Recent progress of amine modified sorbents for capturing CO2 from flue gas☆
2018-04-08XingleiZhaoQianCuiBaodengWangXueliangYanSurinderSinghFengZhangXingGaoYonglongLi
Xinglei Zhao ,Qian Cui ,Baodeng Wang ,Xueliang Yan ,Surinder Singh ,Feng Zhang ,Xing Gao ,Yonglong Li
1 National Institute of Clean and Low Carbon Energy(NICE),Beijing 102211,China
2 NICE America Research,Mountain View,CA,USA
Keywords:CO2 sorbent Amine Flue gas CO2 adsorption CO2 regeneration
A B S T R A C T Under the Paris agreement,China has committed to reducing CO2 emissions by 60%–65%per unit of GDP by 2030.Since CO2 emissions from coal-fired power plants currently account for over 30%of the total carbon emissions in China,it will be necessary to mitigate at least some of these emissions to achieve this goal.Studies by the International Energy Agency(IEA)indicate CCS technology has the potential to contribute 14%of global emission reductions,followed by 40%of higher energy efficiency and 35%of renewable energy,which is considered as the most promising technology to significantly reduce carbon emissions for current coal-fired power plants.Moreover,the announcement of a Chinese national carbon trading market in late 2017 signals an opportunity for the commercial deployment of CO2 capture technologies.
1.Introduction
It is commonly accepted by the international society that anthropogenic CO2emissions are leading to global climate change,notably an increase in global temperatures referred to as global warming.As the largest country in CO2emission amount,China announced its ratification of the Paris climate change agreement in 2015 and promised to cut carbon emissions by 60%–65%per unit of GDP by 2030,compared with 2005 levels[1].Meanwhile,China is also an rapidly developing country and large amount of coal will be required for maintaining its development,which indicates massive CO2emission from coal-fired power plants would continue to be inevitable.
The CO2capture and storage(CCS)technologies from flue gas are considered to be effective and main means to realize the ambitious objectives as planned by Paris climate change agreement.Various CO2capture technologies,including absorption,adsorption,cryogenics,and membranes,have been investigated[2,3].
The benchmark industrial process uses aqueous solutions containing liquid amines(e.g.,Monoethanolamine)to capture CO2from large flue gas sources,which typically have concentrations ranging from 10 vol%to 15 vol%CO2.Heating and cooling of the water solvent in the amine solution during CO2desorption result in high energy requirements for the separation process.The corrosive and volatile nature of the amines also presents problems[4].
Physical sorbent(Zeolites etc.)and pure and metal–organic framework(MOF)have been widely studied in recent years.However,amine modified solid sorbents are gradually attracting more attention because of their lesser energy requirement,higher CO2capacity,higher resistance for contaminants,and higher stability,compared to aqueous amines, zeolites and MOF [5]. Widely studied supports include polymers[6–8],silica materials[9–12],zeolites[13,14],carbons[15–19],carbon nanotubes(CNTs)[20,21]and microporous metal–organic framework(MOF)[22,23]and inorganic–organic hybrid sorbent[24].
Several reviews have been written on post-combustion CO2capture using porous sorbents including those modified by amine[25–29].However,it is a fast-developing field,and the most recent review papers already do not include the state-of-the-art research.This review focuses on more recent developments in the area of amine-modified sorbents to highlight the current issues.
2.Amine Modified CO2 Sorbents and Main Performance
Amine modified solid sorbents were separated into classes based on their preparation method[5,30–33].Class 1 sorbents were prepared by physically impregnating liquid amines within the pores of a support,referred to as wet impregnation[9,34–39].Class 2 sorbents were obtained by chemically grafting the general amines with relatively low molecule weight,hyperbranched amines or amine functional group onto the surface of the support[40–44].In comparison to Class 2 sorbents,Class 1 sorbents have attracted much more attention because they are easier to prepare,and because of their higher CO2adsorption capacity.
The typical preparation procedure of Class 1 sorbents is listed as follows:
The wet impregnation was performed in a sealed vessel under the atmospheric pressure.Specifically,a desired amount of amine was dissolved in a certain amount of solvents such as methanol,ethanol or toluene with the addition of some amount of support,and stirred in a certain period of time at room temperature.After stirring at reflux for longer time,the mixture was evaporated and subsequently dried about 373 K in the air or vacuum dried at lower temperature for some time until the solvent was fully evaporated[45–50].
The typical preparation procedure of Class 2 sorbents,such as aminemodified sorbents based on macroporous silica[51],is also introduced as follows.The preparation procedure was also shown in Fig.1.
1)Synthesis of macroporous silica support.In a typical preparation process of macroporous silica(MPS),Tetraethoxysilane(TEOS)was added to a vessel,followed by dropping Monodispersed sulfonated polystyrene latex(PS)suspension and nitric acid.The resulting mixture was sonicated and centrifuged.The PS templates were removed by calcination at higher temperature such as 600°C for a long time(here,8 h)after drying at room temperature overnight.
2)Preparation of macroporous silica-supported amine sorbents.Ncarboxyanhydride|(NCA)of L-alanine was synthesized in dry THF from triphosgene and L-alanine by following the reported procedure of Daly and Poche [52]. Amine-functionalized macroporous silica was prepared by postsynthetic grafting using 3-aminopropyltrimethoxysilane(APTMS).Typically,silica support was heated and dried under vacuum overnight at 100°C.Then dry toluene followed by APTMS was added.The resulting mixture was stirred constantly at room temperature for 24 h.Finally,the solid products were washed successively with toluene,methanol,methanol/water,and methanol,and finally dried in vacuum.The PA was synthesized using the amines on the silica surface as initiators.The resulting mixture was heated and vigorously stirred at 50°C for 48 h.The powder was then centrifuged and rinsed in THF,DMF,and chloroform.The composite was dried in a 40°C oven and stored as the amine modified sorbent.
The main amines used for sorbent modification were shown in Table 1.The main sorbents included SBA-15,silica,carbon material,Titanium oxides and MOF as shown in previous part.As shown in Table 1,single component amine was used for improving the solid sorbent,which was classified into primary,secondary,primary–secondary and secondary–tertiary component based on their structure.Organic contents ranged between 10%and 70%for the sorbents cited in this paper.Most amines have the molecule weight ranged between 80 and 270 g·mol-1besides PEI,PA and benzoxazine.The amine with the lower molecule has a lower viscosity and corresponding lower resistance,while larger amine loss was expected.Aliphatic polyamines with primary–secondary structure have been widely considered as the main amine modifying for sorbents.The amine with this structure has also been used as the main component in solvent CO2capture process for coal-fired flue gas.
The sorbent selectivity and the heat of adsorption are more important parameters for assessing the potential sorbents[55].Higher selectivity corresponds with higher CO2adsorption capacity.The adsorption capacity of sorbent modified by the amines was also shown in Table 1.When inlet gas with CO2concentration between 10%and 20%at atmospheric pressure was used, the maximum adsorption capacity reached 4.6 mmol·g-1for sorbent of silica mixing with SBA-15,followed by Tetraethylene-pentamine(TEPA)modification,which was much higher than capacity requirement(2 mmol·g-1)for practical applications in the field[56].

Fig.1.Preparation of MPS-supported amine sorbent[49].
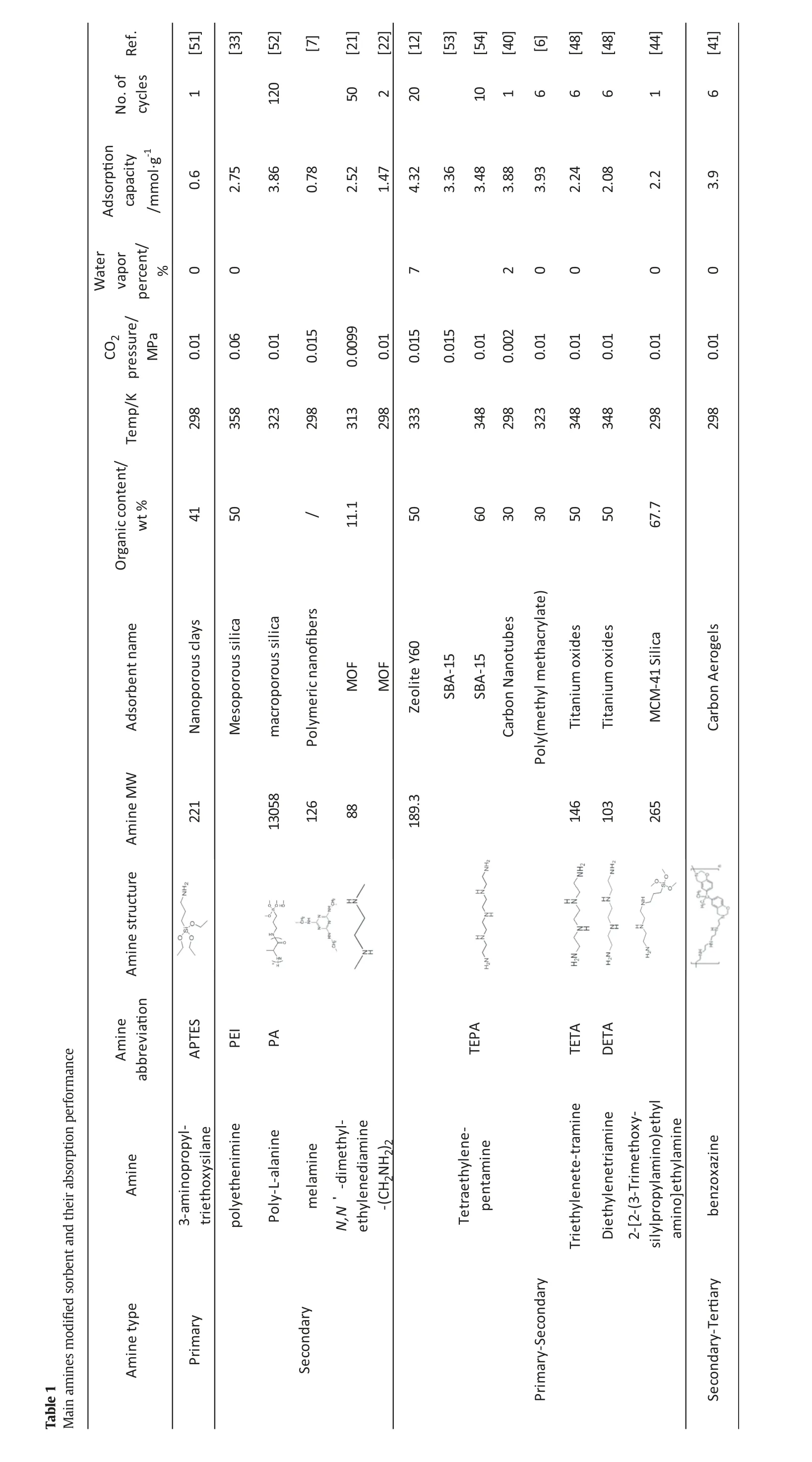
The sorbent modified by aliphatic polyamine with primary–secondary structure was recommended because of their high CO2adsorption capacity.The main reasons for that were concluded as the aliphatic polyamine had higher adsorption dynamics and capacity.The regenerated sorbent has been reused for many times and which showed a good repeatability of CO2adsorption capacity for potential commercial viability.However,it should be noted for any viable process,thousands of cycles will be needed to meet sorbent lifetimes of several years,while the cycles provided in Table 1 are orders of magnitude smaller than those which will be required in industrial practice.
Hahn et al.[57]investigated the mechanism of CO2adsorption on primary,secondary,and primary–secondary amine modified onto porous sorbents.Fig.2 showed their results.Primary amines bonded CO2preferentially through the formation of intermolecular ammonium carbamates,whereas CO2was predominantly stabilized as carbamic acid,when interacting with secondary amines.Ammonium carbamate formation required the transfer of the carbamic acid proton to a second primary amine group to form the ammonium ion and hence two(primary)amine groups were required to bind one CO2molecule.Fig.2 also indicated all reactions required the presence of water to accommodate the charged species.This means that the feed gas stream must be humid in order to prompt the adsorption.
Gibbs free energy was another main parameter to evaluate aminemodified sorbents,which represented the carbamate stability,and the enthalpy of carbamate formation.Higher reaction enthalpy means that adsorption is favored,but it also means that desorption is difficult,while higher Gibbs free energy indicates the better potential for the reverse reaction in regeneration process.The reaction enthalpy and Gibbs free energy for different primary and secondary amino groups were compared in Fig.3.Carbamate formed on modified amino group by attaching alkyl group(Nos.2–7)exhibited less stability than pristine amine(No.1).Reaction enthalpy also increased except for sec-butyl group(No.6)and isopropyl group(No.4).This trend indicated that CO2can desorb more hardly from these amines.On the other hand,polyamines modified with alkanol group(Nos.8–10)had less Gibbs energy and enthalpy,indicating that carbamate was more stabilized and thus lower regeneration energy was anticipated compared with unmodified polyamine. Carbamate with alkanol group(Nos.8–10)was stabilized through the intramolecular hydrogen bonding in this calculation.These results proposed that polyamine with tert-butyl group was the most desirable,and isopropyl and ethyl groups were also good candidates for low-temperature regeneration[58].
3.Main Characterization Method
Many characterization methods have been widely used to evaluate the amine-modified sorbent property such as Thermogravimetric analysis,Micromeritics,X-ray powder diffraction,FT-IR,and general NMR.
3.1.Thermo Gravimetric Analyzer(TGA)

Fig.2.The reaction mechanism between amine and CO2 at the surface of silica sorbent[57].
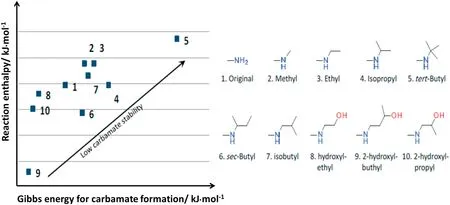
Fig.3.Screening results of functional groups[55].
Thermogravimetric analysis(TGA)was a frequently used method to perform the test of CO2adsorption and regeneration[50,54,59].The typical schematic diagram was shown in Fig.4[56].The mixing gas of CO2and N2was used as the inlet gas for discussing the adsorption performance of sorbent,while pure N2was purged for regeneration purpose.Argon(Ar)and Helium(He)were also used as the inert gases to control CO2concentration during the adsorption process and as carrier gas in the regeneration process.The change of sorbent mass was monitored in order to identify the adsorption and regeneration performance of sorbent.The change of CO2concentration in outlet gas was also a good analysis method to determine the sorbent performance in CO2adsorption and regeneration,which was realized by the online infrared CO2analyzer.
H2O vapor was introduced to the feed gas to investigate its impact on CO2adsorption and desorption performance in the sorbent.TGA was also used to consider the thermal stabilities of sorbent[47],which was realized by control the temperature range of 30–800°C of TGA with a heating rate of 5–10°C·min-1under inert gas atmosphere.The mass loss of sorbent at different temperatures indicated the thermal stabilities.
The organic content in the sample was determined by TGA by following the above procedures,followed by complete combustion of the organic material under air at the same heating rate and gas flow up to 1000°C was also used to evaluate the thermal degradation of aminemodified solid sorbents.Only the mass loss above 200°C was considered as propylamine loss[60].
3.2.Other characterization tools

Fig.4.Schematic of the adsorption–desorption experimental apparatus via(TGA)[56].
The structural properties of amine modified sorbent were determined by nitrogen adsorption at low temperature using nitrogen adsorption–desorption isotherm measurements[60].Prior to each analysis,the samples were degassed under vacuum at 150°C for 5 h.The surface area was determined by the BET method,whereas the pore size distribution was calculated using the Kruk–Jaroniec–Sayari(KJS)approach[61].The pore volume was calculated as the amount of liquid nitrogen adsorbed at P/P0=1[47].
X-ray powder diffraction(XRD)analysis was used for determining the atomic and molecular structure of a crystal within sorbents.While morphology of amine-modified sorbents was analyzed by field-emission scanning electron microscopy and transmission electron microscopy(TEM,JEOL-JEM 2100,200 kV)[47].
Many spectroscopic techniques have been used to study CO2–amine interactions,with FT-IR and general NMR being the most prominent tools[39,49,59,62–65].13C and15N magic-angle spinning(MAS)NMR has also been used to identify the interaction of CO2molecules with the grafted amine groups and the correlation of this information with the CO2adsorbed amounts,adsorption heats,and amine loadings.13C NMR resonances at ca.164 and 160 have been assigned to respectively carbamate and carbamic acid,and the stability of these species has been studied. Peak areas and the amount of13CO2adsorbed allowed the determination of the concentration of carbamate and carbamic acid[53].
Calorimetric measurements were the well-established method of experimentally measuring isosteric heats of adsorption[48,49,66–68].The typical operating procedure was shown as follows:the sample(~0.7 mg)was heated to 100°C with a 5°C·min-1heating rate in N2atmosphere.During this stage,sorbent was made free from preadsorbed moisture and CO2.Furthermore,the sample was cooled to 90°C with a-5°C·min-1cooling rate and maintained for 30 min to achieve a uniform temperature of the sample.Then,N2was interchanged with CO2with a 100 ml·min-1flow rate and maintained for 30 min.
4.Operating Sensitivity Consideration
The preferable operating condition should be identified for each amine-modified solid.Many operating conditions have been considered such as amine loading,adsorption and regeneration temperature,water vapor,structure parameters.
4.1.Amine loading
The bare sorbent has a lower CO2loading via physical adsorption,while the CO2loading has apparently increased with the increasing amine loading into the sorbent,which mainly attributes to chemical adsorption.The amine loading also has a significant impact on the transport properties of the amine sorbent.The intensity of the diffraction peak of the sorbent becomes weak after the amine modification,implying that the pore structure order is better for modified sorbent and higher adsorption performance is expected.There were reports in the literature that suggested that as the amount of amine used in the sorbent grafting solution is increased,the greater the chances of amine clustering in the hydrophobic solutions is during the surface grafting process[69–71].
Wang et al.[72]and Zhang et al.[73]considered the impact of amine loading on CO2adsorption property when CO2was adsorbed via amine modified sorbent.The highest CO2capacity with 40%–60%TEPA loading was observed.The CO2adsorption capacity showed the increasing trend with the increasing amine loading under the maximum CO2adsorption capacity.While the contrary was obtained as CO2capacity continued to increase exceeding the maximum value.The main reason for this change is that the increasing amine loading enabled more CO2molecules captured by sorbent.However,adsorption channel was blocked by amine when its loading reached some value.
4.2.Adsorption and regeneration temperature
The operating conditions for capture and regeneration can affect the working capacity of the sorbent.Temperature swing adsorption(TSA)is a better option because the pressure of coal-fired flue gas was around 0.1 MPa and pressure increasing for pressure swing adsorption(PSA)would apparently raise the energy consumption.An understanding of the role of temperature is therefore necessary to determine the optimal conditions for a particular sorbent to capture CO2in a given process.
There are literature reports of increased capture capacity with increasing temperature,an effect usually attributed to mass transport and diffusional resistance[74,75].In essence,at low temperature,the capacity is limited by the kinetics of CO2diffusing into the polymeric amines.As a result,the capacity does not reach its equilibrium value,and may appear lower than thermodynamics would predict.As the temperature is increased,diffusion rates increase,leading to an apparent increase in the capacity up to some temperature where diffusion is no longer rate limiting.However,Alesi and Kitchin[76]made the contrary conclusion when they considered the impact of adsorption temperature on CO2capture capacity.Decreasing capture capacities ranging from 1.85 to 1.15 mol CO2·(kg sorbent)-1were obtained in a packed bed reactor exposed to 10 vol%CO2in N2at adsorption temperatures ranging from 30 to 70°C.This was expected if the capacity was equilibrium limited,as the adsorption is exothermic.Lee et al.[77]compared several amine-modified mesoporous silica sorbents and found the trends of increasing or decreasing adsorption capacity with temperature increasing for different sorbents.This was explained by the interaction between kinetics and thermodynamics.Therefore,the selection of suitable adsorption temperature should be determined by balancing the kinetics and capacity.
Regeneration temperature played an important role for CO2releasing from sorbent.The higher the regeneration temperature,the leaner the sorbent.Hahn et al.[78]discussed the regeneration properties of TEPA modified SBA-15 sorbents at 50 or 100°C in N2flow.The CO2uptake on TEPA was fully reversible at 100°C,while about 50%CO2was released at 50°C.
Interaction between adsorption and regeneration temperature has been identified in order to realize an optimized operating.The regeneration temperature increases with increasing adsorption temperature because the sorbent has to be leaner in order to remove the same amount of CO2as those in the lower adsorption temperature.With varying the adsorption and regenerator temperature,the lean and rich loading will vary and thus the cyclic capacity(the difference between lean and rich loading)would also vary[68,79].
4.3.Water vapor
Previous studies have shown both an increase and decrease in CO2capacity on supported amine sorbents when comparing humid conditions to dry conditions[80–85].In studies in which the CO2capacity reduced,too much water adsorbing onto the sorbents blocked amine adsorption sites.In most FTIR spectroscopy studies,increases of the CO2capacity under humid conditions were considered to come from the formation of more carbamate species in the presence of humidity,rather than formation of bicarbonate species[59,78,86,87].The tertiary amine sorbents showed a dramatic increase in CO2capacity when water was present in the gas mixture,as water is necessary for bicarbonate formation.Bicarbonate formation on tertiary amine sorbents in the presence of humid CO2was confirmed using in situ FTIR and13C NMR spectroscopy[88].
The carbamate formed during the reaction of CO2with the(primary or secondary)amine group was also considered to further react with CO2and H2O to form a bicarbonate group.Therefore,the presence of water in the flue gas will be an assistant to the enhancement of the CO2adsorption on the amine modified mesoporous materials[13,89].Didas et al.[87]evaluated the adsorption of binary CO2/H2O onto mesoporous silica modified by primary amine under dilute conditions.Bicarbonate was observed on longer time scales of adsorption on the materials with the lowest amine loading,but only after spectral contributions from rapidly forming alkylammonium carbamate species were weakened.That was the first time to show the change of ion spectrum when CO2was adsorbed onto the sorbent in the humid conditions.Water was also thought to improve the kinetics of CO2adsorption by helping CO2diffuse through the sorbent and/or increasing aminopolymer chain mobility[13].
Fayaz and Sayari[90]investigated the long-term effect of water vapor exposure on CO2capture performance of amine-grafted silica,unlike those mentioned in above literatures at the relatively short operating duration.Increasing the duration of water vapor exposure from 3 to 24 h reduced adsorption uptake at 25°C by 56%.However,the CO2uptake reduction was much less severe at higher adsorption temperatures,reaching 21%at 50°C and only 4%at 75°C.Conducting water vapor treatment for 360 h reduced adsorption uptake at 25,50,and 75°C by 83%,61%,and 26%,respectively.For this extreme water vaporing experiment,the decrease in CO2uptake at all adsorption temperatures was attributed to the reduction of the sorbent average pore width,increasing diffusional mass transfer resistance.
4.4.Structural parameters
The structure parameters of sorbents have an important impact on CO2capture process.The main structure parameters include pore volume,pore size,and pore layout.Higher pore volume improves the amine distribution inside the pore,which prompts CO2adsorption performance.Pore size of support also plays an important role during adsorption kinetics.Increased pore size improves the sorption kinetics of sorbent.Moreover,reasonable sorbent structure improves CO2adsorption performance.Sorbents with shorter pore lengths perform much better than materials with longer channels in terms of CO2uptake and adsorption and desorption kinetics.At present,limited work has been done to understand the role of the structural parameter of support on the CO2adsorption performance[11,49,91–94].
Yan et al.[11]increased the sorption capacity from 2.51 to 4.04 mol CO2·kg-1by increasing the pore volume from 1.51 to 1.82 cm3·g-1of PEI-impregnated MCF at 75°C and 0.015 MPa.Wang et al.[91]improved the CO2sorption capacity from 2.39 mol·kg-1to 3.10 mol·kg-1by tailoring the pore size from 5.6 to 7.6 nm of mesoporous silica with constant TEPA loading.However,sorbents with shorter pore length outperformed during adsorption/desorption kinetics over longer channel materials[92].Chen et al.[93]achieved a sorption capacity of 4.18 mol CO2·kg-1at 75°C with 60 wt%PEI loading by using three dimensional hexagonal mesoporous silica(>50 nm)with wormhole-like pore structure as support.Kishor et al.[49]claimed it was advantageous to use the macroporous silica(MPS)-supported amine as the new sorbent.They explained this sorbent was less susceptible to pore blockage and plugging as the amine loading increases compared with microporous sorbent.Also,three dimensional structures with large interconnected pores showed faster sorption kinetics than those of hexagonal sorbent.
5.Main Problems and Challenges
Good progress has been made on the materials science aspects for sorbent development.However,there are a number of engineering challenges which must be addressed,which may benefit from additional materials and process advances:
1)Regeneration method and its impact on the performance of regenerated sorbent
Inert gases such as N2,Ar,He have been used as carrier gas to release CO2from rich sorbent at different temperatures.The advantage of this regeneration method is easy operation and good regeneration performance.However,it is almost impossible to be used in the real condition because the highly concentrated CO2gas was not easily obtained via this method and the large amounts of above carrier gases are not available in a coal-fired power plant.
The selection of carrier gas is a very difficult thing.A good candidate should at least have typical characters such as good availability,low cost,and easy separation with CO2.Water vapor seems to satisfy these requirements.But amine loaded on the sorbent has some solubility in water phase and the loss of amine is another big challenge as water vapor is used as carrier gas.If the water vapor becomes liquid in the adsorption process,the sorbent could be blocked and amine loss would be greater.Moreover,CO2adsorption is affected in the presence of water vapor because water vapor could occupy the CO2adsorption position.A third gas is required for the release of water vapor within sorbent in order to restore the adsorption performance for regenerated sorbents,which will increase the operating cost.
Another carrier gas was attempted for solving the above disadvantages.Ntiamoah et al.[95]used hot CO2gas as the carrier gas after indirect heating.They yielded product purities of >91%CO2and maximum recoveries of 55.5%,76.2%,and 83.6%at specific energy consumptions of 3.4,3.8,and 4.5 MJ·kg-1of CO2for regeneration temperatures of 150,200,and 250°C,respectively.This should be a possible proposal.However,this proposal has higher energy consumption compared to the standard energy consumption of 3.5 MJ·kg-1of CO2for conventional 30 wt%MEA solution.Therefore,the potentially effective carrier gas is still the main challenge for industrial adsorption process.
In considering the drawbacks for carrier gas,indirect heating for sorbent regeneration should be another choice[96].The main advantage for this method is that it can alleviate the above negative effects on adsorption process,such as product purity and operating complexity.However,higher regeneration temperature could be required in order to realize better regeneration in the absence of carrier gas,which could bring higher thermal and oxidative degradation.
2)Long-term stability under operating conditions
Most literature reports were devoted to the measurements of equilibrium or near equilibrium adsorption capacity of materials using CO2-containing gas mixtures.The thermal stability of amine-modified sorbent without CO2adsorption has also been considered.Li et al.[97]researched the thermal stability of N-[3-(Trimethoxysilyl)propyl]diethylenetriamine(DETA)modified silica materials,such as(DETA-P(precipitated silica),DETA-F(fumed silica),DETA-M(MCM-41),and DETA-S(silica gel)),through the thermogravimetric analysis.The thermogravimetric analysis result was shown in Fig.5.They divided thermograms and differential curves into four typical steps;the first was from 30 to 100°C.In this temperature range,the mass loss was mainly attributed to the release of physisorbed water.The second step was from 100 to 200°C,where the mass loss was mainly attributed to the removal of chemisorbed water.The third step is from 200 to 400°C,where the mass loss is mainly due to the degradation of the DETA links grafted onto the silica.The fourth step is at 400–800°C,and the mass loss is mainly due to the complete degradation of the diethylenetriamine links,particularly those deep into the irregular silica pores.
However,only a limited number of contributions dealt with the stability of amine-modified sorbents in the presence of NO2[98],SO2[42],O2[99–101],moisture[102,103],and CO2[102–105]on the carbon dioxide adsorptive properties.Drage et al.[104]investigated the behavior of branched polyethylenimine(PEI)-modified silica in pure CO2at different temperatures.Beyond 135°C,they observed a mass gain paralleled by a decrease in adsorption capacity.This was attributed to the formation of urea linkages.Sayari and Belmabkhout[102]discussed the effect of CO2on several amine-containing sorbents under different temperature swing conditions.They found that the deactivation was due to the formation of stable urea groups at the expense of amine and none of the materials deactivated when humidified gases were used.Sayari et al.[105]investigated the effect of dry CO2on the adsorptive properties of grafted propylamine (PA), N-methylpropylamine (MPA) and propyldiethylenetriamine(PDTA).They found that while PA deactivated readily,MPA was stable even at temperatures as high as 200°C.This finding was associated with the occurrence of isocyanate as intermediate species toward the formation of urea groups.However,the deactivation of TRI was higher than expected based on complete transformation of the primary amine groups into urea linkages.

Fig.5.TGA analysis of DETA-P,DETA-F,DETA-M,and DETA-S[97].
Heydari-Gorji et al.[103]made a multifaceted investigation on thermal,oxidative and CO2-induced degradation of branched PEIimpregnated mesoporous silica.They demonstrated that in the presence of dry CO2,the sorbent degraded through the formation of open chain and/or cyclic ureas,even under mild conditions.Abdelhamid Sayari[106]investigated the stability of a wide variety of mesoporous silicasupported amine-containing sorbents in the presence of carbon dioxide under dry and mild conditions.The main deactivation product included isocyanate,urea and the urea derivatives.The degradation mechanism was shown in Fig.6.
As shown in the above review,secondary amine in the humid adsorption condition was recommended for reducing the degradation of amine.Moreover,most literatures cited in this paper focused on the stability of amine onto the sorbent in the simulated flue gas or at higher temperature than those in the real condition.It is necessary to consider the stability of amine for long term use in the real condition of coal-fired power plant.This work has been considered for solvent-based CO2capture process and which also indicated the sorbent capture technology requires a lot of work to increase the technology readiness level for competing with solvent capture technology.
3)Process and equipment
Temperature swing adsorption(TSA),pressure swing adsorption(PSA),and vacuum swing adsorption(VSA)are three processes considered for CO2capture.In order to realize these processes more efficiently and economically,the fluidized bed,moving bed and moving bed systems have been considered for different research groups[107,108].In temperature swing adsorption,CO2is adsorbed at low temperature and desorbed by raising the temperature.The typical post-combustion CO2capture system was shown in Fig.7[109].Fluidized bed was used to continuously capture CO2in this system.

Fig.6.Proposed mechanisms for CO2-induced degradation of supported amines.

Fig.7.CO2 sorption process using a fluidized bed reactor[94].
Krutka et al.[96]considered process designs that would capture 90%CO2from flue gas in a coal-fired power plant at low CO2partial pressure through using TSA.They chose a bubbling fluidized bed reactor to be the optimal design,as this design had the most efficient heat and mass transfer,and therefore provided the optimal configuration for effective CO2capture.Hasan et al.[110]claimed that it was more cost-effective to use VSA for zeolite 13X than traditional MEA-based absorption.Chaffee et al.[111]found that the most important parameter for determining the amount of CO2captured in VSA process is the ultimate vacuum pressure,rather than the purity of the gas stream.As described in the literatures,fluidized beds,moving beds,bubbling fluidized beds and fixed beds have been considered. However, the fixed bed was recommended for post combustion capture in this study because it is much more affordable and feasible compared with other style beds.
Compared with many researches on sorbent material,the study on adsorption process and equipment should be strengthened including process design and optimization,equipment scale-up,and long-term testing and improvement.
6.Conclusions and Suggestions
The recent progress of sorbent material modified by amine,typical characterization methods,operating condition consideration and main problems and challenges were reviewed in this paper and the following conclusion and suggestions were obtained.
1)Aliphatic polyamine with primary–secondary structure and molecular weight ranged between 80 and 270 was widely considered as the main amine modifying for sorbents.The polyamine with tert-butyl group was the most desirable candidates for low-temperature regeneration.The maximum adsorption capacity is 4.6 mmol·g-1for CO2sorbent of silica mixing with SBA-15,followed by TEPA modification among all papers cited here.
2)Thermo Gravimetric Analyzer(TGA)was a conventional equipment to perform the test of CO2adsorption and regeneration.Other characterization tools such as nitrogen adsorption–desorption isotherm measurements,X-ray powder diffraction,FT-IR and general NMR were used to identify the structural properties between CO2adsorption,while Calorimetrics were useful equipment in measuring isosteric heats of adsorption.
3)Increasing amine loading had a positive effect of CO2adsorption property until the sorbent pore was blocked to some degree.The suitable adsorption temperature was needed to be determined for promoting the adsorption capacity of some sorbent.The CO2adsorption process was stimulated in the presence of water vapor in the flue gas,while the presence in the sorbent before adsorption limited the adsorption.The effective structure parameters such as larger pore size,pore volume and shorter pore length had also beneficial effect for CO2adsorption.
4)The suitable regeneration method should be a big challenge for the advancement of sorbent capture technology because current method including indirect heating,carrier gas has brought a lot of problems.The long term research of thermal and oxidative degradation of amine modified sorbents in the real flue gas condition was another big problem because simulated adsorption conditions were focused in most recent works. The design,optimization and scale-up of adsorption process were also required to pay a lot more attention as a result of the lack of enough demonstration and industrial application experiences.
Acknowledgments
This work was supported by Ministry of Science and Technology of the People's Republic of China under Project No.2017YFB0603301 through the National key research and development program.The authors thank Anthony Ku for the helpful discussions.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Ni/bentonite catalysts prepared by solution combustion method for CO2 methanation☆
- Mass transfer correlations for membrane gas-solvent contactors undergoing carbon dioxide desorption
- Carbon deposition and catalytic deactivation during CO2 reforming of CH4 over Co/MgO catalyst☆
- The CO2 absorption and desorption performance of the triethylenetetramine+N,N-diethylethanolamine+H2O system☆
- Modelling of a post-combustion carbon dioxide capture absorber using potassium carbonate solvent in Aspen Custom Modeller
- High-efficiency and pollution-controlling in-situ gasification chemical looping combustion system by using CO2 instead of steam as gasification agent
