Non-aerated liquid culture promotes shoot organogenesis in Eucalyptus globulus Labill
2018-05-19SalladosSilvadeMachadoAstaritaSantare
T.D.Salla•C.dos S.Silva•K.L.de G.Machado•L.V.Astarita•E.R.Santare´m
Introduction
The genusEucalyptus(Myrtaceae)is among the most important hardwood forest crops worldwide,and due to their fast growth are largely exploited as a source of pulpwood to produce high-quality paper,construction timber,fuel and medicinal compounds(Abril et al.2011;Mabona and Van Vuuren 2013).Eucalyptus globulusLabill is among the most desirable eucalyptus species in pulp industries.It is relatively frost resistant and its wood is low in lignin;cellulose is easily extracted,bleaching ability and pulp yield are some of the main properties that make it a superior raw material(Schwambach et al.2005).Even thoughE.globulushas a wide geographical distribution,it is restricted to limited environments(Resquin et al.2006).Nevertheless,this species is well adapted to southern Brazil where winter frosts are common.
Studies onEucalyptushave been carried out to generate resistance to abiotic stress(Matsunaga et al.2012),to modify the biosynthesis of cellulose and hemicelluloses and to increase biomass accumulation(Quoirin and Quisen 2006).However,plant transformation systems are mainly based on efficient methods for in vitro regeneration.Organogenesis is one of the important techniques associated to genetic transformation,which contributes to the success of obtaining transgenic plants.Reports on efficient regeneration ofEucalyptusspecies through indirect organogenesis are not abundant probably due to the recalcitrance of these species to in vitro manipulation,although success has been reported in some commercially important eucalyptus species such asE.tereticornis(Aggarwal et al.2010),E.grandis(Hajari et al.2006),E.camaldulensis(Dibax et al.2005)andE.saligna(Dibax et al.2010).
As for many other species,several factors affect shoot regeneration;plant growth regulators and type and age of explants are the most studied.Various auxin and cytokinin combinations are effective in promoting plant regeneration steps through indirect organogenesis(Hajari et al.2006;Aggarwal et al.2010;Matsunaga et al.2012;Oliveira et al.2016).6-Benzyladenine(BA),an important plant growth regulator for callogenesis,is the preferred cytokinin to use with 1-naphthaleneacetic acid(NAA)to induce callus and subsequent plant regeneration by someEucalyptusspecies(Bandyopadhyay et al.1999;Nugent et al.2001;Dibax et al.2005).
Regarding the type of explant,young plant material such as hypocotyls and cotyledons has been the choice of explants for callus induction and adventitious shoot formation forEucalyptus.However,regeneration methods are not always reproducible and genotype can markedly affect the morphogenesis capacity.Callus induction is commonly close to 70%(Nugent et al.2001;Dibax et al.2010),and a few studies have reported shoot organogenesis frequency higher than 50%(Nugent et al.2001;Dibax et al.2005;Matsunaga et al.2012).Moreover,the establishment of efficient protocols has been based on organogenesis evaluation at 30–60 days from the onset of experiments,and cultures older than 30 days often show low frequency of shoot formation(Dibax et al.2005).Secretion of phenolic compounds also adversely affects callus culture and regeneration capability of several species(Babaei et al.2013;Kumar et al.2015),includingEucalyptus(Navroski et al.2014),although phenolic production by plantlets may enhance hardening and acclimatization stepsduring micropropagation(Quiala et al.2012).
In various species,organogenesis is enhanced by the use of liquid culture systems,which promote more rapid plant growth(Rathore et al.2014;Marbun et al.2015;Ramı´rez-Mosqueda and Iglesias-Andreu 2016;Cuenca et al.2017).However,mechanical stress and hyperhydricity,which is typically stress-induced,are often disadvantages in these systems(Quiala et al.2012).Nevertheless,liquid culture is an alternative method for shoot multiplication in some species(Quiala et al.2012;Sa´vio et al.2012;Marbun et al.2015).Shoots fromE.globuluswere grown in a liquid medium using a temporary immersion system,although long immersion periods induced hyperhydricity in shoots(Gonza´lez et al.2011).
Despite the several reports on micropropagation ofEucalyptus,reproducible regeneration of plantlets has been dif ficult to achieve,and regeneration systems suitable for genetic engineering are desirable.The aim of this work was to establish a plant regeneration protocol via indirect organogenesis and develop a novel,efficient proliferative culture system for adventitiousEucalyptusshoots using non-aerated liquid medium.To the best of our knowledge,a non-aerated liquid system forEucalyptusshoot propagation has never been reported.
Materials and methods
Seed germination
Seeds ofEucalyptus globulus(larger than 1 mm)were first surface-sterilized in 70%(v/v)ethanol for 30 s,followed by immersion in fungicide solution(3 g L-1Benlate,DuPont,Wilmington,DE,USA)for 20 min.Seeds were then immersed in 1%(v/v)sodium hypochlorite solution for 10 min and rinsed three times in sterile distilled water.Seeds were sown in 250 mL glass flasks containing 25 mL of germination medium composed of¼MS salts and vitamins(Murashige and Skoog 1962),10 g L-1sucrose and 6 g L-1bacteriological agar.No growth regulators were added.Flasks were kept under light conditions for 60 days,until plantlets were used for explant excision.
Callus induction
For callus induction,hypocotyls and leaf segments(1 cm2)were excised from plantlet and used as initial explants cultivated on half-strength MS medium supplemented with 1 g L-1hydrolyzed casein,30 g L-1sucrose,5 g L-1PVP and 6 g L-1agar,hereafter called½MS basal medium(½ MSBM).Based on previous work,BA(4.44 μM)and NAA(16.1 μM)were used as growth regulators(previous experiment;data not shown).Survival rate of explants and callus formation frequency were evaluated.Treatments consisted of hypocotyls and leaf segments distributed in 10 flasks with five explants each,maintained under dark conditions for 60 days.
Shoot induction and proliferation
Induced calli were transferred to shoot induction medium,consisting of ½ MSBM supplemented with 4.44 μM BA and 0,0.5 or 2.7 μM NAA.Cultures were maintained in the dark until the onset of organogenesis,and then shoots induced on callus were excised and transferred to the same medium in light.Subcultures were carried out every 15 days, and the adventitious shoots induced on organogenic callus were counted at 30,60 and 90 days after transfer to shoot induction medium.Each flask contained three calli(1 cm diameter,approximate 0.250 mg each),and 15 flasks were used in each treatment.Shoot proliferation was further carried out on½MSBM supplemented with 4.44 μM BA and 0.5 μM NAA.
Shoot proliferation was also tested comparing½MSBM and liquid system(LiqMSBM).A layer of liquid medium(5 mL/150 mL flask)with 4.44 μM BA and 0.5 μM NAA was used in the system without aeration.The ef ficiency of the system(semisolid and liquid)was evaluated by the number of shoots per cluster every 15 days for 45 days.Initial clusters contained an average of 12 shoots each.Proliferating cultures were kept in the light.
Rooting and acclimatization
Rooting capacity was evaluated on adventitious shoots cultured on½MSBM medium,with sucrose concentration reduced to 10 g L-1.The plant regulators tested were NAA or IBA at either 5 or 16 μM.No growth regulators were added to the control treatment.Experiment consisted of five flasks with three clusters of five to seven shoots each.After rooting,plantlets were isolated,and the rooting frequency and the number and length of roots were recorded.
Acclimatization was achieved by transferring complete plants to vases(500 mL)with sterile vermiculite,with onefourth strength of MS basal salts(Rathore et al.2014)with a plastic cover.Plants were gradually acclimatized over 14 days after transfer to the substrate.Plant survival was determined as the percentage of plants acclimatized after 30 days.
Culture conditions
All culture media were adjusted to pH 5.8 before autoclaving at 120°C for 20 min.Cultures were maintained in the growth room at 26± 2°C.When light was required,cultures were kept under a 16-h photoperiod at photosynthetic flux of 32.6 μmol m-2s-1,provided by cool daylight fluorescent lamps(OSRAM 32 W).Subcultures were carried out every 15 days,in all the culture steps.The antioxidant polyvinylpyrrolidone(PVP)was maintained in the medium composition throughout the protocol to avoid oxidation of the explants.

Table 1 Survival of explants and callus formation in Eucalyptus globulus,cultured on ½ MSBM,supplemented with 4.44 μM BA and 16.1 μM NAA

Fig.1 Organogenic callus(a)and shoot formation(b)obtained from callus induced from leaf segments of E.globulus cultivated on½MSBM supplemented with 4.44 μM BA and either 0.5(white rhombus)or 2.7 μM(black filled square)NAA.Frequency of organogenic callus and average number of shoots per organogenic callus(0.250 g)are expressed as mean±SE from 15 replicates with three calli each.Asterisk indicates signi ficant difference between means at a speci fic time according to independent-samples test at P≤0.05.Regression was used to evaluate the effect of culture
Determination of phenolic compounds in shoots
Samples from adventitious shoots(0.5 g of fresh tissue)were taken from each treatment for proliferation(1/2MSBM orLiqMSBM)at 15-day intervals for 45 days,blot-dried on sterile filter and ground in aqueous methanol solution(80%,v/v).Extracts were filtered and centrifuged at 1250×gfor 15 min.Total phenolic compounds were analyzed in the supernatant by colorimetric method using Folin–Ciocalteu reagent(765 nm)as previously described(Salla et al.2014).Total phenolic compounds were expressed as mg g-1of fresh mass(FM).
Statistical analysis
Data from either medium composition or system of culture and time of culture were analyzed by Two-way analysis of variance(ANOVA).The isolated effect of period of culture was determined by regression analysis.Whenever two treatments were compared,independent-samples test was used.Data from rooting experiment was subjected to oneway ANOVA and Duncan’s test was used to compare means.Data were evaluated for variance homogeneity mean±standard error(SE).Regression and statistical analyses were performed with the SPSS Statistical Software Program(SPSS v.17;SPSS,Chicago,IL,USA).

Results and discussion
Callus induction inE.globuluswas efficiently obtained from leaf and hypocotyl explants(Table 1).Explant survival was above 80%for both explants tested and no oxidation was observed.Callus formation was affected by the type of explant used(p≤0.05).Hypocotyls and leaf segments were responsive to callus formation(Table 1),and hypocotyls were the most suitable explant for inducing callogenesis,resulting in an average of 1.7 times more callus than leaf segments.Despite the higher responsiveness of hypocotyls to callus induction medium,shoots were only obtained on leaf-derived callus(Table 1).Organogenesis in leaf-derived callus ofE.globuluswas induced within 30 days after culturing in the presence of BA and NAA.The highest organogenic callus frequency was observed with 4.44 μM BA+2.7 μM NAA(Fig.1a).However,reduced NAA concentration(0.5 μM)resulted in increased number of adventitious shoots(Fig.1b).Moreover,the extended period of culture(90 days)significantly affected organogenesis(R2=0.9967),and the number shoots induced on 4.44 μM BA+0.5 μM NAA was 6.14,representing 1.8 times higher than the one recorded on 2.7 μM NAA-containing medium(Figs.1a,2a).

Fig.2 Micropropagation of Eucalyptus globulus.a Cluster of shoots on ½ MSBM medium supplemented with 4.44 μM BA+0.5 μM NAA after 90 days of culture.b Shoots on semi-solid½MSBMmedium after 45 days of culture.c Shoot proliferation on liquid non-aerated(LiqMSBM)medium after 45 days of culture.d Plantlet rooted on 16.0 μM IBA after 30 days of culture.e Acclimatized plant.a–c Bar=1 cm;d,e bar=4 cm
Few reports have shown that indirect organogenesis inE.tereticornis,E.globulus,E.nitensandE.calmadulensismay succeed if juvenile tissue is used as initial explant(Bandyopadhyay et al.1999;Dibax et al.2005;Aggarwal et al.2010),since less differentiated cells of young explants are more responsive to the growth regulators and nutritious conditions of the culture medium.Leaf maturity was found to in fluence the organogenic response of leaf segments ofE.tereticornis,and 14-to 16-day-old leaves from in vitro plantlets resulted in 40.5%of organogenic explants(Aggarwal et al.2010).Generally,leaves are not the explants of choice,probably due to tissue differentiation and endogenous hormone balance.However,results suggest that leaves ofE.globulusfrom in vitro 60-day-old plants are still juvenile for callus formation,and a suitable combination of plant growth regulators might trigger de-differentiation,resulting in callus formation and shoot induction.In our study,an optimized culture medium,including antioxidant compounds and appropriate type and concentration of growth regulators,as well as dark cultivation might have accounted for the success of shoot induction onE.globulusleaf explants.It is worth mentioning that older seedlings generated more explants per plant with less labor than the use of explants such as cotyledons or hypocotyls.
Shootmultiplication wascompared on semisolid(MSBM)and non-aerated liquid medium(LiqMSBM)for 45 days.The number of shoots on liquid medium was markedly higher after 30 days(Fig.3a).Proliferation of shoots on MSBS stabilized after 30–45 days.The highest total increment was observed when shoots were cultivated in liquid medium,reaching 53.5 shoots per cluster,representing 1.87 times more shoots than on MSBM medium.No morphological differences were observed between the two systems(Fig.2b,c).The high ef ficiency of adventitious shoot proliferation in liquid medium might be related to the high availability of water and nutrients due to the greatest exposure medium surface area,stimulating shoot proliferation(Marbun et al.2015).Liquid cultures have been efficient,mainly using systems that allow periodic immersion of the tissues in the medium(Ramı´rez-Mosqueda and Iglesias-Andreu 2016;Cuenca et al.2017).Growth and development of the explant were improved due to the more uniform medium distribution in all parts of the tissue from different species,such as pineapple(Zuraida et al.2011),eucalyptus(Gonza´lez et al.2011),acacia(Rathore et al.2014)and oil palm(Marbun et al.2015).However,the system proposed here does not involve immersion,and the base of the shoot cluster is the only part of the plant tissue in contact with the thin-layer of medium.Likely,this system avoids the formation of an aqueous film on the surface of leaves and stems,which could hamper gaseous exchange between the cell surface and flask environment.Moreover,medium absorption occurs at the area of shoot multiplication,namely,at the base of the explant,promoting proliferation.
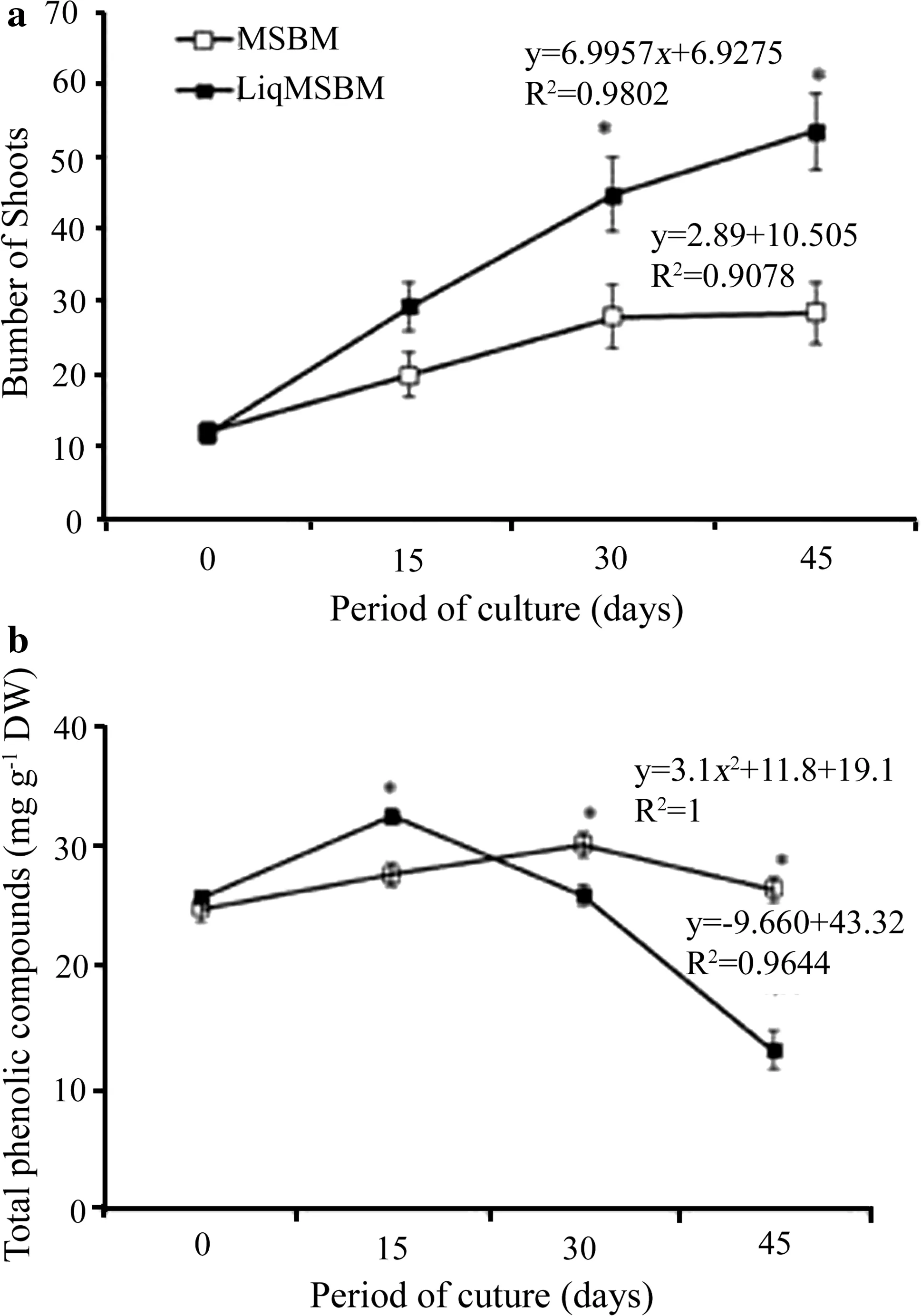
Fig.3 Number of proliferating shoots(a)and total phenolic compounds(b)from E.globulus on either½MSBM(white rhombus)or liquid medium without aeration(LiqMSBM)(black filled square),both supplemented with 4.44 μM BA and 0.5 μM NAA.Data are expressed as mean±SE from 15 replicates with three clusters.Asterisk indicates signi ficant difference between means at a speci fic time according to independent-samples test at P≤0.05.Regression was used to evaluate the effect of culture period
Production of phenolic compounds by tissues in culture is frequently reported in relation to browning of excised explants,which may result in lower rates of regeneration and recalcitrance of the species,mainly wood species(Ahmad et al.2013;Babaei et al.2013;Jones and Saxena 2013;Kumar et al.2015).Browning of tissues is caused by oxidation of polyphenols by polyphenol oxidases(PPO)and peroxidases(POD).This common problem in tissue culture is often minimized by addition of different absorbents or antioxidants such as PVP,citric acid,ascorbic acid or carbohydrates to the medium(Krishna et al.2008;Kumar et al.2015),by the inhibition of phenylalanine ammonia lyase(Jones and Saxena 2013)and several other strategies(Ahmad et al.2013).In eucalyptus cultures,browning is a common problem since explants are usually rich in phenolic compounds(Dibax et al.2005;Navroski et al.2014;Lopes da Silva et al.2015);however,addition of PVP to the medium can overcome this problem,by reducing PPO activity.Excessive PVP,however,can hinder tissue proliferation(Gonza´lez et al.2011).When the content of phenolic compounds was compared between semisolid and liquid medium cultures,shoots cultured on MSBM medium steadily accumulated phenolic compounds(Fig.3b).On the other hand,the content of phenolic compounds significantly decreased with culture time in shoots proliferating inLiqMSMB medium(Fig.3b).Thus,the liquid system seems less stressful for culturingE.globulusshoots while stimulating proliferation.Low production of phenolic compounds could result in less secretion and,consequently,low oxidation of shoots.The novelty of this work is that a thin layer of liquid medium,even with no aeration,was suf ficient to increase shoot multiplication ofE.globulus,and cultures remained stable for up to 45 days with low production of phenolic compounds.
Rooting is one of the most dif ficult steps in the micropropagation of woody species,and forest rooting is often performedex vitrodue to the low frequency of success(Quiala et al.2012).However,in our study,rooting ofE.globulusshoots was obtained in all treatments tested,except in hormone-free medium.The first roots(15 days)were observed on treatment with 5 μM NAA,followed by 5 μM IBA,16 μM NAA and 16 μM IBA,and these later treatments showed similar ef ficiency on rooting(Table 2).Nevertheless,the most roots per shoot cluster(8.6)and the longest roots(7.8 cm)were obtained by adding 16 μM IBA to the medium(Table 2;Fig.2d).Rooted shoots were transferred for acclimatization in sterile vermiculite,and 76%survival rate was obtained(Fig.2e).AlthoughEucalyptus globulusis recalcitrant to rooting,which may compromise the use of in vitro technology,IBA can induce rooting in responsive eucalyptus genotypes(Fett-Neto et al.2001;Brondani et al.2012).InEucalyptus phylacisandE.grandis,5 μM IBA resulted in 37 and 83%of rooting,respectively(Bunn et al.2005;Hajari et al.2006).In another study,rooting rate inE.grandis×E.urophyllawas not superior to 35%using 2.46 μM IBA(Oliveira et al.2016).
Our results showed that leaves from 60-day-old in vitro plants are suitable for callus formation and shoot induction ofE.globulus.Multiplication of adventitious shoots was efficient in liquid medium up to 45 days of culture,creating a less expensive and less laborious system.Eucalyptus plant regeneration has been indicated as the most critical step in developing genetic transformation.Most eucalyptus species are recalcitrant using in vitro methods,probably due to the high production of phenolic compounds and the maturity level of the explants.In the liquid medium used for shoot proliferation,the production of phenolic compounds was lower,and thus a more useful system for regenerating genetically engineeredEucalyptusplants.
Acknowledgements This work was supported by the National Council for Scienti fic and Technological Development(CNPq)/Brazil,under Grant 477538/2013-4.The authors are grateful to Suzano Papel e Celulose(former RioCell,Brazil)for providing seeds ofE.globulusand to Janaı´na Belquis da S.P.Langois for technical assistance.
Compliance with ethical standards
Con flict of interestThe authors declare that they have no con flict of interest.
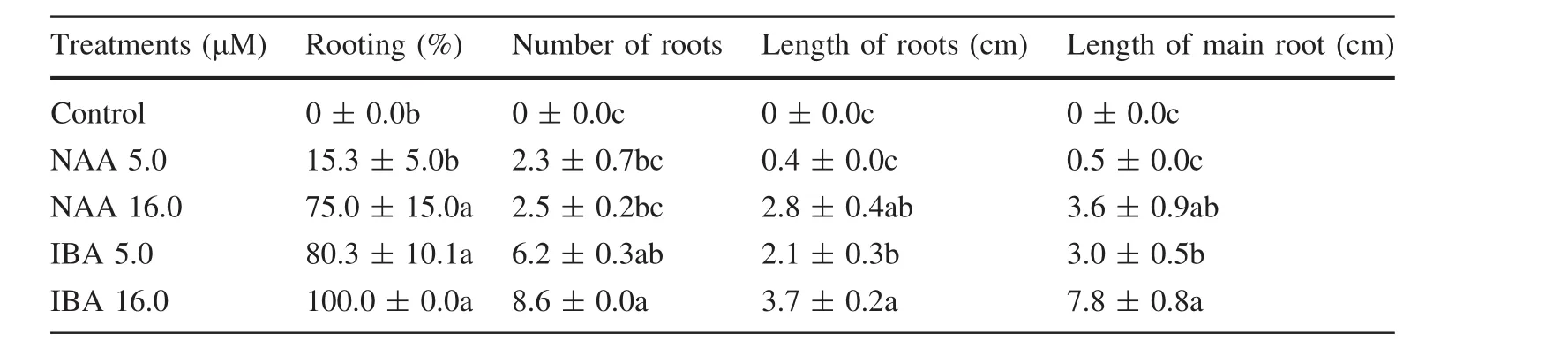
Table 2 Rooting of Eucalyptus globulus shoots on½MSBM medium supplemented with different concentrations of either NAA or IBA after 30 days
References
Abril N,Gion J-M,Kerner R,Mu¨ller-Starck G,Navarro Cerrillo RM,Plomion C et al(2011)Proteomics research on forest trees,the most recalcitrant and orphan plant species.Phytochemistry 72:1219–1242
Aggarwal D,Kumar A,Reddy SM(2010)Shoot organogenesis in elite clones ofEucalyptus tereticornis.Plant Cell,Tissue Organ Cult 102:45–52
Ahmad I,Hussain T,Ashraf I,Nafees M,Maryam Rafay M et al(2013)Lethal effects of secondary metabolites on plant tissue culture.Am Eurasian J Agric Environ Sci 13(4):539–547
Babaei N,Abdullah NAP,Saleh G,Abdullah TL(2013)Control of contamination and explant browning inCurculigo latifoliain vitro cultures.J Med Plants Res 7(8):448–454
Bandyopadhyay S,Cane K,Rasmussen G,Hamill JD(1999)efficient plant regeneration from seedling explants of two commercially important temperate eucalypt species–Eucalyptus nitensandE.globulus.Plant Sci 140:189–198
Brondani GE,Baccarin FJB,Ondas HWW,Stape JL,Gonc¸alves AN,Almeida M(2012)Low temperature,IBA concentrations and optimal time for adventitious rooting of Eucalyptus benthamii mini-cuttings.J J For Res 23(4):583–592
Bunn E,Senaratna T,Sivasithamparam K,Dixon KN(2005)In vitropropagation ofEucalyptus phylacisL.Johnson and K.Hill,a critically endangered relict from Western Australia.In Vitro Cell Dev Biol Plant 41:812–815
Cuenca B,Sa´nchez C,Aldrey A et al(2017)Micropropagation of axillary shoots of hybrid chestnut(Castanea sativa×C.crenata)in liquid medium in a continuous immersion system.Plant Cell,Tissue Organ Cult.doi:10.1007/s11240-017-1285-5
Dibax R,Eisfeld CL,Cuquel FL,Koehler H,Quoirin M(2005)Plant regeneration from cotyledonary explants ofEucalyptus camaldulensis.Sci Agric 624:406–412
Dibax R,Deschamps C,Bespalhok Filho JC,Vieira E,Molinari C,Campos D et al(2010)Organogenesis andAgrobacterium tumefaciens-mediated transformation ofEucalyptus salignawith P5CS gene.Biol Plant 54:6–12
Fett-Neto AG,Fett JP,Goulart LWV,Pasquali G,Termignoni RR,Ferreira AG(2001)Distinct effects of auxin and light on adventitious root development inEucalyptus salignaandEucalyptus globulus.Tree Physiol 21:457–464
Gonza´lez R,Rı´os D,Avile´s F,Sa´nchez-Olate M(2011)Multiplicacio´n in vitro deEucalyptus globulusmediante sistema de inmersio´n temporal.Bosque 32(2):147–154
Hajari E,Watt MP,Mycock DJ,McAlister B(2006)Plant regeneration from induced callus of improvedEucalyptusclones.S Afr J Bot 72:195–201
Jones AMP,Saxena PK (2013)Inhibition of phenylpropanoid biosynthesis inArtemisia annuaL.:a novel approach to reduce oxidative browning in planttissue culture.PLoS ONE 8(10):e76802
Krishna H,Sairam RK,Singh SK,Patel VB,Sharma RR et al(2008)Mango explant browning:effect of ontogenic age,mycorrhization and pre-treatments.Sci Hortic 118:132–138
Kumar GP,Subiramani S,Govindarajan S,Sadasivam V,Manickam V,Mogilicherla K et al(2015)Evaluation of different carbon sources for high frequency callus culture with reduced phenolic secretion in cotton(Gossypium hirsutumL.)cv.SVPR-2.Plant Biotechnol Rep 7:72–80
Lopes da Silva AL,Gollo AL,Brondani GE,Horbach MA,Oliveira LS,Machado MP et al(2015)Micropropagation ofEucalyptus salignaSM.from cotyledonary nodes.Pak J Bot 47:311–318
Mabona U,Van Vuuren SF(2013)Southern African medicinal plants used to treat skin diseases.S Afr J Bot 87:175–193
Marbun CLM,Toruan-Mathiusa N,Re flini Utomo C,Liwang T(2015)Micropropagation of embryogenic callus of oil palm(Elaeis guineensisJacq.)using temporary immersion system.Proc Chem 14:122–129
Matsunaga E,Nanto K,Oishi M,Ebinuma H,Morishita Y,Sakurai N et al(2012)Agrobacterium-mediatedtransformation ofEucalyptus globulususing explants with shoot apex with introduction of bacterial choline oxidase gene to enhance salt tolerance.Plant Cell Rep 31:225–235
Murashige T,Skoog F(1962)A revised medium for rapid growth and bioassays with tobacco tissue cultures.Physiol Plant 15:473–497 Navroski MC,Reiniger LRS,Arau´jo MM,Curti AR,Pereira MO(2014)In vitro establishment and multiplication of genotypes ofEucalyptus dunniiMaiden.Cerne 20:139–146
Nugent G,Chandler SF,Whiteman P,Stevenson TW(2001)Somatic embryogenesis inEucalyptus globulus.Plant Cell,Tissue Organ Cult 67:85–88
Oliveira C,Degenhardt-Goldbach J,Bettencourt GMF,Amano E,Franciscon L,Quoirin M(2016)Micropropagation ofEucalyptus grandisxE.urophyllaAEC 224 clone.J For Res 27:1–11
Quiala E,Canal M-J,Meijo´n M,Rodrı´guez R,Cha´vez M,Valledor L et al(2012)Morphological and physiological responses of proliferating shoots of teak to temporary immersion and BA treatments.Plant Cell,Tissue Organ Cult 109:223–234
Quoirin M,Quisen R(2006)Advances in genetic transformation ofEucalyptusspecies.In:Franche C(ed)Molecular biology tropical plant research signpost Kerala,pp 41–56
Ramı´rez-Mosqueda MA,Iglesias-Andreu LG(2016)Evaluation of differenttemporaryimmersionsystems(BIT®,BIG,and RITA®)in the micropropagation ofVanilla planifoliaJacks.In Vitro Cell Dev Biol Plant 52:154–160
Rathore JS,Rai MK,Phulwaria M,Shekhawat NS(2014)A liquid culture system for improved micropropagation of matureAcacia nilotica(L.)Del.ssp.indicaandex vitrorooting.Proc Natl Acad Sci 84:193–200
Resquin F,Barrichelo LEG,Silva Ju´nior FG,Brito JO,Sansigolo CA(2006)Wood quality for kraft pulping ofEucalyptus globulusorigins planted in Uruguay.Sci For 72:57–66
Salla TD,da Silva TR,Astarita LV,Santare´m ER(2014)Streptomycesrhizobacteria modulate the secondary metabolism ofEucalyptusplants.Plant Physiol Biochem 85:14–20
Sa´vio LE,Astarita LV,Santare´m ER(2012)Secondary metabolism in micropropagatedHypericum perforatumL.grown in non-aerated liquid medium.Plant Cell,Tissue Organ Cult 108:465–472
Schwambach J,Fadanelli C,Fett-Neto A(2005)Mineral nutrition and adventitious rooting in microcuttings of Eucalyptus globulus.Tree Physiol 25:487–494
Zuraida AR,Nurul Shahnadz AH,Harteeni A,Roowi S,Che Radziah CMZ,Sreeramanan S(2011)A novel approach for rapid micropropagation of maspine pineapple(Ananas comosusL.)shoots using liquid shake culture system.Afr J Biotech 10:3859–3866
杂志排行
Journal of Forestry Research的其它文章
- In vitro propagation of conifers using mature shoots
- ‘Relationships between relationships’in forest stands:intercepts and exponents analyses
- Effects of application date and rate of foliar-applied glyphosate on pine seedlings in Turkey
- Assaying the allelopathic effects of Eucalyptus camaldulensis in a nursery bed incorporated with leaf litter
- Effects of soil compaction on growth variables in Cappadocian maple(Acer cappadocicum)seedlings
- Variation and selection analysis of Pinus koraiensis clones in northeast China
