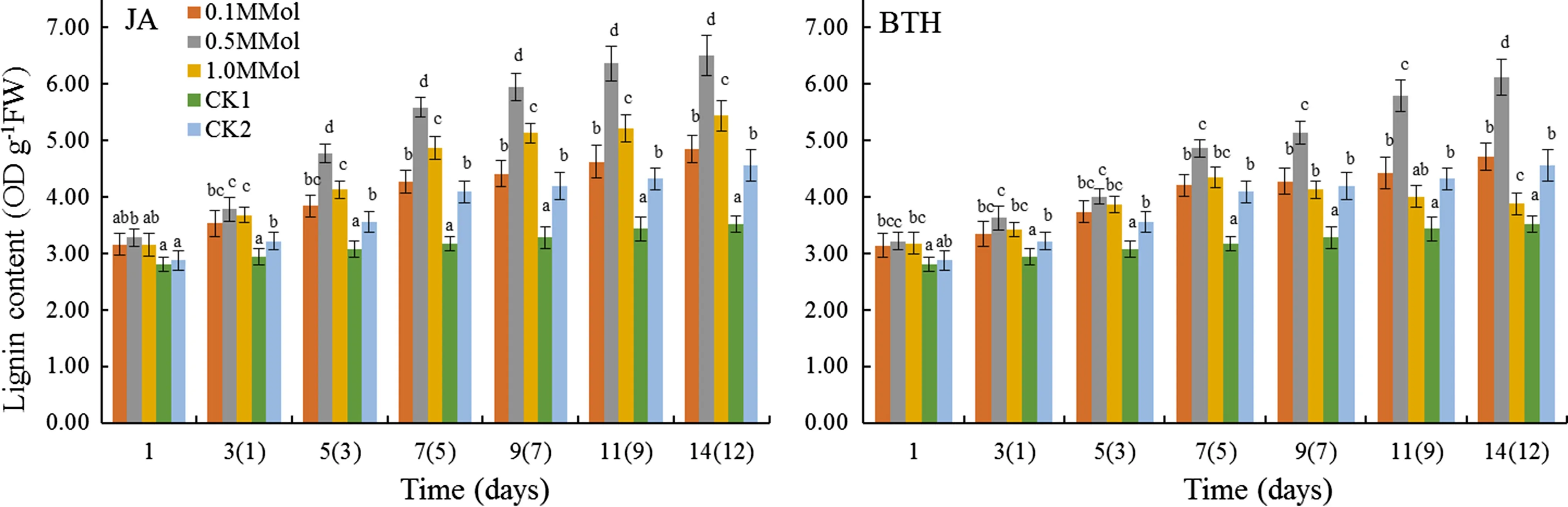
Fig.6 Lignin content of R.rugosa‘Plena’induced by JA and BTH Note:different letters indicates signi ficant differences(p<0.05)among JA or BTH treatments
Plant disease resistance levels depend on a response time and the ratio and quantity of accumulated resistant substancesafterthe expression ofresistance genes.Inductive agents have functions to induce the expression of disease resistance genes and subsequent synthesis and accumulation of disease resistant chemicals(Chen et al.2010a,b).An improvement in disease resistance is the optimal selection for plants in defending against pathogen infections(Zeng et al.2008).Our results demonstrated that different concentrations of JA and BTH induced increased resistance ofR.rugosa‘Plena’leaves toS.pannosa.Leaves treated with 0.5 mmol/L BTH had the maximum resistance toS.pannosa;its inductive effect reached 66.36%.Leaves treated with 0.5 mmol/L JA showed the optimal induction to plant disease resistance with its inductive effect reaching 54.49%.Both JA and BTH can improve the resistance to powdery mildew ofR.rugosa‘Plena’.This result is similar to the research of Chen et al.(2010a,b)and Niu et al.(2011)on melon and wheat.The optimum concentration of induction was different for different species.To conclude,BTH and JA treatments could increase the resistance ofR.rugosa‘Plena’toS.pannosaby improving the activities of the protective enzymes and the accumulation of secondary metabolites.
Plants use different defense signaling pathways to carry out the most effective response to pathogens(Thomma et al.1998).At present,it is generally accepted that there are two main signaling pathways to induce disease resistance in plants.They are salicylate-dependent defense-response pathways and jasmonate-dependentdefenceresponse pathways(Balbi and Devoto 2008).BTH,as a salicylic acid analogue,can induce the production of the salicylic acid pathway(Sun et al.2012).Therefore,we concluded that JA-and BTH-induced resistance ofR.rugosa‘Plena’toS.pannosamight be related to the activation of salicylic acid and jasmonic acid pathways.
References
Aires A,Dias CSP,Carvalho R,Oliveira MH,Monteiro AA,Simo˜es MV,Rosa EAS,Bennett RN,Saavedra MJ(2011)Correlations between diseaseseverity,glucosinolatepro filesand total phenolics andXanthomonas campestrispv.campestrisinoculation of different Brassicaceae.Sci Hortic 129:503–510
Babu AN,Jogaiah S,Ito S,Nagaraj AK(2015)Improvement of growth,fruit weight and early blight disease protection of tomato plants by rhizosphere bacteria is correlated with their bene ficial traits and induced biosynthesis of antioxidant peroxidase and polyphenol oxidase.Plant Sci 231:62–73
Balbi V,Devoto A(2008)Jasmonate signaling network inArabidopsis thaliana:crucial regulatory nodes and new physiological scenarios.New Phytol 177(2):301–318
Bokshi AI,Morris SC,Deverall BJ(2003)Effects of benzothiadiazole and acetylsalicylic acid on β-1 3-glucanase activity and disease resistance in potato.Plant Pathol 52(1):22–27
Bovie C,Ongena M,Thonart P,Dommes J(2004)Cloning and expression analysis of cDNAs corresponding to genes activated in cucumber showing systemic acquired resistance after BTH treatment.BMC Plant Biol 26:4–15
Chen F,Mu XQ,Liang ZS,Zhang HQ,Liao DH(2007)Induced resistance effect of salicylic acid on leaf spot ofAconitum carmichaeliand mechanism.Acta Agric Boreali-Occident Sin 16(2):245–249(in Chinese)
Chen NL,Hu M,Dai CY,Yang SM(2010a)The effects of inducing treatments on phenolic metabolism of melon leaves.Acta Hortic Sin 3(11):1759–1766(in Chinese)
Chen NL,Hu M,Qiao CP,Nai XY,Wang R(2010b)Effects of BTH,SA,and SiO2treatment on disease resistance and leaf HRGP and lignin contents of melon seedlings.Sci Agric Sin 43(3):535–541(in Chinese)
Chen NL,Zhang YX,Wang CL,Dai CY,Zhang JN,Wang XW(2011)Systemic induction of BTH treatment on reactive oxygen species metabolism in melon leaves.Acta Phytophylacica Sin 38(6):499–505(in Chinese)
Cheng ZH,Li YH,Meng HW,Chen P,Du HF(2006)The relationship between BTH-induced resistance to downy mildew in cucumber seedlings and content of HRGP and lignin in cell walls.Sci Agric Sin 39(5):935–940(in Chinese)
Duzan HM,Mabood F,Zhou XM,Souleimanov A,Smith DL(2005)Nod factor induces soybean resistance to powdery mildew.Plant Physiol Biochem 43:1022–1030
Gaige AR,Ayella A,Shuai B(2010)Methyl jasmonate and ethylene induce partial resistance inMedicago truncatulaagainst the charcoal rot pathogenMacrophomina phaseolina.Physiol Mol Plant Pathol 74:412–418
He CY(2001)Induction of enhanced broad-spectrum resistance and defense enzyme activities in rice bybinucleate rhizoctoniaspecies.Acta Phytopathol Sin 31(3):208–212(in Chinese)
Hu ZH,Zhang W,Shen YB,Fu HJ,Su XH,Zhang ZY(2009)Activities of lipoxygenase and phenylalanine ammonia lyase in poplar leaves induced by insect herbivory and volatiles.J For Res 20(4):372–376
Kohler A,Schwindling S,Conrath U(2002)Benzothiadiazoleinduced priming for potentiated responses to pathogen infection,wounding,in filtration of water into leaves requires the NPR1/NIM1 gene in Arabidopsis.Plant Physiol 128:1046–1056
Li DQ,Qin XY,Tian PP,Wang J(2016)Toughening and its association with the postharvest quality of king oyster mushroom(Pleurotus eryngii)stored at low temperature.Food Chem 196:1092–1100
Lin ZF,Li SS,Zhang DL,Lin GZ,Li YB,Liu SX,Chen MD(1995)The changes of pigments,phenolic contents and activities of polyphenol oxidase and phenylalanine ammonia-lyase in the pericarp of postharvest litchi fruit.Acta Bot Sin 30(1):40–45(in Chinese)
Liu X,Zhang SQ,Lou CH(2006)Jasmonic acid signal transduction and it’s relation to abscisic acid signal transduction.Plant Physiol Commun 38(3):285–288(in Chinese)
Lorenzo O,Chico JM,Sa´nchez-Serrano JJ,Solano R (2004)Jasmonate-insensitivel encodes a MYC transcription factor essential to discriminate between different jasmonate-regulated defense response in Arabidopsis.Plant Cell 16(7):1938–1950
Mao XY,Zhang YL,Li MS,Feng ZS(2005)Study on inducement of BTH to resistance effectiveness of Xinjiang Hami melon.Xinjiang Agric Sci 42(3):158–161(in Chinese)
Mortensen LM,Gislerød HR(2005)Effect of air humidity variation on powdery mildew and keeping quality of cut roses.Sci Hortic 104:49–55
Niu JS,Liu J,Ni YJ,Yin J(2011)Induction of PR-1,PR-2,PR-5,Ta-JA2 and wheat powdery mildew resistance in response to MeJA treatment.Acta Phytopathol Sin 41(3):270–277(in Chinese)
Pasini C,D’Aquila F,Curir P,Gullino ML(1997)Effectiveness of antifungal compounds against rose powdery mildew(Sphaerothecapannosavar.rosae)in glasshouses.Crop Prot 16(3):251–256
Pati PK,Rath SP,Sharma M,Sood A,Ahuja PS(2006)In vitropropagation of rose:a review.Biotechnol Adv 24:94–114
Perazzolli M,Dagostin S,Ferrari A,Elad Y,Pertot I(2008)Induction of systemic resistance againstPlasmopara viticolain grapevine byTrichoderma harzianumT39 and benzothiadiazole.Biol Control 47:228–234
Perez L,Rodriguez ME,Rodriguez F,Roson C(2003)Ef ficacy of acibenzolar-S-methyl,an inducer of systemic acquired resistance against tobacco blue mould caused byPeronospora hyoscyamif.sp.tabacina.Crop Prot 22:405–413
Ran LX,Gu WZ,Wu GJ(2004)Role of salicylic acid in induction of resistance against Bacterial Wilt inEucalyptus urophyllaand changes of peroxidase and polyphenol oxidase.For Res 17(1):12–18(in Chinese)
Ren Q,Hu YJ,Li ZY,Jin YJ(2007)Content variation of lignin and peroxidase activities from damagedPinus massioniana.Acta Ecol Sin 27(11):4895–4899(in Chinese)
Rout GR,Samantaray S,Mottley J,Das P(1999)Biotechnology of the rose:a review of recent progress.Sci Hortic 81:201–228
Shivakumar PD,Geetha HM,Shetty HS(2003)Peroxidase activity and isozyme analysis of pearl millet seedlings and their implications in downy mildew disease resistance.Plant Sci 164:85–93
Sillero JC,Rojas-Molina MM,Avila CM,Rubiales D(2012)Induction of systemic acquired resistance against rust,ascochyta blight and broomrape in faba bean by exogenous application of salicylic acid and benzothiadiazole.Crop Prot 34:65–69
Sun RR,Peng Z,Cheng L,Zhu XF,Shao TL,Lu G(2012)Studies on BTH-induced resistance toSclerotinia sclerotiorumin cauliflower and physiology basis. Acta Phytopathol Sin 42(3):281–289(in Chinese)
Terry LA,Joyce DC(2004)Elicitors of induced disease resistance in postharvest horticultural crops:a brief review.Postharvest Biol Technol 32:1–13
Thomma BPHJ,Eggermont K,Penninckx IAMA,Mauch-Mani B,Vogelsang R,Cammue BPA,Broekaert WF(1998)Separate jasmonate-dependent and salicylate-dependent defense-response pathways inArabidopsisare essential for resistance to distinct microbial pathogens. Proc Natl Acad Sci USA 95(25):15107–15111
Uggla M,Carlson-Nilsson BU(2005)Screening of fungal diseases in offspring from crosses betweenRosasectionsCaninaeandCinnamomeae.Sci Hortic 104:493–504
Walters D,Walsh D,Newton A,Lyon G(2005)Induced resistance for plant disease controls:maximizing the ef ficacy of resistance elicitors.Phytopathology 95:1368–1373
Wang J(1994)Development on the researches of induced resistance in plants.J South China Agric Univ 15(4):121–126(in Chinese)
Wang L,Huang LL,Kang ZS,Gao XN(2005)Ef ficiency of cucumber resistance induced by BTH to downy mildew.Acta Phytopathol Sin 35(3):274–277(in Chinese)
Wang C,Hu D,Liu X,She HZ,Ruan RW,Yang H,Yi ZL,Wu DQ(2015)Effects of uniconazole on the lignin metabolism and lodging resistance of culm in common buckwheat(Fagopyrum esculentumM.).Field Crops Res 180:46–53
Xue YL,Ouyang GC,Ao SG(1983)Studies on plant phenylalanine ammonia-lyase(PAL):iV the dynamic changes of PAL activity in rice seedling.Acta Phytophysiol Sin 9(3):301–305 (in Chinese)
Yan JX,Chi DF,Zhang YQ,Pang HY(2013a)Effects of JA and SA on the growth and development as well as defensive enzyme activity ofRosa rugosa.J Beijing For Univ 35(3):128–136(in Chinese)
Yan JX,Chi DF,Zhang YQ,Yu J,Zhang XJ(2013b)Salicylic acid induced resistance ofRosa rugosa‘Plena’toSphaerotheca pannosa(Wallr.).J Northeast For Univ 41(8):95–101 (in Chinese)
Yan JX,Xu LX,Yu J,Zhang YQ,Chi DF(2017)Effects of MeJA on the insect-resistant physiological indexes ofRosa rugosa‘Plena’and the feeding ofMonolepta hieroglyphica.J Northeast For Univ 45(1):77–81(in Chinese)
Yanti Y(2015)Peroxidase enzyme activity of rhizobacteria-introduced shallots bulbs to induce resistance of shallot towards bacterial leaf blight(Xanthomonas axonopodispvallii).Proced Chem 14:501–507
Yu CG,Huang XY,Li TL,Liu ZH(2013)Effect of calcium on lignin synthesis induced by chemical elicitors.J Plant Nutr Fertil 19(6):1445–1449(in Chinese)
Zeng RS,Su YJ,Ye M,Xie LJ,Chen M,Song YY(2008)Plant induced defense and biochemical mechanisms.J South China Agric Univ 29(2):1–6(in Chinese)
Zhang JT,Duan GM,Yu ZY(1987)Relationship between phenylalanine ammonia-Lysae(PAL)activity and resistance to rice blast.Plant Physiol Commun 6:34–37(in Chinese)
Zhang XQ,Yan JX,Yang JY,Ma ZS,Shu WB,Meng QD(2014)Effects of induced resistance ofRosa rugosa‘Plena’on the activities of detoxifying enzymes inMonolepta hieroglyphica(Motschulsky).JNortheastForUniv 42(5):125–128 (in Chinese)
Zhao JH,Sun SJ,Li JZ(2003)A progress on the study of plant induced resistance and elicitors.Plant Prot 29(4):7–10(in Chinese)
Zhao L,Feng CH,Wu K,Chen WB,Chen YJ,Hao XA,Wu YF(2017)Advances and prospects in biogenic substances against plant virus:a review.Pestic Biochem Physiol 135:15–26
杂志排行
Journal of Forestry Research的其它文章
- In vitro propagation of conifers using mature shoots
- ‘Relationships between relationships’in forest stands:intercepts and exponents analyses
- Effects of application date and rate of foliar-applied glyphosate on pine seedlings in Turkey
- Assaying the allelopathic effects of Eucalyptus camaldulensis in a nursery bed incorporated with leaf litter
- Effects of soil compaction on growth variables in Cappadocian maple(Acer cappadocicum)seedlings
- Variation and selection analysis of Pinus koraiensis clones in northeast China
