A meta-analysis of experimental warming effects on woody plant growth and photosynthesis in forests
2018-05-19YonggeYuanLitaoGeHaishuiYangWeizhengRen
Yongge Yuan•Litao Ge•Haishui Yang•Weizheng Ren
Introduction
Forest ecosystems cover approximately 35%of the world’s land surface,contribute to~70%of terrestrial net primary productivity,and are responsible for 66%of worldwide photosynthesis(Meyer and Turner 1992;Melillo et al.1993;Nagy et al.2000;Valentini et al.2000).Studies suggest that climate change,especially increasing temperatures,may fundamentally alter ecosystem functioning through its effect on plant growth and photosynthesis(Eamus and Jarvis 1989).Previous experiments have been carried out in an effort to better understand the effects of climate warming on growth and photosynthesis(Luomala et al.2005;Fu et al.2014).However,a general pattern of these effects is lacking.
Warming could affect production and physiology via impacting temperature-dependent parameters(Fu et al.2014).These include concentrations of photosynthetic pigments,chlorophyll(Chl) fluorescence parameters,stomatal conductance(gs),rates of carboxylation(Vcmax),apparent quantum yield of the photochemical ef ficiency of PSII,and the fluorescence parameter ratio(Fv/Fm)(Yin et al.2008;Yang et al.2013).The effect of warming on these parameters could be positive,negative or neutral depending upon the species and experimental conditions.For example,warming decreasedgsand Fv/Fm ofBetula albosinensisBurkill(Chinese red birch),but had no effect onAbies faxonianaRehd&Wilson(Farges fir)(Duan et al.2014).Studies show that photosynthesis generally bene fits from increases ings,Chl pigments concentration,Vcmaxand AQY,but is inhibited by H2O2and MDA(malondialdehyde)(Shi et al.2010).Thus,changes in growth and photosynthesis under climate warming are the results of overall changes of parameters(Fu et al.2014).
Temperature is also a driving force for nutrient cycling which might affect foliar nutrients,including mineral and mobile nutrients(Coley 1998;Sager and Hutchinson 2005).For example,N(nitrogen),P(phosphorous),K(potassium),S(sulphur),and Cu(copper)concentrations in needles ofPinus sylvestrisL.were decreased by warming treatment,whileCa(calcium),Mg(magnesium),B(boron),Zn(zinc),and Mn(magnesium)concentrations in needles were increased(Luomala et al.2005).However,these trends were condition-dependent.Kellomaki and Wang(1997)found elevated temperatures increased foliar N concentration in Scots pine.Moreover,because foliar nutrients are closely related to biochemical capacity for photosynthesis and growth(Luomala et al.2005),warming could affect them indirectly through changes in nutrients.
Increasing,decreasing or neutral impacts of experimental warming have been observed for biomass and/or photosynthesis in forest ecosystems(Bruhn et al.2007;Yin et al.2008;Bronson and Gower 2010;Yang et al.2011;Lavola et al.2013;Li et al.2013).Yin et al.(2008)found that warming stimulated a signi ficant increase in biomass accumulation and photosynthetic performance ofPicea asperataMast.seedlings.Yang et al.(2011)reported that height and biomass ofAbies fabri(Mast.)Craib.were decreased by arti ficially simulated warming at the nearby Gongga Alpine Forest Ecosystem Observation Station which might be caused by a shortage in nitrogen supply.Bronson and Gower(2010)reported that neither photosynthesis or nor respiration ofPicea mariana(Mill.)BSP was affected after 3 years of warming.This result is consistent with a growing number of experiments which have shown species acclimation to increased temperatures for photosynthesis and/or respiration(Gunderson et al.2000).However,we do not know to what extent warming impacts woody plantbiomass and photosynthesis in forest ecosystems.
A meta-analytic method was used in this study to summarize the effects of climatic warming on woody plant physiology and growth and to evaluate the direction and magnitude of the effects of experimental warming.Studies at a global scale have demonstrated that experimental warming could stimulate plant growth(Wu et al.2011).Forest ecosystems have the most terrestrial net primary productivity(Meyer and Turner 1992;Melillo et al.1993;Nagy et al.2000;Valentini et al.2000)and are large CO2sinks(Valentini et al.2000).We hypothesize that warming is bene ficial to woody plants,and the positive response magnitude would be higher than the global average.
Materials and methods
Data collection
Journal articles published before December 2015 were searched using the Web of Science database,and the keywords ‘forest’and ‘warming’, ‘plant’or ‘forest’,and‘temperature’and ‘plant’.The literature survey was intended to be comprehensive with studies on woody species,including trees,shrubs and lianas.Publication years were restricted between 1996 and 2015.Papers published in Chinese were searched in the CNKI database(http://www.cnki.net/).A total of 522 observations from 52 papers(Table S1and S2)were selected and the database included 24 variables related to woody plant growth and physiology.The final database included data from 25 forest sites.Most data were located between latitudes(30°–60°N and S)(See Table S1).
The collected variables included:(1)plant growth parameters of height,basal diameter,shoot length,speci fic leaf area(SLA),total biomass and ratio of root/shoot(R/S);(2)foliar nutrient content,including nitrogen,carbon/nitrogen(C/N),phosphorus,potassium,calcium,magnesium,aluminum,and manganese;(3)photosynthetic parameters of net photosynthetic rate(Pn),leaf transpiration(E),stomatal conductance(gs),Vcmax,maximum photosynthetic rate(Amax),Fv/Fm,yield,concentration of chlorophyll a(Chla),chlorophyll b(Chlb),and carotenoids(Carot).
For each study,we collected latitude,longitude,elevation,species,warming magnitude and duration,and the response variables.In addition,the variables warming magnitude and experimental duration were divided into three classes to reveal general patterns of plant response to warming(for warming magnitude(°C),≤ 2,2–5,>5;for warming duration(month),≤ 12,12–36,>36).Data in tables were directly extracted,and data with graphs,were digitized with GetData software(http://getdata-graph-digi tizer.com/).For experiments along warming gradients,data were only collected at both ends.We identi fied the means,standard deviations(SD),standard errors(SE),and sample sizes(N).SE was transformed to SD when studies only provided the SE value.When SD or SE was missing,SD was estimated from van Groenigen et al.(2011).We first calculated coefficient of variation(CV)for each dataset and then averaged all CVs.The missing SD was estimated by multiplying the reported mean value by the average CV.
Selection criteria were:(1)only studies conducted in forest ecosystems were included,including natural and planted forests;(2)laboratory incubation studies were not included;only field warming manipulation studies were selected;(3)studies included pair-wise control and experimental treatments.Warming and control plots had the same ecosystem and soil types;(4)the warming method,magnitude and experimental duration were clearly recorded,and the measurements of variables in the control and experimental groups were performed at the same temporal and spatial scales;(5)only data from woody plants were used;(6)at least one of the selected variables was measured;and,(7)for multifactorial studies,only control and warming treatment data were used.
Meta-analysis
In this study,Metawin v.2.0(Rosenberg et al.2000)was used to perform the meta-analysis.As a metric for the response of variables to experimental warming,we used the natural log of the response ratio(R),de fined as the‘effect size’(Hedges et al.1999).For a given variable,R was calculated as the ratio of its value in the warming treatment group¯Xtto that in the control group¯Xc,without and with standardization by warming magnitude(Eqs.1,2).

where Ttand Tcare the airtemperature in the control and warming treatments,respectively.
The variance of logeR(v)was approximated using the following formula:

wheresc2andst2are the standard deviations in the control and warming treatments,ncandntare the sample sizes in the control and warming treatments.

Generally,positive or negative data in ecological studies are more likely to be published than neutral data.Therefore,we examined whether our datasets were publicationbiased or not(Peters et al.2006;Yang et al.2014).First,Spearman correlation analysis was performed between the standardized effect size of raw data and sample size.Spearman correlation analysis assesses how well the relationship between two variables can be described using a monotonic function.If no correlation was found,the dataset had no publication bias;or else,we needed to estimate fail-safe number to con firm whether publication bias would in fluence the final conclusions.A fail-safe number is the number of studies that would have to be added to change the results of the meta-analysis from signi ficant to non-signi ficant(Rosenberg et al.2000).In this study we used Rosenthal’s fail-safe calculation of 5 N+10 where N is the original number of studies.
Results
Publication bias test
Publication bias was examined for each dataset of parameters.Spearman correlation analysis showed signi ficant correlation between standardized effect size and sample size for height,yield and foliar Ca content datasets(Spearmanr=0.435,P=0.016 for height,r=0.711,P=0.021 for yield andr=0.424,P=0.049 for foliar Ca content).Statistics suggested that there was slight publication bias for these three datasets.No publication bias was detected in other datasets as no correlation was found.
The fail-safe numbers were 304.1,149.8 and 268.6 for height,yield and foliar Ca content while the respective 5 N+10 values were 155,60 and 120.This indicated that although there was slight publication bias for these datasets,it would not change the overall meaning of the results.
Growth response to warming
Without standardization by warming magnitude,the effect size and 95%CI of experimental warming on shoot length,height,SLA,and total biomass were 0.1264(0.0757–0.1938),0.0547(0.0313–0.0804),0.0798(0.0529–0.0992),and 0.1542(0.0542–0.3029),respectively,and their 95%CI did not cover zero(Fig.1),suggesting a significantly positive effect of warming on plant growth.Warming increased shoot length by 13.5%,height by 5.6%,SLA by 8.3%,and total biomass by 16.7%.Basal diameter(0.0449,-0.0033 to 0.0752)and R/S(-0.0757,-0.2104 to 0.0203)did not respond to warming treatment.
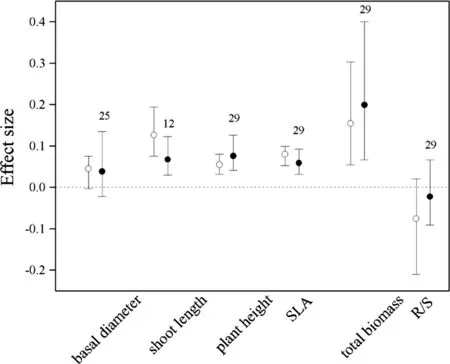
Fig.1 Effect sizes of experimental warming on basal diameter,shoot length,plant height,speci fic leaf area(SLA),and total biomass,without(hollow points)and with(solid points)standardization by warming magnitude.Error bars indicate effect sizes and 95%bootstrap con fidence intervals.The dashed lines are drawn at effect size=0.The sample size for each variable is shown next to the bar
With standardization by warming magnitude,the effect size and 95%CI of experimental warming on shoot length,SLA,plantheightand totalbiomasswere 0.0661(0.0281–0.1245), 0.0577 (0.0299–0.0916), 0.0746(0.0397–0.1241)and 0.1979(0.0649–0.3990),respectively,and their 95%CI did not cover zero,also suggesting a significantly positive effectofwarming on growth.Warming increased shoot length by 6.8%,SLA by 5.9%,height by 7.8%and total biomass by 21.9%.Basal diameter(0.0372,-0.0232 to 0.0595)and R/S(-0.0240,-0.0927 to 0.0648)did not respond to warming treatment(Fig.1).
Photosynthesis and related parameters response to warming
Without standardization by warming magnitude,the effect size and 95%CI was 0.0349(0.0164–0.1180)for net photosynthetic rate(Pn),0.0411 (0.0012–0.1045)for transpiration(E),0.0702(0.0218–0.2748)forgs,0.2226(0.0081–0.5064)forVcmax,0.1265(0.0950–0.2602)forAmax, 0.2976 (0.0927–0.3393) for Chla, 0.2860(0.0414–0.4349)for Chlb,0.4034(0.1107–0.5767)for carotenoid(Carot).Warming significantly increasedPnby 3.6%,transpiration by 4.2%,gsby 7.3%,Vcmaxby 24.9%,Amaxby 13.5%,Chla by 14.2%,Chlb by 34.7%,and carotenoid by 49.7%.The effect of experimental warming on Chla/b(0.0556,–0.0016 to 0.1088),yield(-0.0023,-0.0147 to 0.1663),Fv/Fm(0.0097,-0.0187 to 0.0935)were not signi ficant(Figs.2,3).
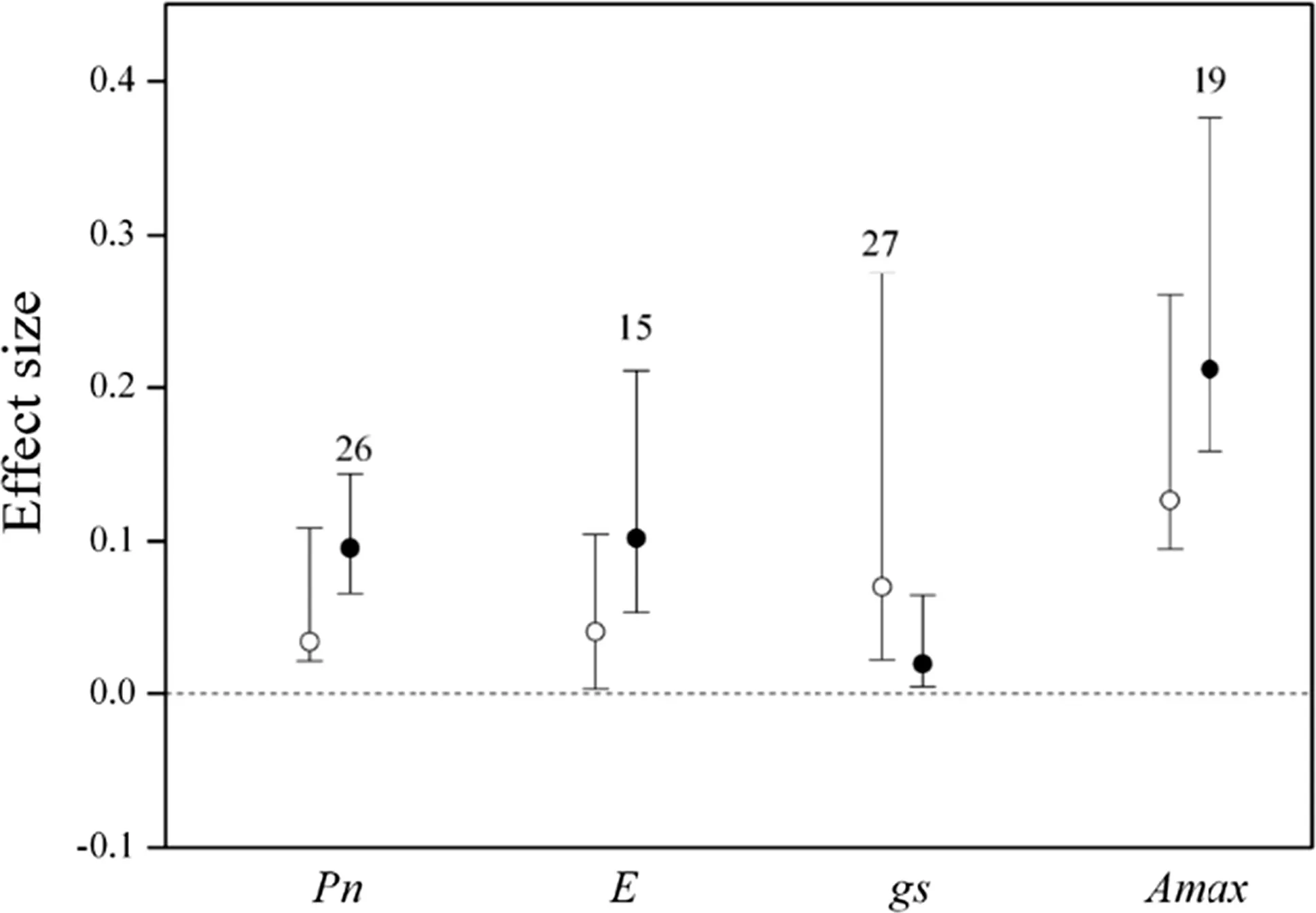
Fig.2 Effect sizes of experimental warming on net photosynthetic rate(Pn),transpiration(E),gs,and Amax,without(hollow point)and with(solid point)standardization by warming magnitude.Error bars indicate effect sizes and 95%bootstrap con fidence intervals.The dashed lines are drawn at effect size=0.The sample size for each variable is shown next to the bar
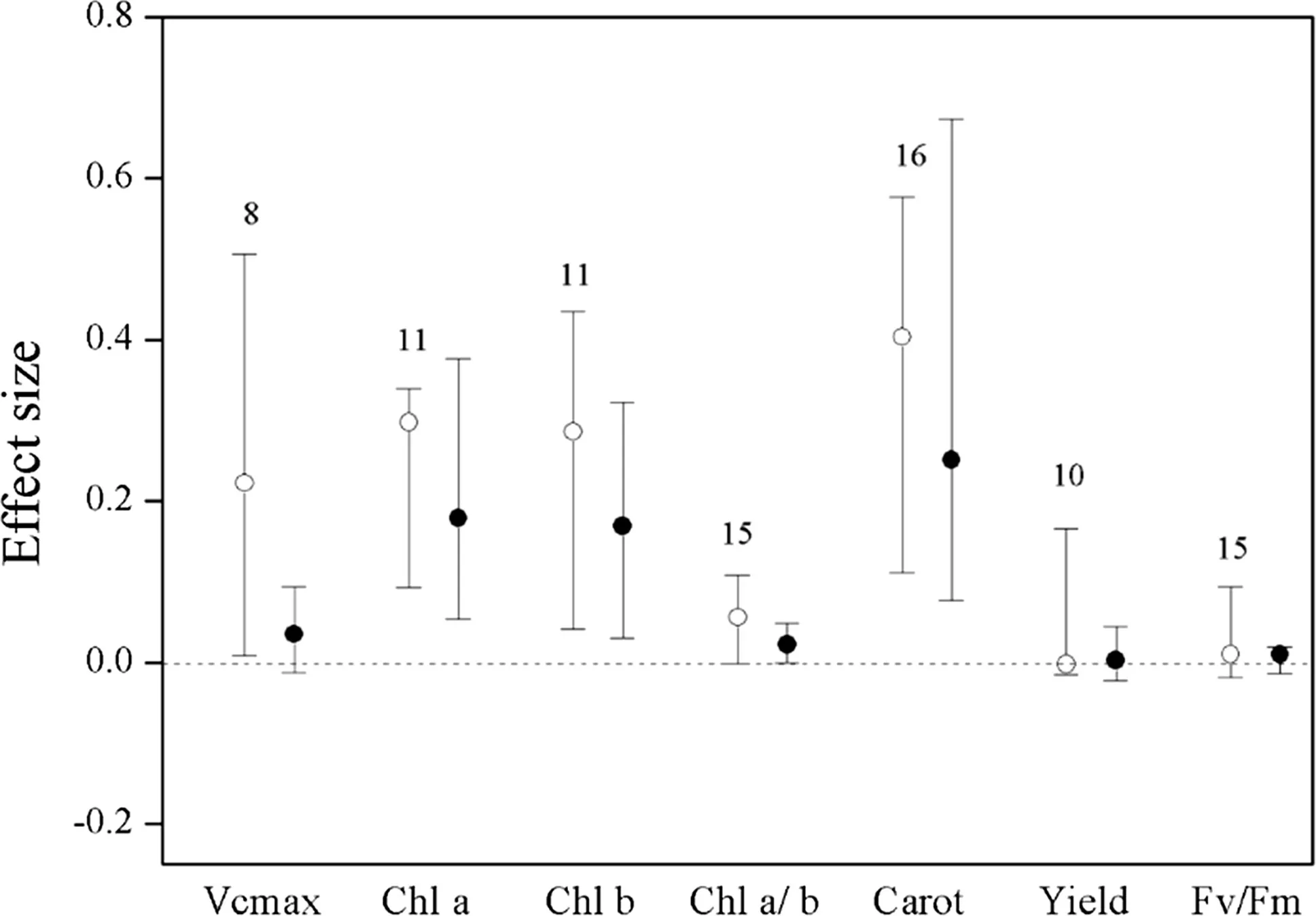
Fig.3 Effect sizes of experimental warming on Vcmax,Chla,Chlb,Chla/b,carotenoid(carot),yield,and Fv/Fm,without(hollow point)and with(solid point)standardization by warming magnitude.Error bars indicate effect sizes and 95%bootstrap con fidence intervals.The dashed lines are drawn at effect size=0.The sample size for each variable is shown next to the bar
With standardization by warming magnitude,the effect size and 95%CI was 0.0946(0.0653–0.1429)forPn,0.1008 (0.0351–0.2132) for transpiration, 0.0179(0.0034–0.0639)forgs,0.2108(0.1574–0.3752)forAmax,0.1768(0.0485–0.3746)for Chla,0.1668(0.0278–0.3211)for Chlb,0.2494(0.0753–0.6435)for carotenoid,respectively,and their 95%CI did not cover zero(Figs.3,4).Warming significantly increased thePnby 9.9%,transpiration by 10.6%,gsby 1.6%,Vcmaxby 21.9%,Amaxby 23.5%,Chla by 19.3%,Chlb by 18.2%,carotenoid by 28.3%.The effect of experimental warming onVcmax,Chla/b,yield,Fv/Fm,was not signi ficant(Figs.2,3).
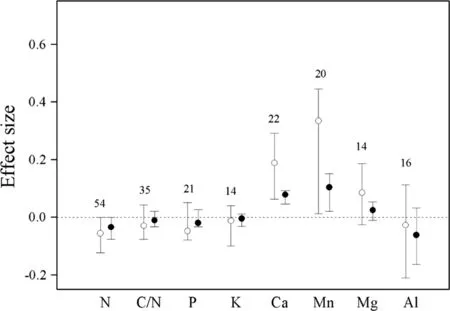
Fig.4 Effect sizes of experimental warming on foliage concentration of N,C/N,P,K,Ca,Mn,Mg,and Al,without(hollow point)and with(solid point)standardization by warming magnitude.Error bars indicate effect sizes and 95%bootstrap con fidence intervals.The dashed lines are drawn at effect size=0.The sample size for each variable is shown next to the bar
Foliage nutrients response to warming
Without standardization by warming magnitude,the effect size and 95%CI of experimental warming on leaf N was-0.0555(-0.1224 to-0.0005),Ca 0.1879(0.0620–0.2906),Mn 0.3337(0.0116–0.4446)and their 95%CI did not cover zero.Warming significantly increased the foliage content of Ca by 20.7%,Mn by 39.6%,and decreased N content by 5.5%.The effect of experimental warming on C/N,P,K,Mg,Al were not signi ficant(Fig.4).
With standardization by warming magnitude,the effect size and 95%CI of experimental warming on leaf N was-0.0369(-0.0789 to-0.0034),Ca 0.0754(0.0428–0.0908),Mn 0.1013(0.0164–0.1464)and their 95%CI did not cover zero.Warming significantly increased the foliage content of Ca by 7.8%,Mn by 10.7%,Mg by 2.3%,and decreased N content by 3.6%.The effect of experimental warming on C/N,P,K,Mg,Al were not signi ficant(Fig.4).
Response to warming magnitude and experimental duration
Warming significantly stimulated woody plant biomass when the magnitude was<5 °C.However,Pnwas not significantly affected when the warming magnitude was>2 °C.When the magnitude was<2 °C,the biomass andPnenhancement was significantly higher than that at the warming magnitudes of 2–5 °C(Fig.S1).
Temperature manipulations caused signi ficant increments in plant biomass at durations of<12 months and>36 months,but not between 12 and 36 months.Pnof woody plants did not show signi ficant enhancement at durations>12 months(Fig.S1).
Discussion
Our meta-analysis provided evidence that when the temperature was between 0.3 and 10°C,growth and photosynthetic rate was significantly increased by 21.9 and 9.9%respectively.These increases were greater than the growth stimulation(12.3%,with a 95%CI of 8.4–16.3%)reported by Lin et al.(2010),andPnstimulation reported by Lu(2013)(4.5%,with a 95%CI of 2.6–6.4%).Lin et al.(2010)and Lu et al.(2013)studied terrestrial plants at a global scale while we focused on forest ecosystems.In forest ecosystems,climatic warming may trigger a stronger positive response of plant growth and photosynthesis than thatofglobalaverages.These resultsveri fied our hypothesis.
Based on our results,increased growth and net photosynthesis by woody plants may have a relationship with changes of temperature-dependent parameters.From our analysis,warming increased the content of photosynthetic pigments(Fig.4).These increases may promote photosynthetic capacity(Carter et al.2000;Yin et al.2008).Warming also increased Fv/Fm and quantum yield,indicating higher excitation energy transfer and higher ef ficiency of using light which re flected higher photosynthetic ef ficiency(Fu et al.2014).As stomatal conductance(gs)could affect photosynthesis,increasedgsin our analysis may indicate higher passage rates of CO2which might have a positive relationship with photosynthetic rates.In addition,other studies have shown that increased temperatures can improve the activity of RuBisCo(Ribulose bisphosphate carboxylase oxygenase)to enhance photosynthesis(Farquhar et al.1980).Increased carboxylation rates(Vcmax)due to warming in our study indicated either enhanced RuBisCo activity or higher concentrations.As leaf nitrogen,which has a close relationship with RuBisCo concentration,did not increase under warming treatments(Farquhar et al.1980),Vcmaxincrease might be caused by improved RuBisCo enzyme activity rather than higher concentrations.
Root/shoot(R/S)ratios re flect the carbon allocation between above-ground and below-ground.Research suggests that R/S ratios may be more reasonable to assess experimental effects on plants than root or shoot biomass alone(Agathokleous et al.2016).From our results,warming did not affect biomass allocation between roots and shoots,and re flected the same stimulation of aboveand below-ground growth.In addition,the effect of warming on R/S ratios was similar to leaf C/N in our study.This similarity might fit the biomass allocation model(Reynolds and Thornley 1982)which shows that R/S ratios should show similar shifts as tissue C/N ratios because when C/N ratios rise,N will become more limiting relative to C and biomass allocation to roots will increase.Speci fic leaf area(SLA)is highly correlated with light exposure(St Clair and Lynch 2004),so increased SLA by warming might have a relationship withPn.
Previous studies have found that climate warming could increase soil organic matter decomposition(Rustad et al.2001;Henry and Moise 2015)which is usually associated with increased release of acidic matte(D’Orangeville et al.2014).The production of acidic matter can result in soil acidi fication(Vancleve et al.1990;Simandi et al.2005).Acidic inputs can lead to the leaching of basic cations such as calcium(Ca)and increase the availability of potentially phytotoxic trace metals such as manganese (Mn)(D’Orangeville et al.2014).Thus increased Mn and Ca concentrations in plant tissues due to warming might be the result of soil acidi fication in forest ecosystems(Sager and Hutchinson 2006;Tian et al.2016).Because foliar concentrations of Ca and Mn are highly correlated with biochemical capacity for plant growth and photosynthesis(Farquhar and Sharkey 1982;Fischer et al.2015;Hochmal et al.2015),the observed changes of plant growth andPnby warming maybe the indirect outcome in changes of foliar Ca and Mn concentrations.
Most data used in our analysis is located between latitudes 30°–60°N and S(See Table S1).The biomass andPnof woody plants varied with warming magnitude and experimental duration.In order to identify the response of plants to climate warming at a global scale more clearly,and to distinguish the role of photosynthetic parameters in this process,multiple field experiments that span various environmental gradients and duration are urgently needed.
Results in this and previous meta-analyses provide evidence that warming generally increases terrestrial net primary productivity(Lin et al.2010)at a forest ecosystem scale and a global scale.The increased growth and net photosynthesis rates by a forest ecosystem could be attributed to the integrated changes of various parameters.Our findings will be useful to predict the response of plant functioning to global climate warming.
References
Agathokleous E,Saitanis CJ,Wang X,Watanabe M,Koike T(2016)A review study on past 40 years of research on effects of tropospheric O3on belowground structure,functioning and processes of trees:a linkage with potential ecological implications.Water Air Soil Pollut 227:33
Bronson DR,Gower ST(2010)Ecosystem warming does not affect photosynthesis or aboveground autotrophic respiration for boreal black spruce.Tree Physiol 30:441–449
Bruhn D,Egerton JJG,Loveys BR,Ball MC(2007)Evergreen leaf respiration acclimates to long-term nocturnal warming under field conditions.Glob Change Biol 13:1216–1223
Carter GA,Bahadur R,Norby RJ(2000)Effects of elevated atmospheric CO2and temperature on leaf optical properties inAcer saccharum.Environ Exp Bot 43:267–273
Coley PD(1998)Possible effects of climate change on plant/herbivore interactions in moist tropical forests.Clim Change 39:455–472
D’Orangeville L,Houle D,Cote B,Duchesne L(2014)Soil response to a 3-year increase in temperature and nitrogen deposition measured in a mature boreal forest using ion-exchange membranes.Environ Monit Assess 186:8191–8202
Duan B,Dong T,Zhang X,Zhang Y,Chen J(2014)Ecophysiological responses of two dominant subalpine tree speciesBetula albosinensisandAbies faxonianato intra-and interspeci fic competition under elevated temperature.For Ecol Manage 323:20–27
Eamus D,Jarvis PG(1989)Direct effects of CO2increases on trees and forests(natural and commercial)in the UK.Adv Ecol Res 19:1–55
Farquhar GD,Sharkey TD(1982)Stomatal conductance and photosynthesis.Ann Rev Plant Physiol Plant Mol Biol 33:317–345
Farquhar GD,Caemmerer VS,Berry JA(1980)A biochemical model of photosynthetic(CO2)assimilation in leaves of C3 species.Planta 149:78–90
Fischer WW,Hemp J,Johnson JE(2015)Manganese and the evolution of photosynthesis.Orig Life Evol Biosph 45:351–357
Fu G,Shen ZX,Sun W,Zhong ZM,Zhang XZ,Zhou YT(2014)A meta-analysis of the effects of experimental warming on plant physiology and growth on the Tibetan Plateau.J Plant Growth Regul 34:57–65
Gunderson CA,Norby RJ,Wullschleger SD(2000)Acclimation of photosynthesis and respiration to simulated climatic warming in northern and southern populations ofAcer saccharum:laboratory and field evidence.Tree Physiol 20:87–96
Hedges LV,Gurevitch J,Curtis PS(1999)The meta-analysis of response ratios in experimental ecology.Ecology 80:1150–1156
Henry HAL,Moise ERD(2015)Grass litter responses to warming and N addition:temporal variation in the contributions of litter quality and environmental effects to decomposition.Plant Soil 389:35–43
Hochmal AK,Schulze S,Trompelt K,Hippler M(2015)Calciumdependent regulation of photosynthesis.Biochimica Et Biophysica Acta-Bioenergetics 1847:993–1003
Kellomaki S,Wang KY(1997)Effects of long-term CO2and temperature elevation on crown nitrogen distribution and daily photosynthetic performance of Scots pine.For Ecol Manage 99:309–326
Lavola A,Nybakken L,Rousi M,Pusenius J,Petrelius M,Kellomaki S,Julkunen-Tiitto R(2013)Combination treatment of elevated UVB radiation,CO2and temperature has little effect on silver birch(Betula pendula)growth and phytochemistry.Physiol Plant 149:499–514
Li Y,Zhang Y,Zhang X,Korpelainen H,Berninger F,Li C(2013)Effects of elevated CO2and temperature on photosynthesis and leaf traits of an understory dwarf bamboo in subalpine forest zone,China.Physiol Plant 148:261–272
Lin D,Xia J,Wan S(2010)Climate warming and biomass accumulation of terrestrial plants:a meta-analysis.New Phytol 188:187–198
Lu M,Zhou XH,Yang Q,Li H,Luo YQ,Fang CM,Chen JK,Yang X,Li B(2013)Responses of ecosystem carbon cycle to experimental warming:a meta-analysis.Ecology 94:726–738
Luomala EM,Laitinen K,Sutinen S,Kellomaki S,Vapaavuori E(2005)Stomatal density,anatomy and nutrient concentrations of Scots pine needles are affected by elevated CO2and temperature.Plant,Cell Environ 28:733–749
Melillo JM,McGuire AD,Kicklighter DW,Moore B,Vorosmarty CJ,Schloss AL(1993)Global climate-change and terrestrial net primary production.Nature 363:234–240
Meyer WB,Turner BL(1992)Human-population growth and global land-use cover change.Annu Rev Ecol Syst 23:39–61
Nagy M,Ogawa K,Hagihara A(2000)Interactive effect of CO2enrichment and temperature on the photosynthesis of field-grown hinoki cypress(Chamaecyparisobtusa)branches.Trees-Struct Funct 14:282–288
Peters JL,Sutton AJ,Jones DR,Abrams KR,Rushton L(2006)Comparison of two methods to detect publication bias in metaanalysis.JAMA 295:676–680
Reynolds JF,Thornley JHM(1982)A shoot-root partitioning model.Ann Bot 49:585–597
Rosenberg MS,Adams DC,Gurevitch J.2000.MetaWin:statistical software for meta-analysis,version 2.0..Sinauer Associated,Sunderland
Rustad LE,Campbell GL,Marion GM,Norby RJ,Mitchell MJ,Hartley AE,Cornelissen JHC,Gurevitch J,NEWSGCTE(2001)A meta-analysis of the response of soil respiration,net nitrogen mineralization,and aboveground plant growth to experimental ecosystem warming.Oecologia 126:543–562
Sager EPS,Hutchinson TC(2005)The effects of UV-B,nitrogen fertilization,and springtime warming on sugar maple seedlings and the soil chemistry of two central Ontario forests.Can J For Res 35:2432–2446
Sager EPS,Hutchinson TC(2006)Responses of secondary chemicals in sugar maple(Acer saccharum)seedlings to UV-B,springtime warming and nitrogen additions.Tree Physiol 26:1351–1361
Shi FS,Wu Y,Wu N,Luo P(2010)Different growth and physiological responses to experimental warming of two dominant plant speciesElymus nutansandPotentilla anserinain an alpine meadow of the eastern Tibetan Plateau.Photosynthetica 48:437–445
Simandi P,Takayanagi M,Inubushi K(2005)Changes in the pH of two different composts are dependent on the production of organic acids.Soil Sci Plant Nutr 51:771–774
St Clair SB,Lynch JP(2004)Photosynthetic and antioxidant enzyme responses of sugar maple and red maple seedlings to excess manganese in contrasting light environments.Funct Plant Biol 31:1005–1014
Tian QY,Liu NN,Bai WM,Li LH,Chen JQ,Reich PB,Yu Q,Guo DL,Smith MD,Knapp AK et al(2016)A novel soil manganese mechanism drives plant species loss with increased nitrogen deposition in a temperate steppe.Ecology 97:65–74
Valentini R et al(2000)Respiration as the main determinant of carbon balance in European forests.Nature 404:861–865
van Groenigen KJ,Osenberg CW,Hungate BA(2011)Increased soil emissions of potent greenhouse gases under increased atmospheric CO2.Nature 475:214–216
Vancleve K,Oechel WC,Hom JL(1990)Response of black spruce(Picea-mariana)ecosystems to soil-temperature modi fication in interior Alaska.Can J For Res 20:1530–1535
Wan SQ,Hui DF,Luo YQ(2001)Fire effects on nitrogen pools anddynamics in terrestrial ecosystems:a meta-analysis.Ecol Appl 11:1349–1365
Wu Z,Dijkstra P,Koch GW,PeN˜Uelas J,Hungate BA(2011)Responses of terrestrial ecosystems to temperature and precipitation change:a meta-analysis of experimental manipulation.Glob Change Biol 17:927–942
Yang L,Yang Y,Wang G,Guo J,Yang Y(2011)Short-term effects of warming on growth and stoichiometrical characteristics ofAbies fabiri(Mast.)Craib seedling in Gongga mountain.Acta Ecol Sin 31:3668–3676
Yang Y,Wang GX,Yang LD,Guo JY(2013)Effects of drought and warming on biomass,nutrient allocation,and oxidative stress inAbies fabriin Eastern Tibetan Plateau.J Plant Growth Regul 32:298–306
Yang H,Zhang Q,Dai Y,Liu Q,Tang J,Bian X,Chen X(2014)Effects of arbuscular mycorrhizal fungi on plant growth depend on root system:a meta-analysis.Plant Soil 389:361–374
Yin H,Liu Q,Lai T(2008)Warming effects on growth and physiology in the seedlings of the two conifersPicea asperataandAbies faxonianaunder two contrasting light conditions.Ecol Res 23:459–469
杂志排行
Journal of Forestry Research的其它文章
- In vitro propagation of conifers using mature shoots
- ‘Relationships between relationships’in forest stands:intercepts and exponents analyses
- Effects of application date and rate of foliar-applied glyphosate on pine seedlings in Turkey
- Assaying the allelopathic effects of Eucalyptus camaldulensis in a nursery bed incorporated with leaf litter
- Effects of soil compaction on growth variables in Cappadocian maple(Acer cappadocicum)seedlings
- Variation and selection analysis of Pinus koraiensis clones in northeast China
