Soybean hairy roots produced in vitro by Agrobacterium rhizogenes-mediated transformation
2018-04-12LiChenYupengCaiXiujieLiuChenGuoShiSunCunxiangWuBingjunJiangTianfuHanWenshengHou
Li Chen, Yupeng Cai, Xiujie Liu, Chen Guo, Shi Sun, Cunxiang Wu,Bingjun Jiang, Tianfu Han, Wensheng Hou,*
a National Center for Transgenic Research in Plants,Institute of Crop Science,Chinese Academy of Agricultural Sciences,Beijing 100081,China
b Ministry of Agriculture Key Laboratory of Soybean Biology(Beijing),Institute of Crop Science,Chinese Academy of Agricultural Sciences,Beijing 100081,China
1.Introduction
Agrobacterium rhizogenes contains a root-inducing(Ri)plasmid that contains root locus(rol)genes in the T-DNA region,including rolA,rolB,rolC,and rolD,and is able to induce hairy roots from the wounded surface of explants after infection[1].The hairy roots can be maintained in culture or hosted in composite plants with untransformed aerial tissue.Agrobacterium rhizogenes-mediated transformation has been widely used in many plants,such as Glycine max[2],Capsicum annuum[3],Lotus corniculatus[4],Prunus[5],Pisum sativum[6],and Catharanthus roseus[7].
Soybean(Glycine max(L.)Merr.)is one of the most important crops and has high oil and protein contents.With the development of biotechnology,advances in breeding,functional research and targeted genetic modifications have become essential for studying soybean.Thus,efficient transformation systems are required to advance soybean research.At present,Agrobacterium tumefaciens-mediated transformation and biolistic methods are the methods most frequently used for soybean transformation[8,9].However,these techniques are too inefficient and labor-intensive to meet the increased demands of research[2].
Several successful soybean studies have been performed using Agrobacterium rhizogenes and taking advantage of the hairy root system.For gene function studies,overexpression of GmACSL2(long-chain acyl-CoA synthetase 2)in soybean hairy roots was observed to reduce lipid and fatty acid content,suggesting that GmACSL2 is an important enzyme that catalyzes the five fatty acids(C16:0,C18:0,C18:1,C18:2,and C18:3 fatty acids)to form acyl-coenzymes [10].Overexpression of TaNHX2(Na+/H+antiporter 2)in hairy roots improves the salinity tolerance of transgenic roots.Under salt stress,a general growth inhibition in hairy roots was observed,but hairy roots transformed with the control vector without TaNHX2 showed much less growth(on a dry-weight basis)than transgenic hairy roots overexpressing TaNHX2[2].For promoter studies,the specificity of the soybean root promoter could be used to detect expression in hairy roots.Using this method,activities,enhancers,repressors,and the core region of the promoter could be easily observed in the hairy root system[11,12].The soybean hairy root system was also used to test the expression efficiency of an RNAi vector and was successfully applied to the CRISPR/Cas9 system[13–15].
Compared with A.tumefaciens-mediated transformation of soybean,the A.rhizogenes-mediated hairy root transformation system has a high transformation efficiency and short transformation period.The procedure can be completed within one month.Hairy roots are usually non-chimeric,because they are derived from single cells and each hairy root consists of uniformly transformed cells[16].Furthermore,hairy roots can grow without exogenous hormones[17].
In this report,the process for producing soybean hairy roots is described and illustrated.Using this method,90%–99% of the infected explants of five different cultivars produced hairy roots within one month,and 30%–60%of the hairy roots induced were transformed.In addition,the formation of calluses from hairy roots can be successfully induced.An efficient in vitro hairy root system was established,it would be an efficient and rapid platform for study of soybean gene function.
2.Materials and methods
2.1.Plant materials
Five soybean cultivars(Williams 82,Jack,Zigongdongdou,Heihe 27,and Zhonghuang 30)were used for Agrobacterium rhizogenes-mediated transformation.
2.2.Plasmid construction
In addition to the desired transgenic construct,plasmids for the transformation of soybean require a linked reporter gene marker.We designed a plasmid with three reporter genes(GUS,GFP,and DsRed2)under the constitutive promoter CaMV 35S for rapidly checking for positive hairy roots.
2.3.Strain
The plasmid vector with the three reporter genes(GUS,GFP,and DsRed2)was mobilized into Agrobacterium rhizogenes K599 via electroporation for use in later soybean infection.
2.4.Preparation of culture medium
The media used in this protocol were(1)solidified YEP medium,composed of 10 g L−1tryptone,5 g L−1yeast extract,5 g L−1NaCl,and 15 g L−1agar(pH 7.0);(2)liquid YEP medium,composed of 10 g L−1tryptone,5 g L−1yeast extract,and 5 g L−1NaCl(pH 7.0);(3)MS liquid medium,composed of 4.33 g L−1MURASHIGE&SKOOG BASAL SALT MIXTURE(PhytoTechnology,M524),30 g L−1sucrose (pH 5.8)and 1 mL L−1of MURASHIGE&SKOOG VITAMIN SOLUTION(PhytoTechnology,M533);(4)germination culture medium(GCM),composed of 3.1 g L−1GAMBORGS BASAL SALT MIXTURE(PhytoTechnology,G768),20 g L−1sucrose,7 g L−1agar(pH 5.8),and 1 mL L−1of GAMBORGS VITAMIN SOLUTION(PhytoTechnology,G249);(5)co-cultivation culture medium(CCM),composed of 0.433 g L−1MURASHIGE&SKOOG BASAL SALT MIXTURE,30 g L−1sucrose,3.9 g L−1MES(Sigma,M3671),7 g L−1agar(pH 5.4),1 mL L−1of MURASHIGE&SKOOG VITAMIN SOLUTION,150 mg L−1DTT(Sigma,D5545),and 0.02 g L−1AS(Sigma,D134406);(6)washing culture medium(WCM),composed of 2.165 g L−1MURASHIGE&SKOOG BASAL SALT MIXTURE,30 g L−1sucrose(pH 5.8),1 mL L−1of MURASHIGE&SKOOG VITAMIN SOLUTION,250 mg L−1cefotaxime sodium salt(Sigma,C7039),and 250 mg L−1carbenicillin disodium salt(INALCO,1758–9317);(7)induction culture medium(ICM),composed of 2.165 g L−1MURASHIGE&SKOOG BASAL SALT MIXTURE,30 g L−1sucrose,0.6 g L−1MES,7 g L−1agar (pH 5.8),1 mL L−1of MURASHIGE&SKOOG VITAMIN SOLUTION,250 mg L−1cefotaxime sodium salt,and 250 mg L−1carbenicillin disodium salt;and(8)callus induction culture medium(CICM),composed of 2.165 g L−1MURASHIGE&SKOOG BASAL SALT MIXTURE,30 g L−1sucrose,0.6 g L−1MES,7 g L−1agar(pH 5.8),1 mL L−1of MURASHIGE&SKOOG VITAMIN SOLUTION,1 mg L−12,4-D(Sigma,D7299),0.2 mg L−16-BA(Sigma,B3408),250 mg L−1cefotaxime sodium salt,and 250 mg L−1carbenicillin disodium salt.All media were autoclaved at 121°C for 15 min.
2.5.Detection of transgenic hairy roots
According to reporter gene in the designed vector,any reporter gene can be used to detect transgenic hairy roots.GUS can be assessed by GUS staining,whereas GFP and DsRed2 can be assessed by fluorescence.Hairy roots were directly screened using a dissecting fluorescence microscope(Nikon SMZ1500),and transgenic hairy roots showed GFP or DsRed2 fluorescence labeling.Histochemical GUS assays were performed following Jefferson[18].The hairy roots were placed in GUS staining solution(50 mmol L−1sodium phosphate,pH 7.0,0.5 mmol L−1potassium ferrocyanide,0.5 mmol L−1potassium ferricyanide, 0.5 mg mL−15-bromo-4-chloro-3-indolyl-β-D-glucuronide(X-Gluc),0.1%Triton X-100,and 20%methanol)and incubated at 37°C overnight.GUS staining was observed under a Nikon SMZ1500 microscope and photographed with a Nikon DS-Fil camera.
3.Results
An overview of the protocol is shown in Fig.1.The process had five primary working phases:(1)the selected soybean seeds were sterilized with chlorine gas for 16–20 h.(2)The sterilized seeds were planted onto GCM and cultured for 4–5 days,depending on genotype.A higher temperature(30°C)will accelerate the germination process,so that a short germination time is more appropriate.During this time,the Agrobacterium strain was prepared.(3)Cotyledons with the wounded points of attachment of the cotyledon and hypocotyls were immersed in a suspension of the Agrobacterium strain for 30 min.(4)The explants were transferred to CCM and cultured for 5 days.(5)After rinsing in WCM,the explants were transferred to ICM.Hairy roots formed in 10–12 days in ICM.

Fig.1–Schematic illustration of the protocol for soybean hairy root induction.The phase column includes sterilization,germation,infection,co-cultivation,and induction.GCM,germination culture medium;CCM,co-cultivation culture medium;ICM,induction culture medium.
3.1.Sterilization of soybean seeds and germination
Healthy seeds,typically with smooth surfaces and without disease lesions,were carefully selected and placed side by side in Petri dishes.The open Petri dishes were placed in a vacuum desiccator in a fume hood(Fig.2-a,b).A 100 mL volume of 10%sodium hypochlorite was placed in a 100-mL beaker in the desiccator,after which 4 mL of 12 mol L−1HCl was slowly added and the desiccator was immediately covered with a lid and sealed with Vaseline.The seeds were exposed to the chlorine gas for 16–20 h(Fig.2-c,d).The time for seed sterilization should not exceed 20 h,as a long sterilization time will result in poor germination.After sterilization,the Petri dishes were removed.During sterilization,the germination culture medium was prepared.The sterilized seeds were cultivated in GCM,with each dish typically containing 20 seeds(Fig.2-e).The seeds were grown in a growth chamber at 28°C for 5 days(Fig.2-f).The entire cotyledons from 5-day-old seedlings were then harvested as explants.
首先,普通村民在监督机构中须占有一定比例。仿照公司模式,监督机构成员由村小组代表大会选举产生,这是为了对监督机构统一领导。但正是因为这样,监督机构很可能变得比较被动,因此需要在其中加入一定比例的普通村民,以保证监督机构的作用发挥到最大化,村民的合法权益也才会得到真正保障。
3.2.Preparation of the Agrobacterium strain for infection
During seed germination,the YEP plates and YEP liquid medium were prepared.Bacteria(from glycerol stock)were streaked onto the surface of the YEP plates containing appropriate antibiotics(Fig.3-a)and the plates were incubated at 28 °C for 2 days(Fig.3-b–d).Next,a single colony was placed into a 20 mL of fresh YEP liquid containing appropriate antibiotics,and incubated at 28°C overnight with shaking at 200 r min−1.The Agrobacterium strain used for infection can be prepared using either of the two methods described below.One method is to use the YEP liquid directly for immersion after incubation.When the OD600of the Agrobacterium culture reaches 0.6,the YEP liquid can be used directly for immersion(Fig.3-e).An alternative method is to resuspend the bacteria in MS liquid medium.When the OD600of the Agrobacterium culture reaches 1.2,the bacteria are collected by centrifugation at 5000 r min−1for 10 min and resuspended in MS liquid medium at an OD600of 0.6 for the immersion step(Fig.3-f).
3.3.Preparation of explants and infection
Five-day-old seedlings were used for infection experiments.Cotyledons with 0.5-cm hypocotyls were cut from seedlings(Fig.4-a,b).Two methods may be used to divide the pair of cotyledons into individual ones.One is to cut from cotyledon to hypocotyls(Fig.4-c)and the other is to cut from hypocotyls to cotyledon(Fig.4-d).The pair of cotyledons is divided into single ones(Fig.4-e),and the points of attachment of the cotyledon and hypocotyls are wounded with a blade(Fig.4-f)previously dipped into the A.rhizogenes K599 strain prepared earlier,such that one cotyledon has seven or eight scars(Fig.4-g).The cotyledons are then immersed in an A.rhizogenes culture and shaken at 50 r min−1at 28 °C for 30 min(Fig.4-h).During seed germination,CCM is prepared.
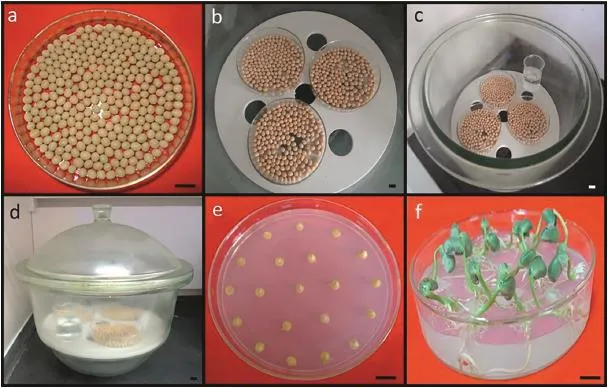
Fig.2–Sterilization of soybean seeds and germination.Healthy seeds were carefully selected and arranged side by side in Petri dishes(a),the open Petri dishes were placed in a vacuum desiccator in the fume hood(b),a 100-mL beaker was placed in the desiccator,and 100 mL of 10%sodium hypochlorite and 4 mL of 12 mol L−1HCl were added(c),the seeds were kept in the chlorine gas for 16–20 h(d),the sterilized seeds were cultivated in GCM(e),and the 5-day-old seedlings were harvested for the explants(f).GCM:germination culture medium.Scale bar,10 mm.
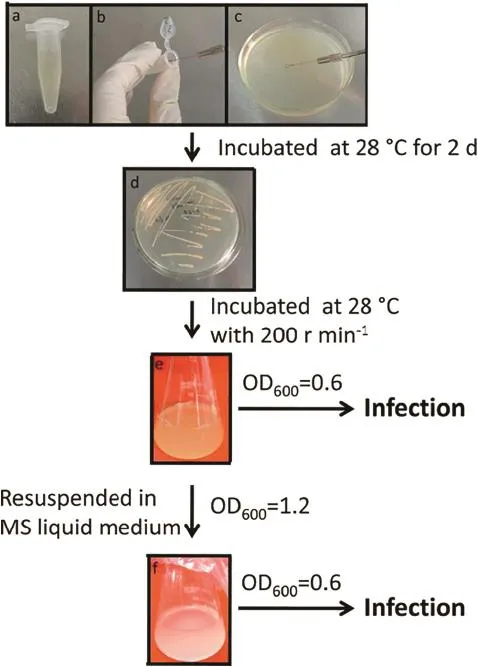
Fig.3 –Preparation of the Agrobacterium strain for infection.Bacteria stored in glycerol at−80 °C(a),inoculating loop soaked bacteria(b),streaking culture on the YEP plates containing the appropriate antibiotics(c),incubation at 28°C for 2 days(d),direct use of YEP liquid for immersion when the Agrobacterium strain reaches an OD600of 0.6(e),and resuspension of the bacterial strain in MS liquid medium at an OD600of 0.6(f).
3.4.Co-cultivation
After infection,the explants were dried on sterile filter paper and then transferred to CCM covered with sterile filter paper and incubated under 16 h light/8 h dark at 22°C(Fig.5-a).During 5 days of co-cultivation,the cotyledons expanded and the Agrobacterium adhered to the surface of the cotyledons(Fig.5-b).It can be seen that the cotyledon is markedly larger than before co-cultivation(Fig.5-c,d).If the cotyledons have not swelled after 5 days of co-cultivation,hairy roots will not emerge.This result may indicate that the Agrobacterium strain is incompetent and that the Agrobacterium will need to be reprepared for successful infection.During the co-cultivation,WCM and ICM were prepared.
3.5.Induction of hairy roots and detection
After co-cultivation,the Agrobacterium adhered to the surface of the cotyledons,so that the cotyledons needed to wash in WCM before being transferred to ICM.The cotyledons can be picked up with a triangular flask(Fig.6-a).Typically,the cotyledons were washed three times until WCM became clear(Fig.6-b),after which explants were dried on sterile filter paper.This step can prevent the Agrobacterium from regrowing during the inducing phase.The cleaned cotyledons were then transferred to ICM.The cotyledon was inserted into the culture medium at a 45°angle,with the abaxial surface downward.Each dish contained five cotyledons(Fig.6-c),and was incubated under a 16 h light/8 h dark at 28°C.The hypocotyls formed calluses during the inducing process(Fig.6-d,e).If a callus did not form,positive hairy roots were not produced.In the present study,hairy roots were produced within approximately 10–12 days and grew very quickly.Within 12–15 days,the hairy roots could spread throughout the culture medium(Fig.6-f).

Fig.4–Preparation of explants and infection.Cotyledons with long hypocotyls were cut from 5-day-old seedlings in the medium(a),cotyledons with 0.5 cm hypocotyls for explants(b),the pair of cotyledons were separated from cotyledon to hypocotyls(c),the pair of cotyledons were separated from hypocotyls to cotyledon(d),the pair of cotyledons was divided into two individual cotyledons(e),the attachment points of the cotyledon and hypocotyls were wounded with a blade(f),one cotyledon has seven or eight scars(g),and the cotyledons were immersed into an A.rhizogenes culture and shaken at 50 r min−1at 28 °C for 30 min(h).Scale bar,10 mm.

Fig.5–Explants at the co-cultivation stage.After infection,the explants were dried on sterile filter paper and transferred to CCM covered with sterile filter paper,then incubated in a 16 h light/8 h dark cycle at 22°C(a),5 days after co-cultivation,the cotyledons were expanded and the Agrobacterium adhered to the surface of the cotyledons(b),the cotyledon was co-cultivated for 1 day(c),and the cotyledon was co-cultivated for 5 days(d).CCM:co-cultivation culture medium.Scale bar,10 mm.
Typically,positive roots may be selected by GUS staining,GFP,and DsRed2(Fig.6-g–i).The positive roots can be used for analyzing the activity of reporter genes,assessing phenotypes,and/or conducting a preliminary study of gene function.
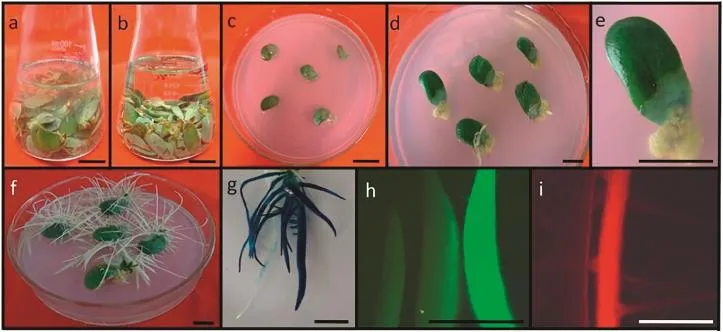
Fig.6–Induction of hairy root formation and detection.The cotyledons were washed in WCM(a),the cotyledons were washed three times until WCM became clear(b),the entire,washed cotyledons were transferred to induction culture medium,with each dish containing five cotyledons(c),the hypocotyls formed a callus in the induction process(d,e),the hairy roots were produced and spread onto the whole culture medium(f),positive roots were selected by GUS staining or GFP or DsRed2(g–i).WCM:washing culture medium.Scale bar,10 mm.
3.6.Analysis of transgenic efficiency
The seeds of five soybean cultivars were used in this protocol for transformation.All tested cultivars were able to produce hairy roots,and the proportion of hairy roots generated was between 90%–99%.Zigongdongdou had the highest generation proportion of almost 99%,followed by Jack and Williams 82 with>95%and Heihe 27 and Zhonghuang 30 with>90%(Table 1).The positive proportion of the hairy roots was between 30%–60%.The highest positive proportion forhairy roots was 58%in Zigongdongdou,and the lowest was 35%in Zhonghuang 30(Table 1).
3.7.Calluses induced by hairy roots
The positive hairy roots were used for callus induction by transfer to CICM.Hairy roots need to be transferred to new callus-inducing medium every 2 weeks,and the callus will produce for approximately 30 days.The callus can be used to test the integration of transgenes.Three reporter genes were detected in callus from positive hairy roots(Fig.7).Callus can be continuously conserved in the culture medium for reproduction and shoot induction research.
4.Discussion
The protocol described in this study is a rapid and efficient approach for inducing soybean hairy roots in vitro.The technology is more practical than other methods,owing to its high productivity and low cost.Transgenic hairy roots canbe obtained in a short period of time(approximately 3–4 weeks)and can be directly used for research.In comparison,the Agrobacterium tumefaciens-mediated transformation approach typically requires up to 3–4 months to obtain a T0plant and the T1transgenic plants needs to be harvested for research[2].

Table 1–The generation rate of hairy roots and the positive rate of hairy roots.
The hairy root system could be an excellent system for molecular studies and genetic engineering in which the production of transgenic plants is not necessary[19],such as the production of recombinant proteins and secondary metabolites[20–22],metabolic engineering and quick functional analyses of genes[23–25],rhizosphere physiology,and biochemistry[26].Recombinant protein production in the hairy root system has proved to be a rapid,low-cost,and reliable method for the production of valuable proteins.Metabolic engineering can elucidate the biosynthetic pathway for phytochemicals in hairy root cultures.Genetic studies can be performed with the hair root system,including foreign gene expression and gene function analyses.Root physiology studies ranging from nitrogen fixation,iron-deficiency,aluminum toxicity,and host-pathogen interactions can be conducted in hairy root cultures[27,28].

Fig.7 –Reporter gene detection in callus from hairy roots.GUS detection(a–d),callus from hairy roots with the GUS reporter gene(a)and control(b).(c)and(d)show GUS staining of(a)and(b),respectively;GFP detection(e–h),callus from hairy roots with the GFP reporter gene(e)and control(f).Calluses in(g)and(h)were placed under fluorescent light to detect GFP for(e)and(f),respectively;DsRed2 detection(i–l),callus from hairy roots with the DsRed2 reporter gene(i)and control(j).Calluses in(k)and(l)were placed under red fluorescent light to detect DsRed2 for(i)and(j),respectively.Scale bar,5 mm.
Kereszt et al.[29]reported a protocol for Agrobacterium rhizogenes-mediated transformation of soybean that primarily focused on the formation of composite plants by A.rhizogenes in field cultivation with hairy roots produced by hypocotyls.In our protocol,the hairy roots are generated from cotyledons and are completely grown in vitro,which can be controlled more easily than in an open condition.Thus,the experiments can be conducted in a relatively stable environment.Mohammadi-Dehcheshmeh et al.[30]have modified Kereszt's protocol by combining both in vitro and in vivo strategies for hairy root transformation,and the transformation frequency is greatly improved compared with Kereszt's protocol.Our protocol requires only preparation of media using common reagents.
The transgenic hairy roots derived from cotyledons in our protocol can be used in rootbiology and gene function studies but are notsuitable for rootnodule studies.The transgenic hairy roots derived from hypocotyls with aerial shoots represent a better system for root nodule and nitrogen fixation studies.
The transgenic hairy roots can be successfully induced to form calluses,and three reporter genes were detected in calluses induced from soybean transgenic hairy root.The callus generated using our method can support a continuous subculture for long-term maintainance of the materials.Shoots can be generated from calluses in some plants,such as Codonopsis lanceolata[31],Rehmannia glutinosa[32],and Medicago truncatula[33].Unlike for other plant species,there has been no report to date of successful plant regeneration from soybean hairy roots,although the recovery of stable soybean transgenic plants from primary-node explants infected by a disarmed A.rhizogenes strain has been reported[34].If this difficulty is solved,the hairy root system will represent a fast and efficient technology for obtaining transgenic soybean plants.
5.Conclusions
This report describes a detailed protocol for A.rhizogenes transformation of soybean.The present protocol has a high transformation efficiency.On average,90%–99% of A.rhizogenes-infected cotyledons generated hairy roots,and they were produced in all soybean cultivars tested.The positive proportion of hairy root formation,as assessed by detecting the reporter gene,reached 30%–60%.The procedure is rapid and simple.Using this method,hairy roots can be obtained within one month and could be used for gene function studies.Several independent transformation events can be obtained by this method,given that every transgenic root originates from a single cell and represents an independent transformation event.The hairy roots can be used to successfully induce calluses.This protocol may become a powerful tool for soybean genetic engineering.
This work was supported by the Major Science and Technology Projects of China(2016ZX08010-004),the Ministry of Science and Technology of China(2016YFD0100504)and the CAAS(Chinese Academy of Agriculture Sciences)Innovation Project.
[1]M.C.Christey,R.H.Braun,Production of hairy root cultures and transgenic plants by Agrobacterium rhizogenes-mediated transformation,in:Leandro Peña(Ed.),Transgenic Plants:Methods and Protocols,Methods in Molecular Biology,Vol.286,Humana Press,Totowa,New Jersey,USA 2005,pp.47–60.
[2]D.Cao,W.S.Hou,W.Liu,W.W.Yao,C.X.Wu,X.B.Liu,T.F.Han,Overexpression of TaNHX2 enhances salt tolerance of‘composite'and whole transgenic soybean plants,Plant Cell Tissue Organ Cult.107(2011)541–552.
[3]J.Aarrouf,P.Castro-Quezada,S.Mallard,B.Caromel,Y.Lizzi,V.Lefebvre,Agrobacterium rhizogenes-dependent production of transformed roots from foliar explants of pepper(Capsicum annuum):a new and efficient tool for functional analysis of genes,Plant Cell Rep.31(2012)391–401.
[4]B.Jian,W.S.Hou,C.X.Wu,B.Liu,W.Liu,S.K.Song,Y.R.Bi,T.F.Han,Agrobacterium rhizogenes-mediated transformation of Superroot-derived Lotus corniculatus plants:a valuable tool for functional genomics,BMC Plant Biol.9(2009)78.
[5]N.Bosselut,C.V.Ghelder,M.Claverie,R.Voisin,J.P.Onesto,M.N.Rosso,D.Esmenjaud,Agrobacterium rhizogenes-mediated transformation of Prunus as an alternative for gene functional analysis in hairy-roots and composite plants,Plant Cell Rep.30(2011)1313–1326.
[6]S.R.Clemow,L.Clairmont,L.H.Madsen,F.Guinel,Reproducible hairy root transformation and spot-inoculation methods to study root symbioses of pea,Plant Methods 7(2011)46.
[7]K.X.Tang,D.H.Liu,Y.L.Wang,L.J.Cui,W.W.Ren,X.F.Sun,Overexpression of transcriptional factor ORCA3 increases the accumulation of catharanthine and vindoline in Catharanthus roseus hairy roots,Russ.J.Plant Physiol.58(2011)415–422.
[8]M.A.W.Hinchee,D.V.Connor-Ward,C.A.Newell,R.E.McDonnell,S.J.Sato,C.S.Gasser,D.A.Fischhoff,D.B.Re,R.T.Fraley,R.B.Horsch,Production of transgenic soybean plants using Agrobacterium-mediated DNA transfer,Nat.Biotechnol.6(1988)915–922.
[9]D.E.MaCabe,W.F.Swain,B.J.Martinell,P.Christou,Stable transformation of soybean(Glycine max)by particle acceleration,Nat.Biotechnol.6(1988)923–926.
[10]L.Yu,X.Tan,B.Jiang,X.Sun,S.Gu,T.Han,W.Hou,A peroxisomal long-chain acyl-CoA synthetase from Glycine max involved in lipid degradation,PLoS One 9(2014),e100144..
[11]L.Chen,B.J.Jiang,C.X.Wu,S.Sun,W.S.Hou,T.F.Han,GmPRP2 promoter drives root-preferential expression in transgenic Arabidopsis and soybean hairy roots,BMC Plant Biol.14(2014)245.
[12]L.Chen,B.J.Jiang,C.X.Wu,S.Sun,W.S.Hou,T.F.Han,The characterization of GmTIP,a root-specific gene from soybean,and the expression analysis of its promoter,Plant Cell Tissue Organ Cult.121(2015)259–274.
[13]Y.P.Cai,L.Chen,X.J.Liu,S.Sun,C.X.Wu,B.J.Jiang,T.F.Han,W.S.Hou,CRISPR/Cas9-mediated genome editing in soybean hairy roots,PLoS One 10(2015),e0136064..
[14]T.B.Jacobs,P.R.LaFayette,R.J.Schmitz,W.Parrott,Targeted genome modifications in soybean with CRISPR/Cas9,BMC Biotechnol.15(2015)16.
[15]X.J.Sun,Z.Hu,R.Chen,Q.Y.Jiang,G.H.Song,H.Zhang,Y.J.Xi,Targeted mutagenesis in soybean using the CRISP-Cas9 system,Sci Rep 5(2015)10342.
[16]P.Costantino,L.Spano,M.Pomponi,E.Benvenuto,G.Ancora,The T-DNA of Agrobacterium rhizogenes is transmitted through meiosis to the progeny of hairy root plants,J.Mol.Appl.Genet.2(1984)465–470.
[17]R.Collier,B.Fuchs,N.Walter,W.K.Lutke,C.G.Taylor,Ex vitro composite plants:an inexpensive,rapid method for root biology,Plant J.43(2005)449–457.
[18]R.A.Jefferson,Assaying chimeric genes in plants:the GUS gene fusion system,Plant Mol.Biol.Rep.5(1987)387–405.
[19]V.Veena,C.G.Taylor,Agrobacterium rhizogenes:recent developments and promising applications,In Vitro Cell.Dev.Biol.Plant 43(2007)383–403.
[20]F.Bourgaud,A.Gravot,S.Milesi,E.Gontier,Production of plant secondary metabolites:a historical perspective,Plant Sci.161(2001)835–891.
[21]M.I.Georgiev,A.I.Pavlov,T.Bley,Hairy root type plant in vitro systems as sources of bioactive substances,Appl.Microbiol.Biotechnol.74(2007)1175–1185.
[22]S.Mehrotra,L.U.Rahman,A.K.Kukreja,An extensive case study of hairy-root cultures for enhanced secondary-metabolite production through metabolic-pathway engineering,Biotechnol.Appl.Biochem.56(2010)161–172.
[23]Z.B.Hu,M.Du,Hairy root and its application in plant genetic engineering,J.Integr.Plant Biol.48(2006)121–127.
[24]M.Ron,K.Kajala,G.Pauluzzi,D.Wang,M.A.Reynoso,K.Zumstein,J.Garcha,S.Winte,H.Masson,S.Inagaki,F.Federici,N.Sinha,R.B.Deal,J.Bailey-Serres,S.M.Brady,Hairy root transformation using Agrobacterium rhizogenes as a tool for exploring cell type-specific gene expression and function using tomato as a model,Plant Physiol.166(2014)455–469.
[25]X.P.Qi,M.W.Li,M.Xie,X.Liu,M.Ni,G.H.Shao,C.Song,A.K.Y.Yim,Y.Tao,F.L.Wong,S.Isobe,C.F.Wong,K.S.Wong,C.Y.Xu,C.Q.Li,Y.Wang,R.Guan,F.M.Sun,G.Y.Fan,Z.X.Xiao,F.Zhou,T.H.Phang,X.Liu,S.W.Tong,T.F.Chan,S.M.Yiu,S.Tabata,J.Wang,X.Xu,H.M.Lam,Identification of a novel salt tolerance gene in wild soybean by whole-genome sequencing,Nat.Commun.5(2014)4340.
[26]R.Rios-Estepa,B.M.Lange,Experimental and mathematical approaches to modeling plant metabolic networks,Phytochemistry 68(2007)2351–2374.
[27]N.N.Ono,L.Tian,The multiplicity of hairy root cultures:prolific possibilities,Plant Sci.180(2011)439–446.
[28]S.Runo,S.Macharia,A.Alakonya,J.Machuka,N.Sinha,J.Scholes,Striga parasitizes transgenic hairy roots of Zea mays and provides a tool for studying plant-plant interactions,Plant Methods 8(2012)20.
[29]A.Kereszt,D.X.Li,A.Indrasumunar,C.D.T.Nguyen,S.Nontachaiyapoom,M.Kinkema,P.M.Gresshoff,Agrobacterium rhizogenes-mediated transformation of soybean to study root biology,Nat.Protoc.2(2007)948–952.
[30]M.Mohammadi-Dehcheshmeh,E.Ebrahimie,S.D.Tyerman,B.N.Kaiser,A novel method based on combination of semi-in vitro and in vivo conditions in Agrobacterium rhizogenesmediated hairy root transformation of Glycine species,In Vitro Cell.Dev.Biol.Plant 50(2014)282–291.
[31]J.A.Kim,Y.S.Kim,Y.E.Choi,Triterpenoid production and phenotypic changes in hairy roots of Codonopsis lanceolata and the regenerated from them,Plant Biotechnol.Rep.5(2011)255–263.
[32]Y.Q.Zhou,H.Y.Duan,C.E.Zhou,J.J.Li,F.P.Gu,F.Wang,Z.Y.Zhang,Z.M.Gao,Hairy root induction and plant regeneration of Rehmannia glutinosa Libosch.f.hueichingensis Hsiao via Agrobacterium rhizogenes-mediated transformation,Russ.J.Plant Physiol.56(2009)224–231.
[33]C.Crane,E.Wright,R.A.Dixon,Z.Y.Wang,Transgenic Medicago truncatula plants obtained from Agrobacterium tumefaciens-transformed roots and Agrobacterium rhizogenestransformed hairy roots,Planta 223(2006)1344–1354.
[34]P.M.Olhoft,L.M.Bernal,L.B.Grist,D.S.Hill,S.L.Mankin,Y.Shen,M.Kalogerakis,H.Wiley,E.Toren,H.S.Song,H.Hillebrand,T.Jones,A novel Agrabacterium rhizogenes-mediated transformation method of soybean[Glycine max(L.)Merrill]using primary-node explants from seedlings,In Vitro Cell.Dev.Biol.Plant 43(2007)536–549.
猜你喜欢
杂志排行
The Crop Journal的其它文章
- Genetic diversity assessment of a set of introduced mung bean accessions(Vigna radiata L.)
- Near-infrared reflectance spectroscopy reveals wide variation in major components of sesame seeds from Africa and Asia
- Alternate phenotype–genotype selection for developing superior high-yielding irrigated rice lines
- F unction of the auxin-responsive gene TaSAUR75 under salt and drought stress
- Development and validation of simple sequence repeat markers from Arachis hypogaea transcript sequences
- Elevated temperature intensity, timing, and duration of exposure affect soybean internode elongation, mainstem node number, and pod number per plant
