Vegetation cover density and disturbance affected arbuscular mycorrhiza fungi spore density and root colonization in a dry Afromontane forest,northern Ethiopia
2018-05-19EmiruBirhaneNakiguliFatumahKidaneGideyAmanuelZenebeSsemwangaMohammed
Emiru Birhane•Nakiguli Fatumah•Kidane Gidey•Amanuel Zenebe•Ssemwanga Mohammed
Introduction
Afromontane forests are known for their species richness around the world(Schmitt et al.2010).In Ethiopia,Afromontane vegetation occupies more than a half of the overall highland area and majority of it being dry Afromontane(Wubet et al.2003).However,the country is highly threatened by increasing rates of deforestation,which has increased the rate of soil erosion and the loss of soil fertility,biodiversity and fragile ecosystems(Chun et al.2003;Lenaerts 2013).These effects of deforestation are increasingly reducing the volume and health of the forestecosystem (Sebhatleab 2012;Lenaerts2013).Restoration and/or re-establishment of this degraded forest ecosystem is,therefore,vital.Restoration of the degraded forest ecosystem can easily be obtained if both the aboveground levels and belowground microorganisms are well conserved(Liu et al.2000).The close linkage between theaboveground systemsand thebelowground soil microorganisms helps to build a healthy ecosystem(Wardle et al.2004).
Belowground soil microorganisms mutually associate with many plant species(Smith and Read 2008);arbuscular mycorrhiza fungi(AMF)are the principal microorganism in these associations(Vani et al.2014).Most woody plant communities(>80%)mutually associatewith these organisms(Zobel and O¨pik 2014);the AMF obtain carbon compoundsfortheirgrowth and development,and numerous bene fits are obtained by plants and soils(Liu et al.2000;Walder et al.2012),such as more water and nutrients via a fungal tree-like network structure(Babikova et al.2013),reduced stress from salts and heavy metals(Hildebrandt et al.2007).Arbuscular mycorrhiza fungi also increase the plants’ability to fight pathogens and pests(Eyles et al.2010).Although AMF provide numerous functions in the natural ecosystem,their spatial and temporal distributions and functioning are still poorly understood(Oliveira and Oliveira 2010).Despite the fact that several research studies have been conducted on how seasons affect the distribution and functioning of AMF in nature(Birhane et al.2010),consistent seasonal patterns in the development of these organisms have not yet been observed(Panwar et al.2011).Moreover,the short-term seasonal patterns and timing of AMF sporulation and colonization have not been investigated thoroughly in the forest ecosystems(Panwar et al.2011).Limited research on the effect of vegetation cover density on AMF has been done in Ethiopia.In addition,Wardle et al.(2004)reported that aboveground systems receive most research attention,while soil microorganisms are often neglected.Hence,a need to understand the in fluence of seasons,vegetation cover density and edaphic and anthropogenic factors on the AMF SD and RC in the Afromontane forest ecosystems is essential.This study was conceived to answer calls for more research-based actions to help restore and re-establish degraded forest ecosystems by maintaining populations of microorganisms aboveground and belowground(Liu et al.2000).
Although many environmental and biotic factors in fluence the distribution and development of these microorganisms,climate, flora and disturbance are the chief factors(Rodrı´guez-Echeverrı´a et al.2008).Physicochemical soil properties and plant species are additional in fluences on AMF activities and distributions(Vyas and Gupta 2014).However,the level of information on the prevalence of AMF in nature is still unsatisfactory(Muthukumar and Udaiyan 2000).Consequently,there is a need for a deeper understanding of these microorganisms for the bene fit of management and maintenance of the health of the entire natural(forest)ecosystem components.This paper,therefore,was aimed at determining the in fluence of seasons,vegetation cover density,edaphic and anthropogenic factors on AMF SD and RC in a forest ecosystem.This paper investigated the AMF SD and RC of trees in wet and dry seasons and in the fenced and unfenced permanent plots having different vegetation cover density.
Materials and methods
Study sites
The research was done in Desa’a dry Afromontane forest located at 39°43′E and 13°45′N in northern Ethiopia(Fig.1).The forest is situated in a semi-arid area with the climate greatly in fluenced by topographic features(Nyssen et al.2005).Annual precipitation in this area is less than 1000 mm with short,seasonal,erratic and irregularly distributed unimodal rainfall with the highest amount between June and September(Aynekulu et al.2011).Annual average rainfall is within 406–700 mm,and the average minimum and maximum temperature varies within 7.5–19.3 °C and 22.6 °C and 33.4 °C,respectively(Fig.2).
The elevation of study site is 1000–2760 m a.s.l(Aynekulu et al.2011).Desa’a forest is located in a matrix of agricultural and heavily grazed land(Sebhatleab 2012).The dominant tree species areJuniperus proceraandOlea europaea(Mokria et al.2015),which are increasingly replaced byAcaciaspecies at lower altitudes.
Study plots
Three permanent study blocks measuring 160×100 m(16,000 m2)with homogeneous soil and vegetation density were established in the study site as described by Giday(2013).The study blocks were established in areas with poor,medium and dense vegetation cover densities.In each of the three study blocks,two management plots of 40×160 m with a 20-m buffer zone between them were established in 2013.One of the two plots from each block was selected randomly and fenced to check for the absence of both human and livestock pressure and deter their potentialeffectonAMF SD andRC.Withinthe 40×160 m plots,40×40 m experimental subplots were established using a split plot design.In the 40×40 m subplots,there were 20×20 m plots for root and rhizosphere soil sampling and 5×5 m plots for physicochemical soil properties.
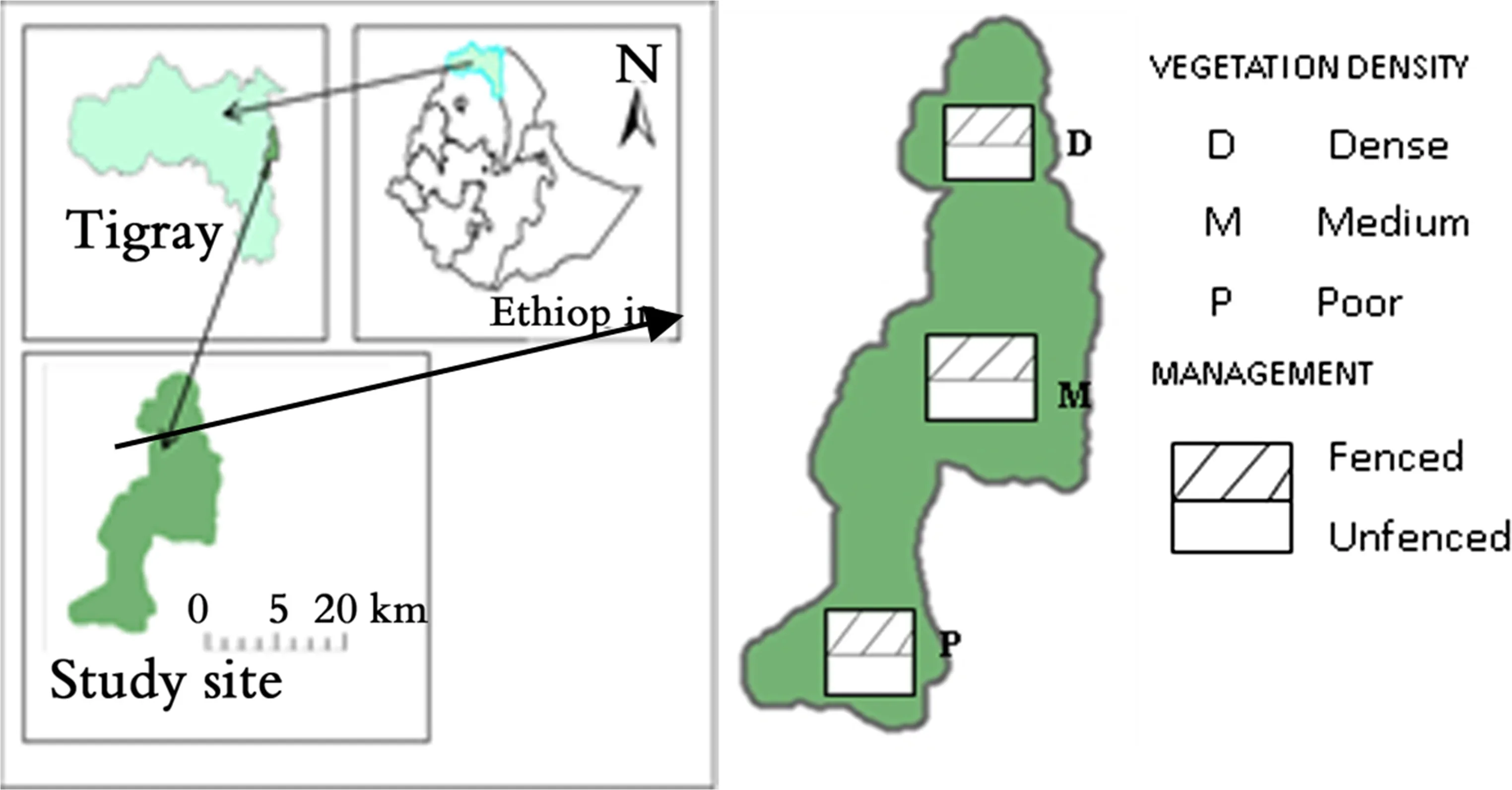
Fig.1 Location of Desa’a Forest in northern Ethiopia
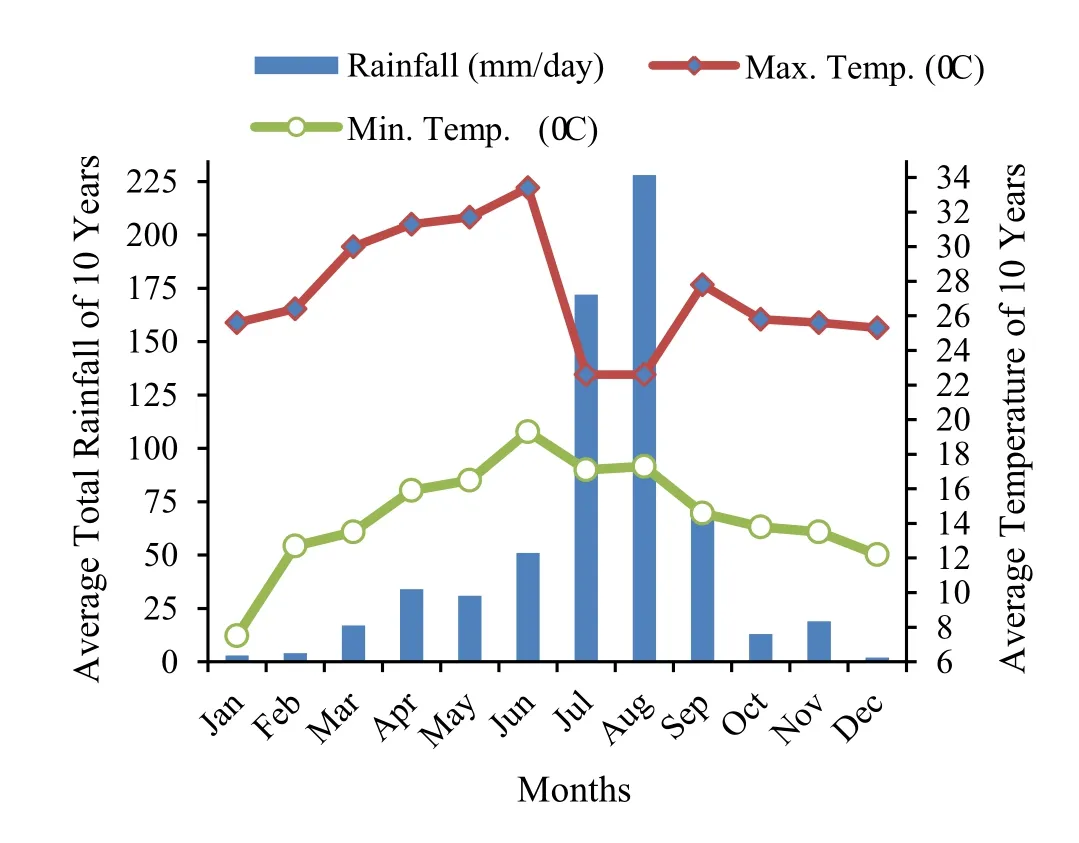
Fig.2 Average total rainfall,maximum and minimum temperature for 10 years(2006–2015)at the study area
Root and rhizosphere soil sampling
Sampling was carried out in both wet and dry seasons in the months of August 2015 and January 2016 respectively.Within the 20×20 m plots,three dominant tree species were identi fied for root and rhizosphere soil samples and replicated twice,for a total of 144 tree species from the 160×40 m plots.In total,488 trees were sampled for each season.For each of the selected tree species,root samples were collected by digging out the soil in four directions with a hand hoe beginning from the tree trunk base around the tree to expose the fine roots.Fine roots were collected around the tree,then mixed well to make a composite sample.The sample was placed in a sampling bottle with 97%ethanol to preserve the roots until processing for colonization analysis.Rhizosphere soils were collected to a depth of 50 cm(Sewnet and Tuju 2013)and mixed to make composite samples.These samples were labelled,then taken to the laboratory for enumerating AMF spores.
AMF root colonization assessment
The ethanol and soil particles were washed from the roots by running tap water through them.They were then cut into 1 cm lengths and placed in autoclave resistant bottles containing 10%KOH and heated at 90°C for 1 h(Phillips and Hayman 1970).These samples were washed again using distilled water then placed in 10%H2O2for 15 min for further bleaching.They were then cleaned using distilled water and placed in 1%HCl for 1 h for acidi fication,then stained using Trypan blue(0.05 g)solution in 1:1:1 lactic acid–glycerol–distilled water for 12 h(Das and Kayang 2008).
The stained root pieces were mounted lengthwise on slides in replicates of nine after being washed and placed into 50%glycerol for 1 h and observed with a compound light microscope at 400×to observe AMF arbuscules,hyphae and vesicles.The gridline intersection method was used to observe fungal structures(Giovannetti and Mosse 1980).The method of Brundrett et al.(1994)was used to analyze AMF colonization.
AMF spore assessment
AMF spores were analysed using the decanting and sieving method described by Gerdemann and Nicolson(1963)using mycorrhizalEndogonespecies spores.The wet sieving and decanting procedures were carried out based on 100 g of air-dried soil in each rhizosphere soil sample.Samples were then centrifuged in water and then in 1:1 sucrose to water(Walker et al.1982).Air-dried,sieved rhizosphere soil(10 g)was added to a container of 100 mL of tap water,shaken for 30 min and left to stand for about 5 min to separate the decant from the filtrate.The suspension was passed through 850,300,100 and 50 μm sieves,placed on each other in the order of decreasing size.The 850 μm sieve was used to remove rocks,woods and other unnecessary material which might have remained during sieving.The suspensions in each of the different sieves were carefully poured into a plastic jar,water was added,then the jar was covered tightly and centrifuged at 2000 rpm for 5 min.Samples were washed again with tap water,then sieved using the 50 μm sieve.The samples were poured into the plastic jar and centrifuged again using 50%sucrose at 2000 rpm for 1 min.Samples were again poured into the 50 μm sieve and rinsed with running tap water to rinse off the sucrose.Lastly,each of these samples was poured on filter paper and left to dry.The filter papers containing spores were kept in inverted Petri dishes divided into sections to simplify spore counting.A stereomicroscope with 100 times magni fication was used for viewing and quantifying the spores.AMF fungal propagules in the form of spores and sporocarps were quanti fied by observing all sieve sizes(300,100 and 50 μm)of the filter papers with the microscope.Total AMF spore density was calculated for 100 g of dried soil.To identify morphospecies,spores were separated by colour and size,then placed in Petri dishes and crushed under a cover slip in a drop of 1:1 polyvinyl alcohol lactoglycerol–Melzer’s reagent(Morton et al.1993),and observed with a compound light microscope at 400×using the density measurement procedures described by Bundrett et al.(1996).
Soil property analysis
Soil for the physiochemical soil analysis was sampled from each of the 5×5 m plots using a soil augur at a depth of 0–50 cm in both the fenced plot(FP)and unfenced plot(NFP)in an ‘‘X’’pattern within a single plot and mixed to form a bulk sample for the whole plot;1000 g of the bulked soil was stored in tightly closed polyethene bags to avoid evaporation.The pH and electrical conductivity(EC)in the soil were determined using a soil suspension at a ratio of 1:5 soil to water(Gee and Bauder 1986).Soil moisture content was obtained using the gravimetric method and 10 g of fresh soil oven-dried at 105°C for 24 h.Organic carbon was determined using the Walkley–Black procedure(Walkley and Black 1934).Available phosphorous was determined using the phosphorous analysis protocol of Olsen et al.(1982).Total nitrogen was determined using the Kjeldahl procedure(Bremner and Mulvaney 1982).
Data analyses
Data were analysed using SPSS statiscical software version 20.0(SPSS,Chicago,IL,USA).Variations in AMF SD and total RC among vegetation cover densities were analysed using two-way an ANOVA.Post-hoc Tukey’s test was used for multiple comparison tests between the vegetation cover densities.Differences in AMF genera were determined using the Kruskal–WallisHtest.Variations in SD and RC between management practices as well as seasons were tested using an independentttest.Before analysis,spore density was log10-transformed.All tests were true at 5%con fidence interval.Correlations in AMF SD and RC with soil variables were tested using Pearson’s correlation test.
Results
Vegetation cover density and edaphic factors
Soil variables did not differ significantly (p>0.05)between the vegetation cover densities except for available potassium and total nitrogen(Table 1).Generally,the soil pH for the poor and medium vegetation cover density was moderately alkaline and strongly alkaline(8.0)for the dense vegetation cover density.Electrical conductivity values were<2 that indicated that the soils were not saline in all the vegetation cover densities.All the nutrients decreased with a decrease in the vegetation cover density(Table 1).
Management practices,season and edaphic factors
None of the soil variables differed significantly(p>0.05)between the FP and NFP except for available phosphorus and total nitrogen.All the nutrients were higher in the FP than in the NFP(Table 2).Apart from available phosphorus,total nitrogen,OC and OM,there were signi ficant(p<0.05)differences in soil moisture content,pH,EC and available potassium between the dry and rainy seasons(Table 2).Soil pH increased significantly with an increase in rain.
AMF spore genera between tree species
Common tree species at all vegetation cover densities includedJ.procera,O.europaea,Maytenus arbutifolia,Carissa spinarumandDodonaea angustifolia.AMF SD varied slightly among the plant species but no signi ficant(p>0.05)differences were found.The SD ranged from 50 to 4467 with an average of 624 spores.Glomus,Acaulospora,Gigaspora,ScutellosporaandEntrophosporawere the spore genera identi fied.Glomuswas the most abundant genus followed byAcaulospora,Gigaspora,ScutellosporaandEntrophosporawith mean spore density of 153,86,25,4,and 0.2,respectively.All the genera differed signi ficantly among the vegetation cover densities whereas the management practices had no signi ficant effect on the AMF spore genera except forGlomus(Table 3).
AMF root colonization between species
All the species were AMF colonized and the internal hyphae,external hyphae,vesicles and arbuscules structures were observed in the roots of the studied plants at a variable degree.Hyphal colonization(HC)was the highestfollowed by mycorrhizal hyphal colonization(MHC),vesicular colonization(VC)and arbuscular colonization(AC)with a mean percentage of 78.74,64.71,42.27 and 37.12,respectively.The AMF structural colonization differed significantly between the species(Table 4).

Table 1 Mean plot soil characteristics(±SE)in each of the vegetation cover density
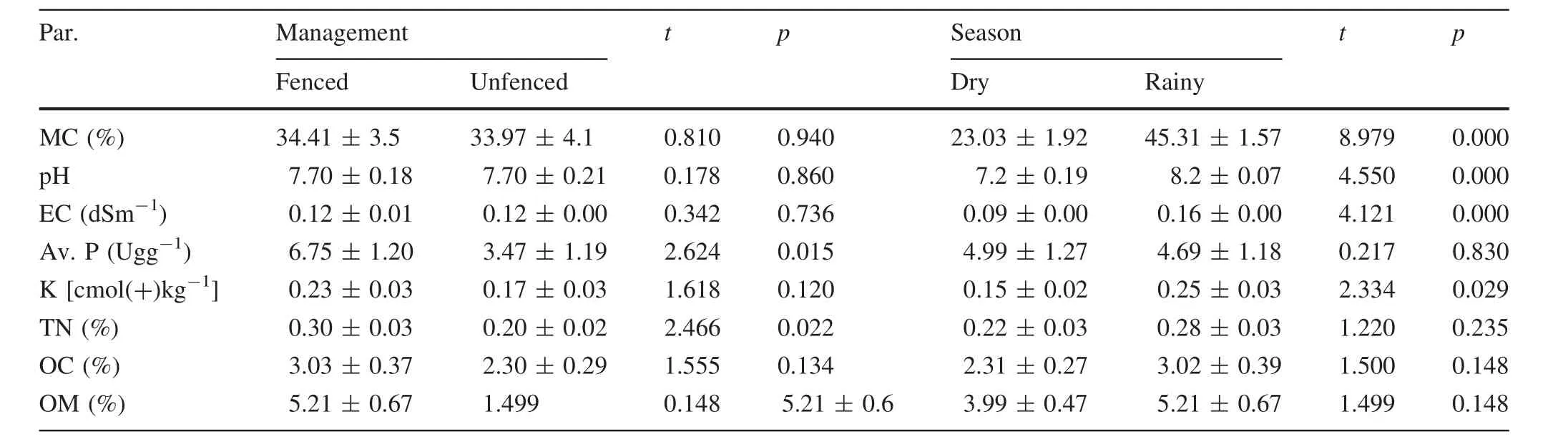
Table 2 Mean soil characteristics(±SE)in management plots during the dry and rainy season(n=22)

Table 3 Number of morphospecies in plots with different vegetation cover densities and management in studied fields
The RC ranged between 4 and 95%and with an average of 54%.Olea europaeahad the highest colonization by AM followed byCarissa spinarumandDodonaea angustifoliawas the least colonized.The total AMF root colonization between species was significantly different(Fig.3).
AMF spore density and root colonization between vegetation cover types
The highest and the lowest spore density were from the poor and dense vegetation cover densities,respectively(Fig.4a),whereas percentage root colonization showed a reverse trend(Fig.4b).The mean spore density of 989,675 and 364 per 100 g soil were observed in poor,medium and dense vegetation densities,respectively.The root colonization percentage ranged between 4–90,0–80 and 0–76 in the dense,medium and poor vegetation cover densities,respectively.Both spore densities and root colonization differed significantly(p<0.05)among the vegetation cover densities.
Management and seasonal effect on AMF spore density and root colonization
AMF SD and RC were significantly(p<0.05)higher in the wet period than in the dry period.The spore densities during the rainy season were 2-fold higher than the spore densities in the dry season(Table 5).AMF SD and RC were significantly higher in the fenced plots than in the unfenced plots(Table 5).The interactions between the main factors were significantly different for both AMF SD and RC(Table 6).
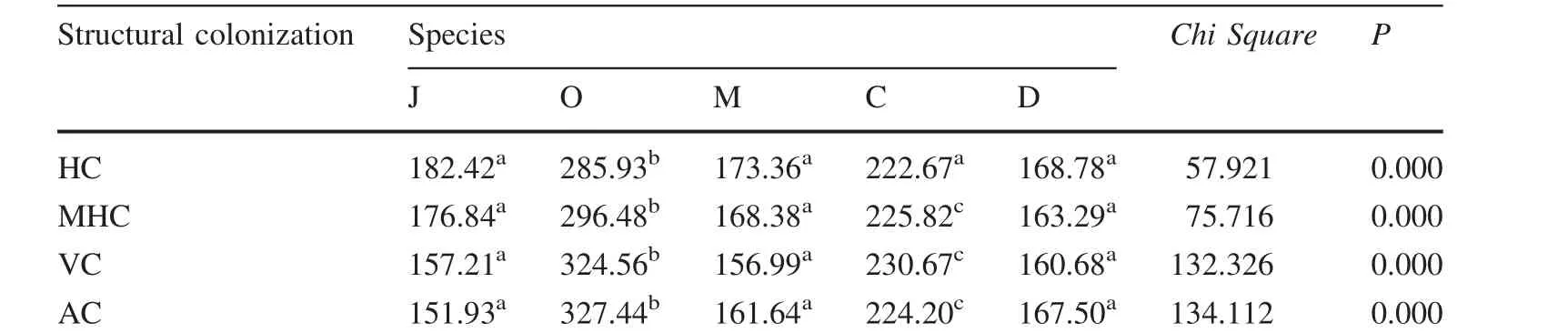
Table 4 AMF root structure colonization and species

Fig.3 Total root colonization percentage between dominant species found in Desaa’dry Afromontane forest.Error bars indicate standard error.J:Juniperus procera,O:Olea europaea,M:Maytenu sarbutifolia,C:Carissa spinarum and D:Dodonaea angustifolia.Bars with different letters differ significantly at p<0.05
Correlation between AMF SD,RC with edaphic factors and elevation
A positive and signi ficant relationship was observed between AMF SD and RC(p<0.05).Soil pH was negatively correlated with both SD and RC(Table 7).Organic carbon ranged from low(1.24)to medium levels(4.82)and showed an inverse relationship with AMF SD and a positive relationship with AMF RC.Both SD and RC inversely related with total nitrogen,available phosphorus and available potassium.There was a negative correlation between altitude and SD,whereas RC was positively correlated with altitude(Table 7).
Discussion
The AMF spore densities recorded in this study were comparable to those observed from the tropics(Tao et al.2004;Muleta et al.2008)and higher than those recorded from the dry tropics(Birhane et al.2010),and in the semiarid area(Mohammad et al.2003).The distribution and variation in AMF spore density are in fluenced by both abiotic and biotic factors(Mohammad et al.2003).AMF spore production varies spatially according to the soil physiochemical properties(Nasrullah et al.2010).Glomushad the highest abundance and found in all rhizosphere soils from all the studied species.Glomusis dominant in different ecosystems and common in semi-arid areas of Ethiopia(Wubet et al.2003),tropical rain forests(Zhao et al.2001),and dry deciduous woodlands(Birhane et al.2010).The domination ofGlomusmight be due to their high reproductive ability compared to other AMF genera(Bever et al.1996).Glomusspecies can adapt to varying soil conditions since they can survive in both acidic and alkaline soils(Pande and Tarafdar 2004)and hence can grow in most environmental conditions with limited trouble.
All the tree species were colonized by AMF,consistent with the study by Wubet et al.(2003)in Ethiopia and by Birhane et al.(2010)in a dry deciduous woodland in northern Ethiopia.Over 80%of the terrestrial plants have a mutual association with AMF(Smith and Read 2008;Zobel and O¨pik 2014).AMF root colonization is higher among wild herbaceous species;over 90% of those examined were colonized by AMF(Gai et al.2006).We observed all types of AMF structures(internal hyphae,external hyphae,vesicles and arbuscules),consistent with the results of Moreira-Souza et al.(2003).Since each structure has a unique role in root colonization(Smith and Read 2008),the presence of all the structure is vital for an effective relationship with the plant host roots.Hyphal colonization(HC)and vesicular colonization(VC)were the highest,which agreed with the findings of Belay et al.(2013).Since hyphae and vesicles are the major structures of AMF and persist longer in the root(Wu et al.2009),this peristence could have been the major reason that they were the chief structures found in the root cortex.The formation and breakdown of arbuscules,however,are quite fast,limiting their abundance in the root cortex.

Fig.4 Variation of AMF SD(a)and RC(b)between vegetation cover densities in forests of Northern Ethiopia.Error bars indicate standard error.Different letters above the bars indicate signi ficant differences among vegetation densities at p<0.05
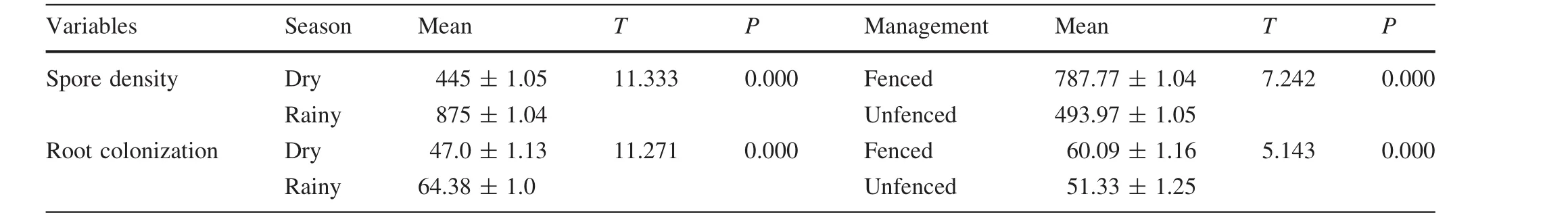
Table 5 Mean AMF spore density(per 100 g soil)and RC(%)in the dry and wet periods and between management practices(α=0.05)

Table 6 Analysis of variance on the effect of vegetation cover density,season and management and their interaction on AMF spore density and the effect of vegetation cover density and management and their interaction on AMF root colonization(%)of the five common species found in the study site
The root colonization percentage ranged from 4 to 95,similar to the 0–95%found by Birhane et al.(2010),and to the 3.5–96.3%reported by Carrenho et al.(2007).Generally,arid environments are stressed with limited water and nutrients hence the association of microorganisms with the plant host species tend to increase(Thrall et al.2007).The roots ofOlea europaeawere the most highly colonized of the species probably because AMF root colonization is species speci fic(Likar et al.2008;Vyas and Gupta 2014).Other factors such as host physiological attributes,level of reliance on mycorrhiza,and the soil physiochemical properties surrounding the host can be attributed to the variation in root colonization among species(Wu et al.2009).
Maximum AMF SD was recorded in poor vegetation density and the minimum AMF SD in the dense vegetation density,whereas the percentage RC showed a reverse trend.The hypothesis that AMF SD and RC in dry Afromontane forest vary across vegetation cover density was accepted.Various studies have indicated that vegetation cover density in fluence the functioning and development of AMF(Yimer et al.2006;Rodrı´guez-Echeverrı´a et al.2008).In a harsh environment with lower productivity,plant dependence on soil microbes becomes high(Thrall et al.2007),which could have been the reason for the higher spore density in the poor vegetation cover density.Furthermore,other factors like the nutrient level in the soil and soil pH conditions can never be overlooked(Gong et al.2012).Soil nutrient content levels in fluenced spore production in the present study,as vegetation cover densities and management practice(Table 1 and 2)with higher nutrient levels appeared to have lower spore density(Fig.4a).
The hypothesis that AMF SD and RC are significantly higher in the fenced plots compared to the non-fenced plots received clear empirical support.The observed results showed similarity with those of Birhane et al.(2010)and Oechel et al.(2000),who reported higher AMF SD and more RC in the exclosures and undisturbed land,respectively.The low AMF spore density in the non-fenced plots could be attributed to the disturbances in the soil due to grazing and vegetation removal that affected both soil and soil organisms,disrupting the entire ecosystem interaction.AMF availability and activity can be affected by vegetation removal,which can result in a signi ficant decrease in SD and RC(Boddington and Dodd 2000).Interference within environment affects both the aboveground ecosystem and soil microorganism functioning(Oechel et al.2000),due to the breakdown of AMF hyphal network during cultivation and grazing,which leads to a reduction in mycorrhizal colonization of the root(Mcgonigle and Miller 1996).
Both SD and RC were highest and differed significantly between the wet period from the dry period,which supports our hypothesis that AMF SD and RC within the dry Afromontane forest ecosystem vary by season.The present results contradicted those of Birhane et al.(2010)and Sivakumar(2013)who observed a low SD in the wet period,but agreed with the findings of Oliveira and Oliveira(2010)and Muchane et al.(2012)who observed higher spore density and root colonization in the wet period than in the dry.These discrepancies in the findings could be due to differences in climate,in time and space,soil fertility and types,elevation,and latitude and longitude of the study sites.Similarly,Lugo and Cabello(2002)also reported more vesicles,arbuscular and highest colonization in the wet period similar to our results.Increased precipitation tends to enhance AMF abundance because photosynthetic materials are readily available to the roots and rhizomes and will enable AMF to sporulate abundantly(Kivlin et al.2013).Furthermore,the higher AMF spore population and root colonization indicate better growth during the rainy season due to the high rate of root development,which provides additional openings on the root for AMF access(Zangaro et al.2013).The observedresults are strongly supported by the fact that increased soil moisture with increasing precipitation during the rainy seasons is directly proportional to AMF root colonization(Oliveira and Oliveira 2010).
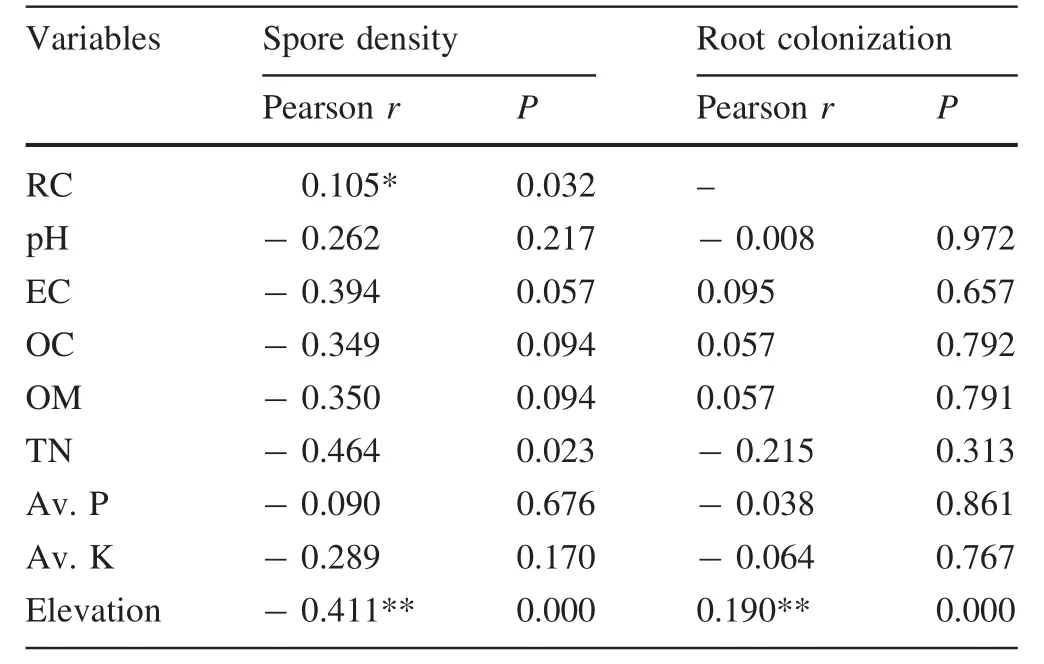
Table 7 Correlation between spore density and root colonization with soil variables and elevation
Spore density and root colonization were positively and significantly correlated,similar to the results of Muthukumar et al.(2003a,b)and Songachan et al.(2011)who also reported an increase in AMF SD as RC increased.Variations in the spatial distribution of AMF spores has been reported as the major reason for this relationship(Zhao et al.2001).
Higher AMF spore density was found in neutral and slightly alkaline soil conditions,as did Sreevani and Reddy(2004).Soil pH was negatively correlated with both SD and RC,consistent with the results of Songachan et al.(2011),but not with the findings of Burni et al.(2011)and Panwar and Tarafdar(2006)who reported a direct relationship between pH and SD.Soil pH has been reported to in fluence AMF reproduction potential as well as their structural composition(An et al.2008),but is also linked to other factors such as the host plant.However,extremes in soil pH are reported to suppress spore production and root colonization(Postma et al.2007).
Organic carbon and SD were negatively related,similar to the high spore production found in soil of fields of mungbean and soybean where OC content was low(Hindumathi and Reddy 2011).OC and AMF RC showed a positive relationship in our study in line with the high correlation found between OC and spore production by Liu et al.(2000)and Sivakumar(2013).AMF are thought to positively in fluence the soil carbon pool(Wilson et al.2009)and over the long-term may increase carbon storage(Iversen et al.2012).Of the total carbon fixed during photosynthesis,AMF are reported to use 30%(Drigo et al.2010).Vegetation cover densities with higher OC had higher percentages of root colonization(Table 1 and Fig.4b).The AMF SD and RC were inversely related to the available phosphorus,in good accord with findings by Panwar and Tarafdar(2006)and Birhane et al.(2010).Certain AMF species sporulate abundantly under low P availability in the soil(Table 1)and as observed by Kahiluoto et al.(2001).AMF RC had a negative relationship to available phosphorus,consistent with the findings of Lekberg and Koide(2005),but contradicted with that of Nogueira and Cardoso(2006)and Khade and Rodrigues(2009)who reported a positive relationship between phosphorus and RC.
Total nitrogen had an inverse relationship with both AMF RC and SD in agreement with those of Deepak et al.(2015),Khade and Rodrigues(2008,2009),who also found a negative association between SD and total nitrogen.However,the results contradicted those of Abubacker et al.(2014)who found a positive relationship between SD and total N.AMF abundance and diversity change greatly with nitrogen content(Egerton-Warburton and Allen 2000).For example,high and low N content in the soil has been reported to respectively suppress and induce AMF SD and RC(Bago et al.2004).Based on current observations,vegetation cover densities with higher total N are associated with low spore density(Table 1,Fig.4a),which further supports the argument.
AMF SD and RC were negatively correlated with available potassium like those of Khanam et al.(2006)and Khade and Rodrigues(2009),but they contradicted those of Gaur and Kaushik(2011),and Abubacker et al.(2014)who determined a positive relationship between available potassium and SD.A negative correlation between available potassium and RC was also reported by Ardestani et al.(2011)in their analysis of the in fluence of different K concentrations on RC.An inverse relationship between RC and available potassium could be due to the fact that AMF tends to lose their potential to develop their structural components such as arbuscules as the K concentration levels increase(Vogel-Mikusˇet al.2005).Generally,high levels of nutrient elements in soil have been reported to decrease mycorrhizal colonization(Liu et al.2000).
There was an inverse relationship between altitude and SD,whereas RC was positively correlated with altitude,supporting the hypothesis that AMF SD and RC in the dry Afromontane forest vary following an altitude gradient.The current results contradicted the decrease in AMF RC with increasing altitude found by Posada et al.(2008),which might be due to a difference in species diversity becauseOlea europaeaspecies,that was most colonized,was found at a higher altitude than all the other species.Human in fluence is also lower with the increase in the altitude.However,poor soil status and unfavourable ecological conditions increase with increasing altitude(Ko¨rner 2003).Plants,therefore,require associations that can assist them in acquiring more water and nutrients in such harsh environments(Pellissier et al.2013).The inverse relationship between SD and altitude might also have resulted from soil erosion.Forces that move soil from one location to another in fluence AMF spores(Smeenk and Ianson 2010).In the current study,soil erosion could have caused the higher number of spores at the low altitude.
Conclusion
Vegetation cover density strongly in fluenced AMF SD and RC.Poor vegetation cover density is more prone to human invasion;soils and vegetation in this block are therefore unstable,which disrupt the ecosystem and microbial activities.AMF SD and RC increased in the wet period and were lower in the dry period.AMF SD and RC changed within the seasons,and precipitation has a positive in fluence on the rate of belowground microbial activity.Although edaphic factors in fluenced SD and RC,management practices had a higher in fluence through vegetation removal and the subsequent effect on the soil.significantly higher SD and RC were observed in the fenced plots as compared to the unfenced plots.Thus,soil disturbance and poor management of the forest ecosystems are some of the major factors affecting AMF development and distribution in the forest ecosystem.Hence,proper management is vital in the AMF development and enhancing ecosystem health at large.
AcknowledgementWe would like to acknowledge the Transdisciplinary Training for Resource Ef ficiency and Climate Change Adaptation in Africa(TRECCAfrica),which funded the second author for the MSc.studies in Climate and Society at Mekelle University.We are also grateful to TRECCAfrica and Norwegian Programme for Capacity Development in Higher Education and Research for Development(NORHED)project which supported the field research including experimentation and data collection and analysis.The valuable suggestions made by anonymous referees is gratefully acknowledged.
References
Abubacker M,Visvanathan M,Srinivasan S(2014)Impact of pesticides on AMF spore population and diversity in banana(Musa spp.).Soils 2:1279–1286
An GH,Miyakawa S,Kawahara A,Osaki M,Ezawa T(2008)Community structure of arbuscular mycorrhizal fungi associated with pioneer grass speciesMiscanthus sinensisin acid sulfate soils:habitat segregation along pH gradients.Soil Sci Plant Nutr 54:517–528
Ardestani NK,Zare-Maivan H,Ghanati F(2011)Effect of different concentrations of potassium and magnesium on mycorrhizal colonization of maize in pot culture.Afr J Biotech 10:16548–16550
Aynekulu E,Denich M,Tsegaye D,Aerts R,Neuwirth B,Boehmer H(2011)Dieback affects forest structure in a dry Afromontane forest in northern Ethiopia.J Arid Environ 75:499–503
Babikova Z,Gilbert L,Bruce TJ,Birkett M,Caul field JC,Woodcock C,Pickett JA,Johnson D(2013)Underground signals carried through common mycelial networks warn neighbouring plants of aphid attack.Ecol Lett 16:835–843
Bago B,Cano C,Azco´n-Aguilar C,Samson J,Coughlan AP,Piche´Y(2004)Differential morphogenesis of the extraradical mycelium of an arbuscular mycorrhizal fungus grown monoxenically on spatially heterogeneous culture media.Mycologia 96:452–462
Belay Z,Vestberg M,Assefa F(2013)Diversity and abundance of arbuscular mycorrhizal fungi associated with acacia trees from different land use systems in Ethiopia.Afr J Microbiol Res 7:5503–5515
Bever JD,Morton JB,Antonovics J,Schultz PA(1996)Hostdependent sporulation and species diversity of arbuscular mycorrhizal fungi in a mown grassland.J Ecol 84:71–82
Birhane E,Kuyper TW,Sterck FJ,Bongers F(2010)Arbuscular mycorrhizal associations inBoswellia papyrifera(frankincensetree)dominated dry deciduous woodlands of Northern Ethiopia.For Ecol Manage 260:2160–2169
Boddington C,Dodd J(2000)The effect of agricultural practices on the development of indigenous arbuscular mycorrhizal fungi.II.Studies in experimental microcosms.Plant Soil 218:145–157
Bremner JM,Mulvaney CS(1982)Nitrogen-total.In:Page AL,Miller RH,Keeney DR(eds)Methods of soil analysis.Part 2.Chemical and microbiological properties.American Society of Agronomy/Soil Science Society of America,Wisconsin/Madison,pp 595–624
Brundrett M,Melville L,Peterson L(eds)(1994)Practical methods in mycorrhiza research.Mycologue Publication of University of Guelph,Guelph,pp 161–174
Bundrett M,Ashwath N,Jasper D(1996)Mycorrhizas in the Kakadu region of tropical Australia.Plant Soil 184:173–184
Burni T,Hussain F,Sharief M(2011)Arbuscular mycorrhizal fungi(amf)associated with the rhizosphere ofMentha arvensisl.,andM.longifoliahuds.Pak J Bot 43:3013–3019
Carrenho R,Trufem SFB,Bononi VLR,Silva ES(2007)The effect of different soil properties on arbuscular mycorrhizal colonization of peanuts,sorghum and maize.Acta Bot Bras 21:723–730
Chun OK,Kim DO,Lee CY(2003)Superoxide radical scavenging activity of the major polyphenols in fresh plums.J Agric Food Chem 51:8067–8072
Das P,Kayang H(2008)Stamp pad ink,an effective stain for observing arbuscular mycorrhizal structure in roots.World J Agric Sci 4:58–60
Deepak V,Vyas R,Giri V,Karanth KP(2015)A taxonomic mystery for more than 180 years:the identity and systematic position of Brachysaura minor.Vertebr Zool 65:371–381
Drigo B,Pijl AS,Duyts H,Kielak AM,Gamper HA,Houtekamer MJ,Boschker HT,Bodelier PL,Whiteley AS,van Veen JA(2010)Shifting carbon flow from roots into associated microbial communities in response to elevated atmospheric CO2.Proc Natl Acad Sci 107:10938–10942
Egerton-Warburton LM,Allen EB(2000)Shifts in arbuscular mycorrhizal communities along an anthropogenic nitrogen deposition gradient.Ecol Appl 10:484–496
Eyles A,Bonello P,Ganley R,Mohammed C(2010)Induced resistance to pestsand pathogensin trees.New Phytol 185:893–908
Gai J,Feng G,Cai X,Christie P,Li X(2006)A preliminary survey of the arbuscular mycorrhizal status of grassland plants in southern Tibet.Mycorrhiza 16:191–196
Gaur S,Kaushik P(2011)Analysis of vesicular arbuscular mycorrhiza associated with medicinal plants in Uttarakhand state of India.World Appl Sci J 14:645–653
Gee GW,Bauder JW(1986)Particle-size analysis.In:Page AL,Miller RH,Keeney DR(eds)Methods of soil analysis:Part 1—physical and mineralogical methods 1.American Society of Agronomy/SoilScienceSocietyofAmerica,New York/Madison,pp 383–411
Gerdemann J,Nicolson TH(1963)Spores of mycorrhizal Endogone species extracted from soil by wet sieving and decanting.Trans Br Mycol Soc 46:235–244
Giday GK(2013)Management interventions to assist restoration of degraded dry Afromontane forest in Nothern Ethiopia(PhD thesis).Groep Wetenschap&Technologie Press,KU Leuven,KU,Belgium,pp 1–181
Giovannetti M,Mosse B(1980)An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots.New Phytol 84:489–500
Gong M,Tang M,Zhang Q,Feng X(2012)Effects of climatic and edaphic factors on arbuscular mycorrhizal fungi in the rhizosphere ofHippophae rhamnoidesin the Loess Plateau,China.Acta Ecol Sin 32:62–67
Hildebrandt A,Al Au fiM,Amerjeed M,Shammas M,Eltahir EA(2007)Ecohydrology of a seasonal cloud forest in Dhofar:1.Field experiment.Water Resour 43:1–13
Hindumathi A,Reddy BN(2011)Occurrence and distribution of arbuscular mycorrhizal fungi and microbial flora in the rhizosphere soils of mungbean[Vvigna radiata(L.)Wwilczek]and soybean[Gglycine max(L.)Merr.]from Adilabad,Nizamabad and Karimnagar districts of Andhra Pradesh state,India.Adv Biosci Biotechnol 2:275–286
Iversen CM,Keller JK,Garten CT,Norby RJ(2012)Soil carbon and nitrogen cycling and storage throughout the soil pro file in a sweetgum plantation after 11 years of CO2-enrichment.Glob Change Biol 18:1684–1697
Kahiluoto H,Ketoja E,Vestberg M,Saarela I(2001)Promotion of AM utilization through reduced P fertilization 2.Field studies.Plant Soil 231:65–79
Khade SW,Rodrigues B(2008)Spatial variations in arbuscular mycorrhizal(AM)fungi associated withCarica papayaL.in a tropical agro-based ecosystem.Biol Agric Hortic 26:149–174
Khade SW,Rodrigues BF(2009)Arbuscular mycorrhizal fungi associated with varieties ofCarica papayaL.in tropical agrobased ecosystem of Goa,India.Trop Subtrop Agroecosyst 10:369–381
Khanam D,Mridha M,Solaiman A(2006)A comparative study of arbuscular mycorrhizal association with different agricultural crops among four AEZs of Bangladesh.J Agril Res 44:147–161
Kivlin SN,Emery SM,Rudgers JA(2013)Fungal symbionts alter plant responses to global change.Am J Bot 100:1445–1457
Ko¨rner C(2003)Alpine plant life:functional plant ecology of high mountain ecosystems.Springer Science&Business Media.Ilia Chavchavadze State University Publishing,Tbilisi,pp 9–20
Lekberg Y,Koide R(2005)Is plant performance limited by abundance of arbuscular mycorrhizal fungi?A meta-analysis of studies published between 1988 and 2003.New Phytol 168:189–204
Lenaerts L(2013)Insights into agency and social interactions in natural resource management:extended case studies from northern Ethiopia.Natural resource management.Wageningen University/Society & NaturalResources,Leuven/Heverlee,pp 2–19
Likar M,Bukovnik U,Kreft I,Chrungoo NK,Regvar M(2008)Mycorrhizal status and diversity of fungal endophytes in roots of common buckwheat(Fagopyrum esculentum)and tartary buckwheat(F.tataricum).Mycorrhiza 18:309–315
Liu Y,Su MY,Yan XH,Liu WT(2000)The mean-square slope of ocean surface waves and its effects on radar backscatter.J Atmos Ocean Technol 17:1092–1105
Lugo MA,Cabello MN(2002)Native arbuscular mycorrhizal fungi(AMF)from mountain grassland(Co´rdoba,Argentina)I.Seasonal variation of fungal spore diversity.Mycologia 94:579–586
Mcgonigle TP,Miller MH(1996)Development of fungi below ground in association with plants growing in disturbed and undisturbed soils.Soil Biol Biochem 28:263–269
Mohammad MJ,Hamad SR,Malkawi HI(2003)Population of arbuscular mycorrhizal fungi in semi-arid environment of Jordan as in fluenced by biotic and abiotic factors.J Arid Environ 53:409–417
Mokria M,Gebrekirstos A,Aynekulu E,Bra¨uning A(2015)Tree dieback affects climate change mitigation potential of a dry Afromontane forest in northern Ethiopia.For Ecol Manage 344:73–83
Moreira-Souza M,Trufem SF,Gomes-da-Costa SM,Cardoso EJ(2003)Arbuscular mycorrhizal fungi associated withAraucaria angustifolia(Bert.)O.Ktze.Mycorrhiza 13:211–215
Morton J,Bentivenga S,Wheeler W(1993)Germ plasm in the International Collection of Arbuscular and Vesicular-arbuscular Mycorrhizal Fungi(INVAM)and procedures for culture development,documentation and storage.Mycotaxon 48:491–528
Muchane MN,Muchane M,Mugoya C,Clet W(2012)Effect of land use system on Arbuscular Mycorrhiza fungi in Maasai Mara ecosystem,Kenya.Afr J Microbiol Res 6:3904–3916
Muleta D,Assefa F,Nemomissa S,Granhall U(2008)Distribution of arbuscular mycorrhizal fungi spores in soils of smallholder agroforestry and monocultural coffee systems in southwestern Ethiopia.Biol Fertil Soils 44:653–659
Muthukumar T,Udaiyan K(2000)Arbuscular mycorrhizas of plants growing in the Western Ghats region,Southern India.Mycorrhiza 9:297–313
Muthukumar T,Sha L,Yang X,Cao M,Tang J,Zheng Z(2003a)Distribution of roots and arbuscular mycorrhizal associations in tropical forest types of Xishuangbanna,southwest China.Appl Soil Ecol 22:241–253
Muthukumar T,Sha L,Yang X,Cao M,Tang J,Zheng Z(2003b)Mycorrhiza of plants in different vegetation types in tropical ecosystems of Xishuangbanna,southwest China.Mycorrhiza 13:289–297
Nasrullah MS,Robina K,Burni T(2010)Occurrence and distribution of AMF in wheat and Maize crops of Malakand Division of North west Frontier Province.Pak J Bot 42:1301–1312
Nogueira MA,Cardoso EJBN(2006)Plant growth and phosphorus uptake in mycorrhizal Rangpur lime seedlings under different levels of phosphorus.Pesqui Agropecu Bras 41:93–99
Nyssen J,Vandenreyken H,Poesen J,Moeyersons J,Deckers J,Haile M,Salles C,Govers G(2005)Rainfall erosivity and variability in the northern Ethiopian Highlands.J Hydrol 311:172–187
Oechel WC,Vourlitis GL,Hastings SJ,Zulueta RC,Hinzman L,Kane D(2000)Acclimation of ecosystem CO2 exchange in the Alaskan Arctic in response to decadal climate warming.Nature 406:978–981
Oliveira AND,Oliveira LAD(2010)In fluence of edapho-climatic factors on the sporulation and colonization of arbuscular mycorrhizal fungi in two Amazonian native fruit species.Braz Arch Biol Technol 53:653–661
Olsen S,Sommers L,Page A(1982)Methods of soil analysis.Part 2.Chemical and microbiological properties of phosphorus.Agronomy monograph,vol 9.American Society of Agronomy/Academic Press,Madison,pp 403–430
Pande M,Tarafdar J(2004)Arbuscular mycorrhizal fungal diversity in neem-based agroforestry systems in Rajasthan.Appl Soil Ecol 26:233–241
Panwar J,Tarafdar J(2006)Distribution of three endangered medicinal plant species and their colonization with arbuscular mycorrhizal fungi.J Arid Environ 65:337–350
Panwar V,Meghvansi M,Siddiqui S(2011)Short-term temporal variation in sporulation dynamics of arbuscular mycorrhizal(AM)fungi and physico-chemical edaphic properties of wheat rhizosphere.Saudi J Biol Sci 18:247–254
Pellissier L,Pinto E,Niculita-Hirzel H,Moora M,Villard L,Goudet J,Guex N,Pagni M,Xenarios I,Sanders I(2013)Plant species distributions along environmental gradients:do belowground interactions with fungi matter?Front Plant Sci 4:500
Phillips JM,Hayman D(1970)Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection.Trans Br Mycol Soc 55:158–161
Posada R,Franco L,Ramos C,Plazas L,Sua´rez J,A´lvarez F(2008)Effect of physical,chemical and environmental characteristics on arbuscular mycorrhizal fungi inBrachiaria decumbens(Stapf)pastures.J Appl Microbiol 104:132–140
Postma JW,Olsson PA,Falkengren-Grerup U(2007)Root colonisation by arbuscular mycorrhizal, fine endophytic and dark septate fungi across a pH gradient in acid beech forests.Soil Biol Biochem 39:400–408
Rodrı´guez-Echeverrı´a S,Hol WG,Freitas H,Eason WR,Cook R(2008)Arbuscular mycorrhizal fungi ofAmmophila arenaria(L.)Link:spore abundance and root colonisation in six locations of the European coast.Eur J Soil Biol 44:30–36
Schmitt CB,Denich M,Demissew S,Friis I,Boehmer HJ(2010)Floristic diversity in fragmented Afromontane rainforests:altitudinal variation and conservation importance.Appl Veg Sci 13:291–304
Sebhatleab M(2012)Land use land cover change detection and deforestation susceptibility analysis of Desa’a forest.M.Sc.thesis.Bahir Dar University Press,Addis Ababa,Ethiopia,pp 96–104
Sewnet TC,Tuju FA(2013)Arbuscular mycorrhizal fungi associated with shade trees andCoffea arabicaL.in a coffee-based agroforestry system in Bonga,Southwestern Ethiopia.Afr Focus 26:111–131
Sivakumar N(2013)Effect of edaphic factors and seasonal variation on spore density and root colonization of arbuscular mycorrhizal fungi in sugarcane fields.Ann Microbiol 63:151–160
Smeenk J,Ianson D(2010)Mycorrhizae in the Alaska landscape.University of Alaska Fairbanks Cooperative Extension Service and United States Department of Agriculture,Alaska,pp 1–8
Smith SE,Read DJ(2008)Mineral nutrition,toxic element accumulation and water relations of arbuscular mycorrhizal plants.Mycorrhizal symbiosis.Academic Press,London,pp 145–148
Songachan L,Kayang H,Lyngdoh I(2011)Colonization of arbuscular mycorrhizal fungi in moderately degraded subtropical forest stands of Meghalaya,Northeast India.J Agric Technol 7:1673–1684
Sreevani A,Reddy B(2004)Arbuscular mycorrhizal fungi associated with tomato(Lycopersicom esculentumMill.)as in fluenced by soil physico-chemical properties.Philipp J Sci 133:115
Tao L,Jianping L,Zhiwei Z(2004)Arbuscular mycorrhizas in a valley-type savanna in southwest China.Mycorrhiza 14:323–327
Thrall PH,Hochberg ME,Burdon JJ,Bever JD(2007)Coevolution of symbiotic mutualists and parasites in a community context.Trends Ecol Evol 22:120–126
Vani SM,Amballa H,Bhumi NR(2014)Arbuscular mycorrhizal fungi associated with rhizosphere soils of brinjal cultivated in Andhra Pradesh,India.Int J Curr Appl Sci 3(5):519-529
Vogel-MikusˇK,Drobne D,Regvar M(2005)Zn,Cd and Pb accumulation and arbuscularmycorrhizalcolonisation of pennycress Thlaspi praecox Wulf.(Brassicaceae)from the vicinity of a lead mine and smelter in Slovenia.Environ Pollut 133:233–242
Vyas D,Gupta RK(2014)Effect of edaphic factors on the diversity of VAM fungi.Trop Plant Res 1:14–25
Walder F,Niemann H,Natarajan M,Lehmann MF,Boller T,Wiemken A(2012)Mycorrhizal networks:common goods of plants shared under unequal terms of trade.Plant Physiol 159:789–797
Walker C,Mize CW,McNabb HS Jr(1982)Populations of endogonaceous fungi at two locations in central Iowa.Can J Bot 60:2518–2529
Walkley A,Black IA(1934)An examination of the Degtjareff method for determining soil organic matter,and a proposed modi fication of the chromic acid titration method.Soil Sci 37:29–38
Wardle DA,Bardgett RD,Klironomos JN,Seta¨la¨H,Van Der Putten WH,Wall DH(2004)Ecological linkages between aboveground and belowground biota.Science 304:1629–1633
Wilson GW,Rice CW,Rillig MC,Springer A,Hartnett DC(2009)Soil aggregation and carbon sequestration are tightly correlated with the abundance of arbuscular mycorrhizal fungi:results from long-term field experiments.Ecol Lett 12:452–461
Wu Y,Liu T,He X(2009)Mycorrhizal and dark septate endophytic fungi under the canopies of desert plants in Mu Us Sandy Land of China.Front Agric China 3:164–170
Wubet T,Kottke I,Teketay D,Oberwinkler F(2003)Mycorrhizal status of indigenous trees in dry Afromontane forests of Ethiopia.For Ecol Manage 179:387–399
Yimer F,Ledin S,Abdelkadir A(2006)Soil organic carbon and total nitrogen stocks as affected by topographic aspect and vegetation in the Bale Mountains,Ethiopia.Geoderma 135:335–344
Zangaro W,Rostirola LV,de Souza PB,de Almeida Alves R,Lescano LEM,Rondina ABL,Nogueira MA,Carrenho R(2013)Root colonization and spore abundance of arbuscular mycorrhizal fungi in distinct successional stages from an Atlantic rainforest biome in southern Brazil.Mycorrhiza 23:221–233
Zhao ZW,Xia YM,Qin XZ,Li XW,Cheng LZ,Sha T,Wang GH(2001)Arbuscular mycorrhizal status of plants and the spore density of arbuscular mycorrhizal fungi in the tropical rain forest of Xishuangbanna,southwest China.Mycorrhiza 11:159–162
Zobel M,O¨pik M(2014)Plant and arbuscular mycorrhizal fungal(AMF)communities—which drives which? J Veg Sci 25:1133–1140
杂志排行
Journal of Forestry Research的其它文章
- In vitro propagation of conifers using mature shoots
- ‘Relationships between relationships’in forest stands:intercepts and exponents analyses
- Effects of application date and rate of foliar-applied glyphosate on pine seedlings in Turkey
- Assaying the allelopathic effects of Eucalyptus camaldulensis in a nursery bed incorporated with leaf litter
- Effects of soil compaction on growth variables in Cappadocian maple(Acer cappadocicum)seedlings
- Variation and selection analysis of Pinus koraiensis clones in northeast China
