Influence of salinity on the early development and biochemical dynamics of a marine fish, Inimicus japonicus*
2018-05-07GONGXu龚续HUANGXuxiong黄旭雄WENWen温文
GONG Xu (龚续) , HUANG Xuxiong (黄旭雄) , , WEN Wen (温文)
1 Key Laboratory of Freshwater Fishery Germplasm Resources, Ministry of Agriculture, Shanghai 201306, China
2 Shanghai Engineering Research Center of Aquaculture, Shanghai 201306, China
3 Shanghai University Knowledge Service Platform, Shanghai Ocean University Aquatic Animal Breeding Center (ZF1206),Shanghai 201306, China
4 National Demonstration Center for Experimental Fisheries Science Education (Shanghai Ocean University), Shanghai 201306, China
1 INTRODUCTION
Salinity is a dominant factor that affects the distribution of species in the marine environment because it is closely related to the osmoregulation,energy budget, feeding, growth, and development of fish (Morgan, 1998; Bœuf and Payan, 2001; Kamler,2002; Yuan and Cui, 2004). Salinity, especially in coastal areas, often drastically changes as a result of extreme weather events (e.g., deluges and typhoons),which profoundly affect marine broodstock reproduction, embryo incubation and larval growth, as well as aquaculture yields (Berlinsky et al., 2004).Numerous studies have focused on the effects of salinity on the early development of marine fish and have demonstrated that the tolerance levels to salinity variations during the early developmental stages of marine fish are species-specific (Wang et al., 2002; Shi et al., 2004, 2009). In addition, the tolerance to salinity within the same species is stage-dependent (Fashina-Bombata and Busari, 2003). Wang (2002) reported that the optimal hatching and lowest malformation rates ofPagrosomusmajor’s fertilised eggs occur at a salinity range of 32 to 33, whereas the survival rate of larvae was greater at a lower salinity range (17 to 22).The adaptation of fish to salinity stress is achieved through osmoregulation; however, no definite theory has been established that clarifies the physiological and hormonal regulatory mechanisms that exist in various fish species (Bœuf and Payan, 2001).Osmoregulation in the early life stages of fish may not be the same as in adult fish, and it may even be deficient in juveniles (Varsamos et al., 2005; Yang and Chen,2006; Bodinier et al., 2010). Thus, a suitable environmental salinity level is essential to the early development and growth of fish larvae.
Previous studies on optimising the incubation conditions for marine embryos and yolk-sac larvae focused on limited aspects of their hatching performances or changes in their physiological metabolisms. Few attempts have been made to identify the various substrates that are oxidised during development. The yolk pellets in marine fish eggs mainly consist of proteins, free amino acids, lipids and carbohydrates (Vetter et al., 1983; Rønnestad and Fyhn, 1993; Wiegand, 1996). The utilisation of yolk substances at the embryonic and early yolk-sac larval stages in oviparous animals, including fish, is vital for their normal development (Ohkubo et al., 2008). The utilisation of yolk reserves is determined by the metabolic rate of fish larvae, which is affected by intrinsic factors, such as egg size and genes, and external factors, such as dissolved oxygen, pH,salinity, and temperature (Kamler, 2002). Additionally,the nutritional compositions of juvenile fish are significantly affected by environmental factors (Zeng et al., 2014). However, the effects of these factors on the nutritional substrate dynamics and energy budgets of marine fish during ontogenesis have rarely been summarised.
Inimicusjaponicus(devil stinger) is an economically important marine fish in southern China that has promising breeding potential. Huang et al. (2013)proposed a pattern of catabolic substrate oxidation,which occurs during development, that may be typical for marine pelagic fish eggs that do not contain oil globules. Temperature has significant effects on the early development and endogenous biochemical dynamics ofI.japonicus(Wen et al., 2013), and it is susceptible to temperature during ontogenesis,responding to higher environmental temperatures with shorter developmental durations and increased rates of energy consumption. Lin (2008) reported that 27 to 31 and 23 to 27 were the ideal salinity ranges for the embryogenesis and larval development ofI.japonicus,respectively, based on survival. Further studies are needed to illustrate the consumption of endogenous reserves at different salinity levels. Thus, the current study presents an integrated investigation of the biological, morphological, and biochemical data derived from a single batch of developing embryos and yolk-sac larvae ofI.japonicusthat was incubated at different salinity levels. This study helps to better understand the utilisation sequence of endogenous nutrients and to determine more reliable salinity ranges for this species.
2 MATERIAS AND METHOD
2.1 Experimental animals
The eggs and larvae ofI.japonicusused for the experiments were obtained from broodstocks, which were captured from the Xiamen Sea zone, China,after spawning was induced by hormones, as described by Wen et al. (2013).
2.2 Experimental design and apparatus
Experimental salinity levels of 21, 25, 29, 33, and 37 were selected for the incubation ofI.japonicuseggs and larvae. Hyperosmotic seawater (salinity levels of 33 and 37) was prepared by adding the appropriate amount of sea salt to the filtered local natural seawater (salinity levels of 29). Hypoosmotic seawater (salinity levels of 21 and 25) was prepared by adding aerated fresh water to the filtered local natural seawater. A salinometer (Atago, Japan) was used to determine the salinity level of each treatment.Six replicated cylindrical tanks, each with a volume of 10 L, were used for each treatment and were placed in a 105-L concrete tank filled with seawater at 21±0.5°C, as measured by digital thermostats(±0.2°C). Then, fertilised eggs were assigned to each 10-L incubation tank at an approximate density of 200–250 eggs/L. Continuous light at an intensity of 500–1 200 lx was applied above all of the tanks (Liu and Quan, 2005; Lin, 2008). The ammonia and dissolved oxygen concentrations in each incubation tank were maintained below 0.1 mg/L and above 5.0 mg/L, respectively, and the pH was maintained between 8.2 and 8.5 through daily water exchanges(80%–100%) and continuous aeration. The experiment lasted from fertilisation to the moment when approximately 80%–90% of the larvae opened their mouths with movable jaws. In addition, 1-L beakers containing 1 L of sea water and 100 eggs were set, in triplicate, in a 105-L concrete tank without aeration for the developmental performance assays (hatching rate and hatching time) at a corresponding salinity.Additionally, 1-L beakers containing 1 L of sea water and 100 yolk-sac larvae, in triplicate, under the same conditions were used to assay larval survival at corresponding salinity levels. The experiment lasted from fertilisation to the moment when 80%–90% of the larvae opened their mouths with movable jaws.
2.3 Morphological measurements
From the onset of hatching, 20 larvae from each replicate were sampled every 12 h to measure the full lengths and the yolk volumes according to the methods of Cetta and Capuzzo (1982) and Wen et al.(2013).
2.4 Biochemical analyses
2.4.1 Sample collecting and pre-processing
The fertilised eggs were sampled prior to the start of the experiment. Subsequently, newly hatched larvae and open-mouthed larvae were sampled. At each sampling time, a sample of approximately 5 g and a subsample of 100 individuals were collected from each replicate. We rinsed the collected samples and subsamples with distilled water and then dried them at -46°C using a freeze-dry system (Labconco,USA). The samples were ground into powder, and dry weights of the subsamples were measured using a high-precision balance (Sartorius, Germany; weights recorded to the nearest 0.01 mg) to determine single egg or larva weight in a specific treatment.
2.4.2 Biochemical composition and energy determinations
The samples were homogenised in pre-cooled distilled water at 4°C to quantify the protein content.The fertilised eggs and newly hatched larvae were homogenised with a dilution ratio of 1/80 (w/v), and the open-mouthed larvae were homogenised with a dilution ratio of 1/160 (w/v). The homogenates were centrifuged at 4 000 r/min for 15 min at 4°C. The supernatants were then used to determine the protein concentrations using the Coomassie Brilliant Blue colorimetric method (Bradford, 1976). Bovine serum albumin was used as the standard.
To determine the carbohydrate content, the protein was precipitated with trichloroacetic acid (15% and 5%) and pelleted by centrifugation at 4 000 r/min for 15 min at 4°C. The supernatant was hydrolysed with hydrochloric acid (6 mol/L) at 96°C in a water bath.Then, the solution was neutralised with sodium hydrate(6 mol/L), and the volume was adjusted to 10 mL with distilled water. The carbohydrate concentration was then quantified in the supernatant using a colorimetric method with a phenol-sulphuric acid reagent (Dubois et al., 1956). In this method, glucose was used as a standard, and its standard curve was applied to evaluate the carbohydrate concentration of each sample.
Lipids were extracted using chloroform:methanol(2:1, v/v) containing 0.01% butylated hydroxytoluene as the antioxidant (Folch et al., 1957), and the extracted lipids were vacuum-dried to a constant weight to determine the total lipid content.
All of the biochemical determinations were performed in triplicate for each replication, and the results were converted to μg per individual (μg/ind.)using the dry weight of a single egg or larva in a specific treatment. The theoretical gross energy value was calculated from the biochemical composition using conversion factors of 5.65, 9.45, and 4.20 kcal/g dry weight for proteins, lipids, and carbohydrates,respectively (Henken et al., 1986). The gross energy value is presented as 10-3calories per individual(cal/ind.).
2.5 Statistical analysis
The results are displayed as the means±standard deviations. Normality and homoscedasticity were checked, and the significance of the data was tested by an one-way analysis of variance followed by Duncan’s new multiple range test, using SPSS 11.0(SPSS Inc., USA). We choseP=0.05 as the significance level. A polynomial regression was applied to the hatching rate (%) and survival rate of open-mouthed larvae (%) to calculate the optimum salinity levels for embryo and yolk-sac larval incubations (Shi et al.,2008). Based on the optimum hatching and survival rates, ranges of ±10% were determined as having suitable salinity levels for the incubation of embryos and yolk-sac larvae, respectively (Yan et al., 2011).
3 RESULT
3.1 Impact of salinity on the survival and growth of embryos and yolk-sac larvae
The fertilised eggs ofI.japonicushatched in a salinity range of 21–37. The hatching time was 40 h after fertilisation. The salinity level had a significant influence on the hatching rate of this species (Fig.1).The hatching rate significantly increased as the salinity rose from 21 to 29 (P<0.05), but no significantdifferences were observed between the salinity of 29-,33-, and 37-treatments (>0.05). Within the salinity range of 21–37, the regression equation for the hatching rate (%) based on salinity was as follows:y=-0.4866x2+33.015x−468.22 andR2=0.989 [Fig.1(hollow diamond)]. The optimum salinity for embryonic incubation was 33.9, with a hatching rate of 91.8%. A suitable salinity range was between 30.5 and 37.3 (applying ±10%).
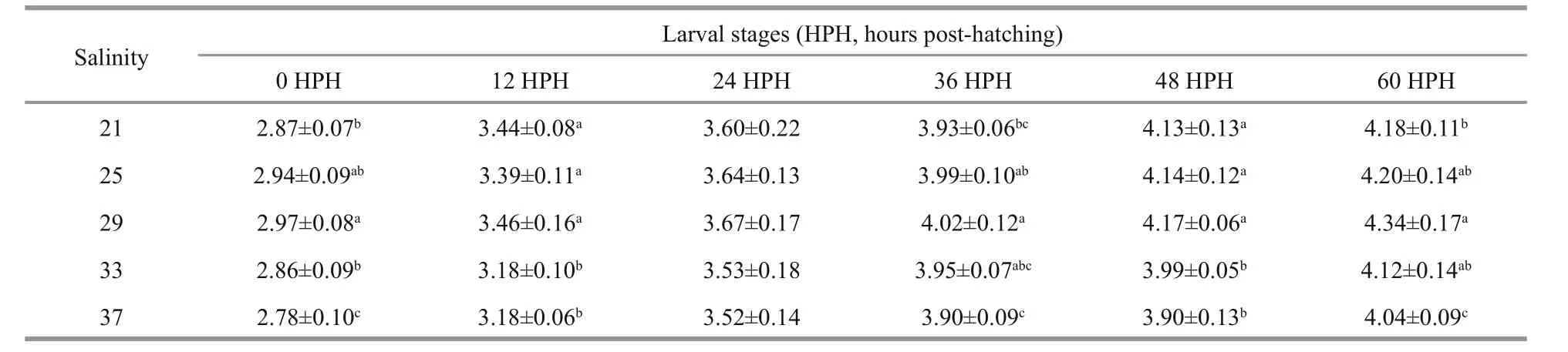
Table 1 Full lengths of developing yolk-sac larvae of I. japonicus incubated at different salinity levels (mm)
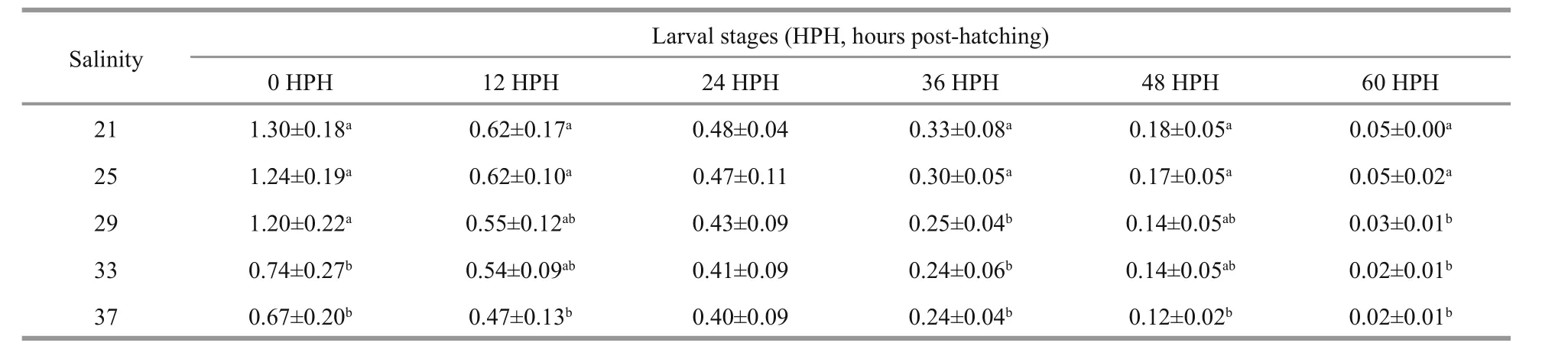
Table 2 Yolk-sac volumes of developing yolk-sac larvae of I. japonicus incubated at different salinity levels (mm 3)
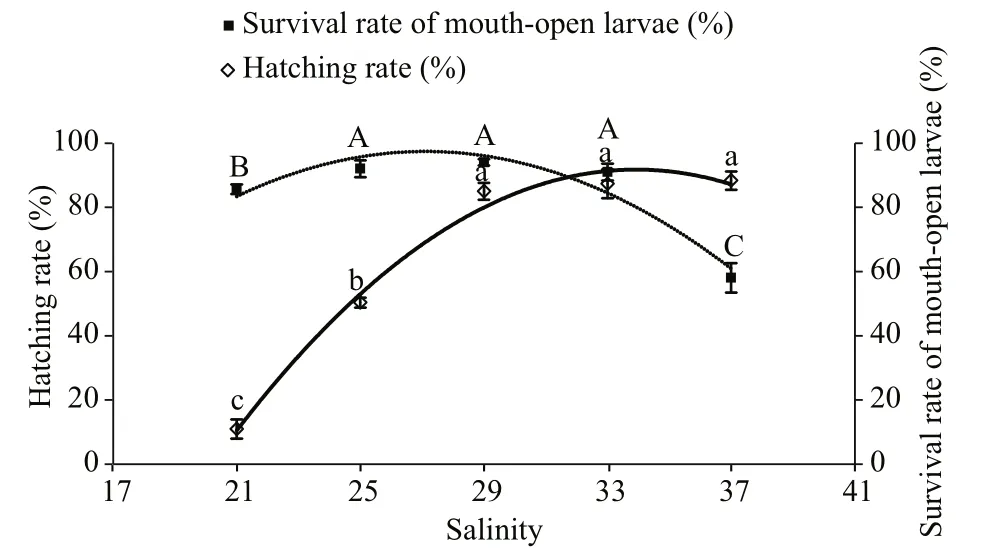
Fig.1 Hatching and survival rates (%) of open-mouthed I.japonicus larvae incubated at different salinity levels
The yolk-sac larvae opened their mouths at 60 h after hatching, and this time was not affected by different salinity levels. The survival rate of openmouthed larvae (%) increased as the salinity rose from 21 to 29 and decreased when the salinity surpassed 29(Fig.1). The highest survival rate was 94.0% at salinity of 29, and no significant differences were observed among salinity levels of 25, 29, and 33 (P>0.05). The lowest survival rate was 58.0% at a salinity of 37(P<0.05). Within the experimental range, the regression equation for the survival of open-mouthed larvae (%) based on salinity was as follows:y=-0.3735x2+20.255x−177.2 andR2=0.916 6 [Fig.1 (solid square)].The optimum salinity for yolk-sac larvae incubation was 27.1, with a survival rate of 97.4%. A suitable salinity range was 24.4–29.8.
Significant differences were observed in the full lengths of yolk-sac larvae when incubated at different salinity levels (Table 1). The full lengths of newly hatched larvae (0 hours post-hatching) and openmouthed larvae (60 hours post-hatching) increased as the salinity rose from 21 to 29 and then significantly decreased as the salinity continued to rise from 29 to 37 (P<0.05). Yolk-sac larvae that developed in an environment with a salinity level of 37 were always the shortest when compared with the lengths of larvae from the other treatments at the same developmental stage. The yolk-sac consumption of larvae was also significantly affected by salinity(Table 2). Unlike what was observed for the fulllengths of larvae, the yolk-sac volumes of newly hatched larvae had a decreased with increasing salinity. Incubation at the salinity range of 21–29 did not significantly change the yolk volume of newly hatched larvae (P>0.05). However, when the salinity level was greater than 29, the yolk volume of newly hatched larvae significantly decreased (P<0.05). In open-mouthed larvae, a negative correlation was observed between the yolk-sac volume and the environment’s salinity level.
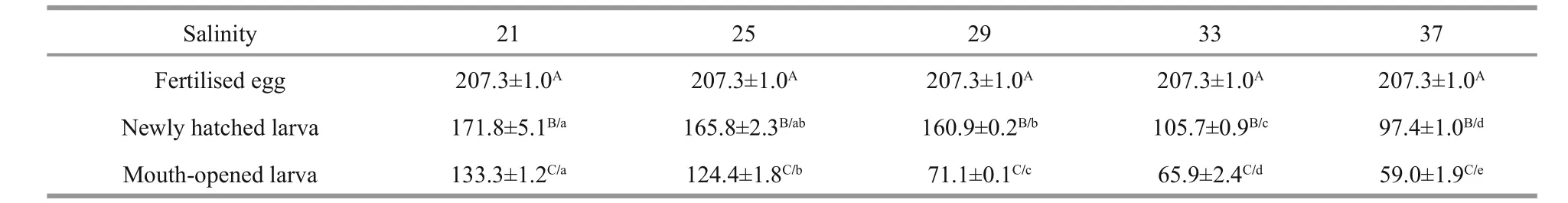
Table 3 Dry weights of I. japonicus fertilised eggs and larvae (μg/ind.)

Fig.2 Nutrient contents (μg/ind.) of developing I. japonicus fertilised eggs and larvae incubated at different salinities
3.2 Impact of salinity on biochemical and energetic dynamics
Table 3 shows that the dry weights of individualI.japonicussharply decreased with development and that the dry weights of newly hatched larvae or openmouthed larvae decreased significantly as the salinity increased.
The individual fertilised eggs contained ~17.8 μg of protein (Fig.2a). The total protein content in newly hatched larvae sharply decreased throughout embryogenesis under all of the treatment conditions(P<0.05). The larvae that were incubated at a salinity level of 29 had the significantly greatest protein content (~8.7 μg/ind.) at hatching, and the larvae that were incubated at the salinity levels of 33 and 37 contained significantly lower protein contents (5.2–5.5 μg/ind.) than those from the other two treatment conditions (7.4–8.0 μg/ind.). Protein was accumulated in larvae under all of the treatment conditions during the yolk-sac larval stage. The protein content of the open-mouthed larvae increased as the environment’s salinity level rose from 21 to 25 and then significantly decreased when the salinity level surpassed 25(P<0.05).
The individual fertilised eggs contained ~1.2 μg of carbohydrates (Fig.2b). Under the experimental salinity levels, the carbohydrate contents (1.2–2.4 μg/ind.) in the newly hatched larvae and openmouthed larvae were higher than in the fertilised eggs.Salinity had a significant effect on the use of carbohydrates inI.japonicus. In newly hatched larvae,the carbohydrate contents increased when the salinity level rose from 21 to 29 and then significantly decreased under salinity conditions of 33 and 37(P<0.05). During the yolk-sac larval stage,carbohydrate utilisation was positively correlated with the salinity level. No differences were observed in the carbohydrate contents of larvae incubated at the salinity levels of 21 and 25 (~2.2 μg/ind.) (P>0.05),while the larval carbohydrate contents were depleted at a salinity level of 29 (~1.4 μg/ind.) (P<0.05).
The individual fertilised eggs contained ~19.1 μg of lipids (Fig.2c). After embryogenesis, only larvae incubated at the salinity levels of 25 and 29 had greater lipid contents (22.1–25.3 μg/ind.) than that of fertilised eggs. Newly hatched larvae incubated at a salinity level of 29 had the greatest lipid contents(~25.3 μg/ind.). No significant differences were observed in the lipid contents of newly hatched larvae incubated at the salinity levels of 21, 33, and 37(14.2–15.6 μg/ind.) (P>0.05). The data also indicated that the lipid contents in open-mouthed larvae incubated at the salinity levels of 21, 29, 33, and 37 were not significantly different (9.8–11.1 μg/ind.).These values were significantly lower than the value seen at a salinity level of 25 (~18.7 μg/ind.) (P<0.05).
The theoretical energy value of an individual was calculated as the energy from protein, carbohydrates and lipids (Fig.3). As the salinity level increased, the gross energy value of the newly hatched larvae and open-mouthed larvae showed an obvious tendency to increase under low salinity conditions and then decrease under higher salinity conditions. The greatest energy values in the newly hatched larvae and openmouthed larvae occurred when the larvae were incubated at salinity levels of 29 (~298.2×10-3cal/ind.)and 25 (~336.9×10-3cal/ind.), respectively. These values were both greater than the values observed in fertilised eggs.I.japonicusthat were incubated at a salinity level of 37 suff ered the greatest energy cost(~115.2×10-3cal/ind.) during embryogenesis, but no significant differences were found in the consumption of gross energy when they were incubated at salinity levels of 21, 33, and 37 (P>0.05). At the larval stage,the energy depletion sharply increased when the water salinity level surpassed 25 (P<0.05).
4 DISCUSSION
For the large-scale seed production of a fish species in a hatchery system or in the wild, it is imperative to know the effects of environmental parameters on larval rearing. Salinity is an important factor that aff ects the survival, metabolism and distribution of fish during their development.
4.1 Growth and survival
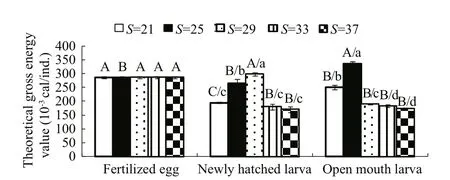
Fig.3 Theoretical gross energy values (10 - 3 cal/ind.) of the developing I. japonicus eggs and larvae when incubated at different salinity levels
The influence of salinity on the growth and survival of a variety of marine fish species has been extensively researched and reviewed (Hart and Purser, 1995;Conides and Glamuzina, 2001; Kamler, 2002;Labonne et al., 2009). Although the retardation of hatching at low and high salinity levels has been reported (Holliday and Blaxter, 1960; Yang and Chen,2006; Xu et al., 2009), no effect of salinity on the ontogenetic rate was reported in the majority of these studies (Bœuf and Payan, 2001; Kamler, 2002). This is in accordance with the observations in this study.The salinity levels that supported hatching (21–37)did not alter the hatching time ofI.japonicusembryos.Shi et al. (2008) revealed that low salinity levels negatively affect the buoyancy of marine eggs and the hatching rate. In addition, our observations and those from other studies (Sha et al., 1981; Huang et al.,2013; Wen et al., 2013) demonstrated thatI.japonicusbelongs to the group of fish whose buoyant eggs contain no oil globules. As a result, the fertilised eggs of this species did not spontaneously become buoyant at the relatively low salinity level (21) in the present study. The greatest hatching rate of ~88.3% was observed in fertilised eggs incubated at a salinity level of 37. This was, however, not significantly greater than the ~85.0% and ~87.3% hatching rates under the 29- and 33- salinity conditions, respectively. Using the regression equation for hatching rate based on salinity [Fig.1 (hollow diamond)], we concluded that 30.5–37.3 was a suitable salinity range for the embryogenesis ofI.japonicus. The salinity range is relatively narrow when compared with those of other marine fish, includingClupeaharengusL. (1.6–60;Holliday and Blaxter, 1960),C.harenguspallasi(4.5–42; Alderdice and Hourston, 1985) andOplegnathusfasciatus(21–42; Cai et al., 2010). This narrow range may be related to the characteristic absence of oil globules in this species, which results in a poor osmoregulation capability (Sucré et al.,2013). Lin (2008) investigated the salinity tolerance ofI.japonicususing a salinity range of 15–39 and found that 27–31 was ideal for the incubation ofI.japonicuseggs. This range is lower than the range defined in the present study. Evidence indicates that the parent fish can maintain the osmotic balance of the eggs before spawning through blood circulation(Alderdice et al., 1979; Davenport et al., 1981;Kjörsvik et al., 1984). Thus, the salinity level that the embryos can tolerate is related to the salinity level present during the rearing of the broodstocks (Hart and Purser, 1995). Holliday (1969) also observed that before spawning, the gametes of teleosts are either isosmotic with, or hypoosmotic to, the body fluid of the parent fish. We considered the differences between our results and those of Lin (2008) to be related to the salinity under which the broodstocks were reared.
The salinity tolerance of a specific fish decreases during development, from embryo to yolk-sac larva(Wang, 2002; Shi et al., 2004). The salinity tolerance ofI.japonicusalso appears to be dependent on the developmental stage. The eggs and larvae of a specific species have different isosmotic points because of their different body compositions. It is the plasma osmolality that determines the osmotic relationships among osmoregulators, and differences in salinity tolerance among the developmental stages are related to the changes in osmotic concentrations (Wang et al.,2012b). Based on the regression equation for the survival rate of open-mouthed larvae based on salinity, we determined that a salinity range from 24.4 to 29.8 was suitable for the larviculturing of this fish.Lin (2008), however, reported a slightly lower salinity range of 23–27 for this same species. This may result from the different incubation temperatures used in Lin’s experiment, 21–23°C, and that of the present study, 21°C. The combined effects of salinity and temperature on the performance of embryonic and larval development have been demonstrated in other fish, such asSparussarba(Mihelakakis and Kitajima,1994) andRhombosoleatapirina(Hart and Purser,1995), and the larval tolerance to a higher salinity may be restricted under warmer temperatures(Kamler, 2002; Overton et al., 2008). However,further studies are needed to clarify the combined effects of salinity and temperature on development and the exact effect of temperature on salinity tolerance in the embryos and larvae ofI.japonicus.
Decreasing survival rates in an increasingly saline environment, as shown forI.japonicuseggs and yolk-sac larvae, is a characteristic of marine pelagic fish (Wang et al., 2002; Shi et al., 2004, 2008; Fielder et al., 2005; Okamoto et al., 2009; Bodinier et al.,2010), and it this may be caused by the increasing maintenance requirements for osmoregulation at higher salinity levels. A relatively low salinity level is an approximation of the osmotic concentration in marine fish bodies (Ostrowski et al., 2011), and fish regulate their plasma ions to produce an internal osmotic balance in their body fluids that allows for a greater survival rate (Brett, 1979). Under intolerably high or low salinity levels, larval physiological functions, such as those of gills, are significantly affected (Fielder et al., 2007; Zhuang et al., 2012) and the malformation rate is increased (Okamoto et al.,2009; Cai et al., 2010).
Additionally, salinity influences egg size and larval growth performance. Shi et al. (2008) recorded a significant variation in the egg size ofPampus punctatissimusafter putting the eggs into water having different salinity levels for 30 min. TheP.punctatissimusegg size significantly decreased as the salinity level increased. The larval growth rate is affected by salinity in many marine fish, includingC.harengus(Holliday and Blaxter, 1960),P.major(Wang, 2002),PercafluviatilisL. (Overton et al.,2008) andO.fasciatus(Shi et al., 2009). The present study did not measure egg size at each salinity level,but the morphology of the yolk-sac larvae support the previously reported observations that the full lengths and yolk volumes are significantly decreased under high salinity treatments. The reduction in full lengths and the depletion of yolk volume recorded at salinity levels greater than the body fluid’s osmo-concentration are ascribed to dehydration, as indicated by the eggs and yolk volumes observed in the hatched fry of the mentioned species. Bœuf and Payan (2001)hypothesised that larvae need to consume more energy to maintain their osmotic balance under hyperor hypo-osmotic conditions, thus reducing the energy used for growth. However, the energy cost of osmoregulation is lower in an isosmotic medium,where the gradient between body fluid and water is minimal, and this saved energy is substantial enough to facilitate better growth. To summarise, to optimise hatching and larval growth, the environmental salinity forI.japonicusembryos should be between 29 and 33, and the incubation salinity for yolk-sac larvae should be adjusted to a lower level of 25–29.
4.2 Biochemical and energetic dynamics
Yolk reserves exclusively provide fish with nutritional and energetic substrates during embryogenesis and later stages. It appears that the timing and sequence of nutrient use during the early development of fish is species specific. For marine fish that do not contain oil globules, such asGadus morhua(Finn et al., 1995a) andHippoglossus hippoglossus(Finn et al., 1995b), the eggs support 70% of their energy dissipation by catabolising yolk protein and amino acids, while lipids are consumed to a lesser extent and can even be synthesised (Rønnestad et al., 1992). In the present study, protein was largely consumed during the embryogenesis ofI.japonicusunder all treatment conditions, and the lipids were relatively conserved. Rønnestad et al. (1992)demonstrated that carbohydrates are not primary catabolic substrates in marine eggs and larvae,although they may provide energy from the onset of embryogenesis until the gastrula stage. InI.japonicus,the carbohydrates appeared to be synthesised during the embryonic period rather than the fertilisation stage, which may be related to their use during and after embryogenesis. Ohkubo et al. (2008) observed in developingAnguillajaponicathat body protein was greatly consumed during the intensive growth stages of the larvae. The open-mouthed larvae ofI.japonicusalso had increased protein contents under all of the treatment conditions. These protein content levels were equal to, or even higher than, the level observed in the fertilised egg. In contrast, the total lipid content sharply decreased throughout the yolksac larval stages. Thus, protein was the primary catabolic substrate for the embryogenesis ofI.japonicus, and, thereafter, in the larval stages, lipids composed more of the energy resources.
The utilisation of nutritional substrates in developing fish is not only related to the ontogenetic stages, it is also affected by environmental factors.Changes in the nutrient composition of fish bodies have become an important area of interest in the field of marine ecosystem dynamics in recent years (Wang et al., 2012a). The consumption of the yolk in nutritional stages by marine organisms is closely related to salinity, which plays roles in the biochemical and physiological composition of organisms mainly by changing their energy budget for regulating the osmotic balance (Bœuf and Payan, 2001). Most information regarding the osmoregulatory physiology of teleost fish is based on juvenile fish (Laiz-Carrión et al., 2005; Saoud et al., 2007; Hu et al., 2008; Xu et al., 2008; You et al., 2009; Liu et al., 2010; Tian et al.,2010; Yu et al., 2011a, b). Previous studies reported that different fish species have their own isosmotic points, thereby preserving various capabilities for regulating the ion concentration within their environment. In general, fish appear to consume more energy for metabolism when the environmental salinity level is much higher or lower than their isotonic point (Morgan, 1998; Bœuf and Payan,2001). As mentioned previously, the adaptation of larvae to salinity aff ects the variation in the morphometric features, such as full lengths and yolk volumes. Correspondingly, the biochemical composition of fish also reflects the salinity level’s effect on the use of nutritional substances. A study onOdontobutispotamophilaillustrated that increases in salinity induce a more rapid decline in the dry weights of juvenile fish, as well as greater decreases in protein and lipid contents (Hu et al., 2008). In the present work, the dry weights and protein contents of the newly hatched larvae ofI.japonicuswere at their lowest values at salinity levels of 33 and 37,respectively. The hydrolysis of protein to free amino acid may be accelerated to meet the greater energy requirement in the hypertonic environment (Wang et al., 2012b). In addition,I.japonicusembryos that were incubated at lower (21) or higher (33 and 37)salinity levels had lower lipid and carbohydrate contents than when they were incubated at moderate salinity levels (25 and 29) (Fig.2). The open-mouthed larvae incubated at a salinity level of 25 maintained the significantly greatest nutrient content compared with larvae incubated at the other salinity levels.Additionally, the larvae that were incubated at 21 had greater protein and carbohydrate contents than the larvae incubated at a salinity levels between 29 and 37. Thus, the larvae consumed less yolk reserves under lower salinity conditions, which further explains the observed greater growth rate (Section 3.1).
The gross energy content in fish is also closely related to environment. You et al. (2009) reported that salinity changes the allocation of growth energy and metabolic energy inPlatichthysstellatus, and Jobling (1988) divided the standard metabolism of fish into two separated parts, one that provides the energy for tissue generation and growth, and another part that maintains homeostasis in the internal environment. The optimal salinity level for rearing a species is that at which the fish can obtain the most effective energy allocation model (Wang et al.,2012a). If the conditions are far from the optimum for growth, fish appear to consume more energy to obtain a balance, and the extent of the energy consumption is related to the deviation from the optimum. Morgan (1998) studied the effects lowsalinity stress on juvenileCoryphaenahippurusand reported a reduction in oxygen uptake and gill Na+-K+-ATPase activity at low salinity levels. In the same study, the transfer ofOreochromismossambicusfrom fresh water to sea water increased the levels of plasma growth hormone, gill Na+-K+-ATPase activity and oxygen uptake (Morgan, 1998). Additionally,gill Na+, K+-ATPase activity, plasma chloride and 15‰ osmolality show decreasing trends that correspond to greater growth and food conversion efficiency levels in turbot under high salinity levels (Imsland et al., 2001). Shifting the salinity from 10 to 15 and from 20 to 25 increased the oxygen uptake in juvenileSiganuscanaliculatus(Zhang et al., 2009). Numerous studies have demonstrated that salinity stress significantly increases the metabolic levels and energy consumption levels in both freshwater and marine fish. Most studies concerning the energy budget and its relationship to growth under different environmental conditions are based on juvenile fish because it is hard to evaluate the energy budget during the larval stage owing to methodologyrelated difficulties and unclear mechanisms. Protein,lipids and carbohydrates are the substrates for biological energy (Henken et al., 1986). Using a calculation of total theoretical energy value that was based on these main nutritional substrates, we compared the effects of salinity on the energy content in developingI.japonicus. This provides basic information for studying the energy budget of this species. In the present study, the total energy content in newly hatched larvae and open-mouthed larvae declined significantly when the environmental salinity level surpassed 29 (Fig.3). Thus, the larvae ofI.japonicuswere more stressed under high salinity conditions. There is a mixed feeding period during the transition from endogenous nutrition to exogenous feeding, which occurs shortly after the first feeding(Kamler, 2002). The excessive consumption of endogenous energy negatively aff ects post-larval growth and survival. Thus, considering the salinity tolerance of larvae from a total energy content perspective is of vital importance. Further studies should focus on the physiological and biochemical statuses of fish to clarify the quantitative relationships among the components of the energy budget in response to ecological factors. The present study provides useful information for optimising larviculture incubation conditions.
5 CONCLUSION
ForI.japonicusculturing, a salinity range of 29–37 is suitable for embryonic incubation, but the optimal range is 29–33 when metabolic substrate efficiency levels are taken into consideration. Salinity levels greater than 29 appear unsuitable for yolk-sac larvae because of the increased mortality rate and energy cost. It is critical to incubate the yolk-sac larvae ofI.japonicusat a salinity range of 25–29 to ensure a successful larviculture.
6 ACKNOWLEDGMENT
The authors thank Mr. CHEN Qingkai for his assistance during larviculturing.
Alderdice D F, Hourston A S. 1985. Factors influencing development and survival of Pacific herring (Clupea harenguspallasi) eggs and larvae to beginning of exogenous feeding.Can.J.Fish.Aquat.Sci.,42(S1): 56-68.
Alderdice D F, Rao T R, Rosenthal H. 1979. Osmotic responses of eggs and larvae of the Pacific herring to salinity and cadmium.HelgoländerWiss.Meeresunters.,32(4): 508-538.
Berlinsky D L, Taylor J C, Howell R A, Bradley T M, Smith T I J. 2004. The effects of temperature and salinity on early life stages of black sea bassCentropristisstriata.J.World Aquacult.Soc.,35(3): 335-344.
Bodinier C, Sucré E, Lecurieux-Belfond L, Blondeau-Bidet E,Charmantier G. 2010. Ontogeny of osmoregulation and salinity tolerance in the gilthead sea breamSparusaurata.Comp.Biochem.Physiol.AMol.Integr.Physiol.,157(3):220-228.
Bœuf G, Payan P. 2001. How should salinity influence fish growth?Comp.Biochem.Physiol.CToxicol.Pharmacol.,130(4):411-423.
Bradford M M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding.Anal.Biochem.,72(1-2): 248-253.
Brett J R. 1979. Environmental factors and growth.In: Hoar W S, Randall D J eds. Fish Physiology. Academic Press,New York, USA. p.599-675.
Cai W C, Ou Y J, Li J E. 2010. effects of water salinity on embryonic development of rock breamOplegnathus fasciatus.ChineseJ.Ecol.,29(5): 951-956. (in Chinese with English abstract)
Cetta C M, Capuzzo J M. 1982. Physiological and biochemical aspects of embryonic and larval development of the winter flounderPseudopleuronectesamericanus.Mar.Biol.,71(3): 327-337.
Conides A J, Glamuzina B. 2001. Study on the early larval development and growth of the red porgy, “Pagrus pagrus” with emphasis on the mass mortalities observed during this phase.SciMar., 65(3): 193-200.
Davenport J, Lønning S, Kjørsvik E. 1981. Osmotic and structural changes during early development of eggs and larvae of the cod,GadusmorhuaL.J.FishBiol.,19(3):317-331.
DuBois M, Gilles K A, Hamilton J K, Rebers P A, Smith F.1956. Colorimetric method for determination of sugars and related substances.Anal.Chem.,28(3): 350-356.
Fashina-Bombata H A, Busari A N. 2003. Influence of salinity on the developmental stages of African catfishHeterobranchuslongifilis(Valenciennes, 1840).Aquaculture,224(1-4): 213-222.
Fielder D S, Allan G L, Pepperall D, Pankhurst P M. 2007. The effects of changes in salinity on osmoregulation and chloride cell morphology of juvenile Australian snapper,Pagrusauratus.Aquaculture,272(1-4): 656-666.
Fielder D S, Bardsley W J, Allan G L, Pankhurst P M. 2005.The effects of salinity and temperature on growth and survival of Australian snapper,Pagrusauratuslarvae.Aquaculture,250(1-2), 201-214.
Finn R N, Fyhn H J, Evjen M S. 1995a. Physiological energetics of developing embryos and yolk-sac larvae of Atlantic cod (Gadusmorhua). I. Respiration and nitrogen metabolism.Mar.Biol.,124(3): 355-369.
Finn R N, Rønnestad I, Fyhn H J. 1995b. Respiration, nitrogen and energy metabolism of developing yolk-sac larvae of Atlantic halibut (HippoglossushippoglossusL.).Comp.Biochem.Physiol.APhysiol.,111(4): 647-671.
Folch J, Lees M, Stanley G H S. 1957. A simple method for the isolation and purification of total lipides from animal tissues.J.Biol.Chem.,226(1): 497-509.
Hart P R, Purser G J. 1995. effects of salinity and temperature on eggs and yolk sac larvae of the greenback flounder(RhombosoleatapirinaGünther, 1862).Aquaculture,136(3-4): 221-230.
Henken A M, Lucas H, Tijssen P A T, Machiels M A M. 1986.A comparison between methods used to determine the energy content of feed, fish and faeces samples.Aquaculture,58(3-4): 195-201.
Holliday F G T, Blaxter J H S. 1960. The effects of salinity on the developing eggs and larvae of the herring.J.Mar.Biol.Assoc. U.K.,39(3): 591-603.
Holliday F G T. 1969. The effects of salinity on the eggs and larvae of teleosts.In: Hoar W S, Randall D J eds. Fish Physiology. Academic Press, New York, USA. p.293-311.
Hu X C, Zhao Y L, Zhou Z L. 2008. effect of salinity on the biochemical composition and energy budget in starved juvenile sleeperOdontobutispotamophila(Günther).Fisheries Sci.27(3): 109-113.
Huang X X, Feng L F, Wen W, Chen Q K, Wei L K. 2013. The changes in lipid and fatty acid profiles of devil stingerInimicusjaponicasduring the development of embryo and yolk-sac larvae.J.Fish.China,37(4): 526-535. (in Chinese with English abstract)
Imsland A K, Foss A, Gunnarsson S, Berntssen M H.G,Fitzgerald R, Bonga S W. Ham E V, Nævdal G, Stefansson S O. 2001. The interaction of temperature and salinity on growth and food conversion in juvenile turbot(Scophthalmusmaximus).Aquaculture,198(3): 353-367.
Jobling M. 1988. A review of the physiological and nutritional energetics of cod,GadusmorhuaL., with particular reference to growth under farmed conditions.Aquaculture,70(1-2): 1-19.
Kamler E. 2002. Ontogeny of yolk-feeding fish: an ecological perspective.Rev.FishBiol.Fish.,12(1): 79-103.
Kjörsvik E, Davenport J, Lönning S. 1984. Osmotic changes during the development of eggs and larvae of the lumpsucker,CydopteruslumpusL.J.FishBiol.,24(3):311-321.
Labonne M, Morize E, Scolan P, Lae R, Dabas E, Bohn M.2009. Impact of salinity on early life history traits of three estuarine fish species in Senegal.Estuar.Coast.ShelfSci.,82(4): 673-681.
Laiz-Carrión R, Sangiao-Alvarellos S, Guzmán J M, Martín del Río M P, Soengas J L, Mancera J M. 2005. Growth performance of gilthead sea breamSparusauratain different osmotic conditions: implications for osmoregulation and energy metabolism.Aquaculture,250(3-4): 849-861.
Lin X J. 2008. The effects of salinity on development of fertilizer eggs and larvae ofInimicusjaponicus.J.Fujian Fish., (4): 24-26. (in Chinese with English abstract)
Liu W, Zhi B J, Zhan P R, Guan H H, Qin D L. 2010. effects of salinity on haematological biochemistrical indices and liver tissue in juvenileOncorhynchusketa.ChineseJ.Appl.Ecol.,21(9): 2 411-2 417. (in Chinese with English abstract)
Liu Z Y, Quan H F. 2005. Research on the technique for artificial breeding ofInimicusjaponicus.J.Shanghai Fish.Univ.,14(1): 30-34. (in Chinese with English abstract)
Mihelakakis A, Kitajima C. 1994. effects of salinity and temperature on incubation period, hatching rate, and morphogenesis of the silver sea bream,Sparussarba(Forskål, 1775).Aquaculture,126(3-4): 361-371.
Morgan J D. 1998. Energetic aspects of osmoregulation in fish.Diss.Abst.Int.Pt.B-Sci.Eng.,59: 486B.
Ohkubo N, Sawaguchi S, Nomura K, Tanaka H, Matsubara T.2008. Utilization of free amino acids, yolk protein and lipids in developing eggs and yolk-sac larvae of Japanese eelAnguillajaponica.Aquaculture,282(1-4): 130-137.
Okamoto T, Kurokawa T, Gen K, Murashita K, Nomura K,Kim S K, Matsubara H, Ohta H, Tanaka H. 2009. Influence of salinity on morphological deformities in cultured larvae of Japanese eel,Anguillajaponica, at completion of yolk resorption.Aquaculture,293(1-2):113-118.
Ostrowski A D, Watanabe W O, Montgomery F P, Rezek T C,Shafer T H, Morris Jr J A. 2011. effects of salinity and temperature on the growth, survival, whole body osmolality, and expression of Na+/K+ATPase mRNA in red porgy (Pagruspagrus) larvae.Aquaculture,314(1-4):193-201.
Overton J L, Bayley M, Paulsen H, Wang T. 2008. Salinity tolerance of cultured Eurasian perch,PercafluviatilisL.:effects on growth and on survival as a function of temperature.Aquaculture,277(3-4): 282-286.
Rønnestad I, Fyhn H J, Gravningen K. 1992. The importance of free amino acids to the energy metabolism of eggs and larvae of turbot (Scophthalmusmaximus).Mar.Biol.,114(4): 517-525.
Rønnestad I, Fyhn H J. 1993. Metabolic aspects of free amino acids in developing marine fish eggs and larvae.Rev.Fish.Sci.,1(3): 239-259.
Saoud I P, Kreydiyyeh S, Chalfoun A, Fakih M. 2007. Influence of salinity on survival, growth, plasma osmolality and gill Na+-K+-ATPase activity in the rabbitfishSiganusrivulatus.J.Exp.Mar.Biol.Ecol.,348(1-2): 183-190.
Sha X S, Ruan H C, He G F. 1981. The development of the egg and larval stages of the lumpfish,Inimicusjaponicus(C.and V.).Oceanol.Limnol.Sinica,12(4): 365-373. (in Chinese with English abstract)
Shi Z H, Chen B, Peng S M, Chen C, Wang J G, Fu R B, Liu M H. 2008. The morphological change under salinity stress in development of yolk sac larvae ofEpinephelus malabaricus.Oceanol.Limnol.Sinica,39(3): 222-227.(in Chinese with English abstract)
Shi Z H, Huang X X, Fu R B, Wang H P, Luo H Z, Chen B, Liu M H, Zhang D. 2008. Salinity stress on embryos and early larval stages of the pomfretPampus punctatissimus.Aquaculture,275(1-4): 306-310.
Shi Z H, Peng S M, Yin Y Q, Luo H Z, Ni M L. 2009.Morphological changes of embryo and yolk sac larvae of barred knifejaw (Oplegnathusfascltus) under salinity stress.ChineseJ.Ecol.,28(3):471-476. (in Chinese with English abstract)
Shi Z H, Xia L J, Wang J G, Lu J X, Zhao R X, Wang H P, Xie L F. 2004. effect of salinity on embryonic development and larval growth ofDentextumifronsTemminck et Schlegel.J.Fish.China,28(5): 599-602. (in Chinese with English abstract)
Sucré E, Bossus M, Bodinier C, Boulo V, Charmantier G,Charmantier-Daures M, Cucchi P. 2013. Osmoregulatory response to low salinities in the European sea bass embryos: a multi-site approach.J.Comp.Physiol.B,183(1): 83-97.
Tian X L, Wang G D, Dong S L, Fang J H. 2010. effects of salinity and temperature on growth, osmophysiology and energy budget of tongue sole (CynoglossussemilaevisGünther).J.Fish.Sci.China,17(4): 771-782. (in Chinese with English abstract)
Varsamos S, Nebel C, Charmantier G. 2005. Ontogeny of osmoregulation in postembryonic fish: a review.Comp.Biochem.Physiol.AMol.Integr.Physiol.,141(4): 401-429.
Vetter R D, Hodson R E, Arnold C. 1983. Energy metabolism in a rapidly developing marine fish egg, the red drum(Sciaenopsocellata).Can.J.Fish.Aquat.Sci.,40(5):627-634.
Wang H S, Fang Q S, Zheng L Y. 2002. effects of salinity on hatching rates and survival activity index of the larvae ofEpinephelusakaara.J.Fish.China,26(4): 344-350. (in Chinese with English abstract)
Wang H S. 2002. effects of salinity on egg development and growth, larval and juvenile survival rate ofPagrosomus major.J.Fish.Sci.China,9(1): 33-38. (in Chinese with English abstract)
Wang S L, Zhou Y, Zhu X M. 2012a. Research advances on several factors affecting energy budget of fish.Chinese Fish.Qual.Stand.,2(4): 61-67. (in Chinese with English abstract)
Wang Y R, Li E C, Chen L Q, Wang X D, Zhang F Y, Gao L J,Long L N. 2012b. effect of acute salinity stress on soluble protein, hemocyanin, haemolymph glucose and hepatopancreas glycogen ofEriocheirsinensis.Acta Hydrobiol.Sinica,36(6): 1 056-1 062. (in Chinese with English abstract)
Wen W, Huang X X, Chen Q K, Feng L F, Wei L K. 2013.Temperature effects on early development and biochemical dynamics of a marine fish,Inimicusjaponicus.J.Exp.Mar.Biol.Ecol.,442: 22-29.
Wiegand M D. 1996. Composition, accumulation and utilization of yolk lipids in teleost fish.Rev.FishBiol.Fish.,6(3): 259-286.
Xu L W, Feng J, Guo Z X, Lin H Z, Guo G X. 2008. effect of salinity on hematology and gill Na+-K+ATPase activity of juvenile cobia,RachycentroncanadumLinnaeus.Mar.Environ.Sci.,27(6): 602-606. (in Chinese with English abstract)
Xu Y J, Liu X Z, Wang Y Y, Qu J Z. 2009. effects of temperature and salinity on embryonic development and starving tolerance of newly hatched larvae of rock breamOplegnathusfasciatus.Prog.Fish.Sci.,30(3): 25-31. (in Chinese with English abstract)
Yan J Q, Huang X X, Ma S J, Huang Z Z, Lü W Q. 2011.Culture condition and cellular biochemical composition of two microalgaeOocystissolitariaandSelenastrumsp.ChineseJ.Ecol.,30(12): 2 761-2 766. (in Chinese with English abstract)
Yang Z, Chen Y F. 2006. Salinity tolerance of embryos of obscure puff erTakifuguobscurus.Aquaculture,253(1-4):393-397.
You H Z, Yang Z Q, Ge S G, Jiang Z Q. 2009. effect of different salinity on biochemical composition and energy budget forPlatichthysstellatusjuveniles.HebeiFisheries,185(5): 16-19. (in Chinese with English abstract).
Yu D G, Yang Y Q, Wang H Y, Xie J, Yu E M, Wang G J, Gong W B. 2011b. The effect of salinity change on physiology and biochemistry ofEpinepheluscoioides.J.Fish.China,35(5): 719-728. (in Chinese with English abstract)
Yu N, Li J E, Ou Y J, Fan C Y, Zhang J S. 2011a. effects of salinity stresses on gill Na+/K+-ATPase (NAK) activity and body moisture in juvenile grey mulletMugilcephalus.ChineseJ.Zool.,46(1): 93-99. (in Chinese with English abstract)
Yuan C Y, Cui Q M. 2004. A review: influence of salinity on development and growth of aquatic animals in aquaculture.Fish.Sci.,23(5): 41-42. (in Chinese with English abstract)
Zeng L, Lei J L, Liu B, Hong W S, Ai C X. 2014. effects of salinities on muscle amino acid and fatty acid composition of juvenile turbot(Scophthalmusmaximus). Marine Sciences.38(12): 40-47 (in Chinese with English abstract)
Zhang L Z, Yang J H, Liu J Y, Zhuang P, Zhao F, Qu L. 2009.effects of water temperature, salinity, pH, and anaesthetics on oxygen consumption rate of juvenileSiganus canaliculatus.ChineseJ.Ecol.,28(8): 1 494-1 498. (in Chinese with English abstract)
Zhuang Q Q, Zhao J L, Zhao L H, Chang J J. 2012. effects of salinity stress on the adjustment of branchial chloride cells inOreochromisniloticus.ChineseJ.Ecol.,31(10):2 619-2 624. (in Chinese with English abstract)
杂志排行
Journal of Oceanology and Limnology的其它文章
- An aftereffect of global warming on tropical Pacific decadal variability*
- Analysis of monthly variability of thermocline in the South China Sea*
- A numerical study of the South China Sea Warm Current during winter monsoon relaxation*
- Predicting the sinkage of a moving tracked mining vehicle using a new rheological formulation for soft deep-sea sediment*
- Chemical characterization of fractions of dissolved humic substances from a marginal sea—a case from the Southern Yellow Sea*
- The morphological and molecular detection for the presence of toxic Cylindrospermopsis (Nostocales, Cyanobacteria) in Beijing city, China*
