Evaluation of cardiolipin nanodisks as lipid replacement therapy for Barth syndrome
2018-03-28NikitaIkonFongFuHsuJenniferShearerTrudyForteRobertRyan
Nikita Ikon,Fong-Fu Hsu,Jennifer Shearer,Trudy M.Forte,Robert O.Ryan,3,✉
1Children's Hospital Oakland Research Institute,Oakland,CA 94609,USA;
2Department of Medicine,School of Medicine,Washington University,St.Louis,MO 63110,USA;
3Department of Biochemistry,University of Nevada,Reno,NV 89557,USA.
Introduction
Barth Syndrome(BTHS)is a rare,life threatening X-linked recessive disorder characterized by cardiomyopathy,skeletal muscle weakness,low weight gain,neutropenia,and 3-methylglutaconic aciduria[1].The underlying cause of BTHS has been traced to mutations in the tafazzin(TAZ)gene[2-3],which encodes a phospholipid transacylase,termed tafazzin[4].Loss of tafazzin activity leads to a deficiency in cardiolipin(CL),an important phospholipid component of the mitochondrial inner membrane(IM).Additional changes include increased CL molecular species heterogeneity and accumulation of monolyso(ML)CL[5].Given the key structural role CL plays in the IM of mitochondria[6],it is not surprising that BTHS subjects manifest ultrastructural changes to this organelle[7],as well as defective energy metabolism,particularly in cardiac tissue and skeletal muscle[8-9].Normally,heart and skeletal muscle mitochondria are highly enriched in a single CL molecular species,tetralinoleoyl CL[10].Establishment and maintenance of this molecular species composition is dependent upon acyl chain remodeling reactions that involve tafazzin transacylase activity[11].When tafazzin is missing or defective,major changes in CL content and composition occur.
To date,treatment of BTHS is largely symptomatic and directed toward alleviating problems associated with cardiomyopathy,skeletal myopathy and neutropenia.However,earlier research suggests that compensating for defective tafazzin activity may be a feasible approach.For example,Valianpour et al.[12]investigated whether linoleic acid supplementation of the growth medium for cultured BTHS fibroblasts would have an effect on tetralinoleoyl CL levels.These authors reported a time and dose dependent increase in tetralinoleoyl CL following supplementation with linoleic acid,suggesting that a deficiency in tafazzin-mediated CL remodeling can be bypassed by increasing substrate availability for direct de novo synthesis of tetralinoleoyl CL.
To investigate the underlying cause of neutropenia in BTHS,Makaryan et al.[13]used HL60 myeloid progenitor cells as a model system.When these cells were transfected with a TAZ-specific short-hairpin RNA(shRNA),increased apoptosis was observed.Subsequently,in an attempt to bypass the de ficiency,TAZ knockdown(KD)HL60 cells were incubated with CL nanodisks(ND),water soluble nanoscale particles formed upon incubation of an aqueous dispersion of tetralinoleoyl CL with an amphipathic apolipoprotein[14-16].ND-mediated delivery of tetralinoleoyl CL to cultured TAZ KD HL60 cells delivered exogenous CL to mitochondria and attenuated the apoptotic response[16].
Based on these in vitro results,it was hypothesized that in vivo administration of CL-ND may affect mitochondrial CL levels.A doxycycline(dox)-inducible taz shRNA KD mouse has been reported that recapitulates the BTHS phenotype.These mice exhibit an abnormal CL pro file,mitochondrial structural abnormalities,impaired weight gain and adult-onset cardiomyopathy[17-19].At eight weeks of age,Acehan et al.[17]observed a dramatic decrease in CL content in cardiac and skeletal muscle as well as a shift toward saturated CL molecular species.There was also a substantial increase in levels of MLCL,resulting in a markedly elevated MLCL/CL ratio.Pronounced defects in both cardiac and skeletal muscle were noted at eight months,including extreme morphological changes in mitochondria and severe left ventricular dysfunction.Taken together,this murine model of BTHS recapitulates phenotypic features of the human disorder.Herein,these mice were employed in experiments designed to test the hypothesis that administration of CL-ND over a 10 week period normalizes the content and composition of CL in key tissues and,thereby,confers protection against manifestation of the BTHS disease phenotype.
Materials and methods
CL-ND formulation
Tetralinoleoyl CL[(18:2)4-CL](5 mg,Avanti Polar Lipids Inc,Alabaster,AL,USA)was transferred to a glass tube and solvent evaporated under a stream of N2 gas,forming a thin film on the vessel wall.Residual solvent was removed under vacuum.The prepared lipid was dispersed in PBS(20 mmol/L sodium phosphate,150 mmol/L sodium chloride,pH 7.0)followed by the addition of 2 mg recombinant murine apolipoprotein(apo)A-I[20]in a final volume of 0.55 mL.Bath sonication of the turbid CL-apoA-I mixture induced sample clearance,an indication that CL-ND had formed[16].CL-ND preparations were sterile- filtered(0.22µm)prior to administration to mice.
Animals
C57BL/6J taz shRNA KD mice and wild type(WT)littermates were obtained from The Jackson Laboratory.WT female and hemizygous male tet-on shRNA KD mice were used for breeding.Breeding females were maintained on dox(625 mg/kg in chow)from one week before mating until pups were weaned,except during breeding(to prevent dox-related infertility in breeding males).Upon weaning,pups were placed on dox for the remainder of the study.
CL-ND toxicity testing
WT C57BL/6 mice were administered a single intraperitoneal(IP)injection of 0,30,60,90 and 150 mg/kg CL in the form of CL-ND.After 24 hours,blood was collected by submandibular vein bleed,centrifuged at 8,000 g at 4°C for 10 minutes;plasma was recovered and stored at-80°C until use.Plasma alanine transaminase(ALT)and aspartate transaminase(AST),as well as creatinine and blood urea nitrogen(BUN),were analyzed by the UC Davis Comparative Pathology Laboratory.
CL-ND injection studies
Only male mice were used in experiments and all were maintained on dox throughout the treatment period.CL-ND administration was by IP injection,starting when pups reached 4 weeks of age and continued for 10 weeks.At the conclusion of the treatment phase,mice were euthanized,tissues harvested and frozen(-80°C)until extracted.The animal protocol employed was approved by Children's Hospital Oakland Research Institute's Institutional Animal Care and Use Committee.Five groups of mice were injected as follows:Group 1:taz shRNA KD mice(n=6)administered a weekly bolus injection of 90 mg/kg CL(as ND).Group 2:taz shRNA KD mice(n=6)administered a daily injection(5 days per week)of 18 mg/kg CL(as ND).Group 3:taz shRNA KD mice(n=5)administered a weekly injection of PBS(correspond-ing to the volume of the CL-ND injection).Group 4:WT mice(n=6)administered a weekly bolus injection of 90 mg/kg CL(as ND).Group 5:WT mice(n=6)administered a weekly injection of PBS(corresponding to the volume of the CL-ND injection).Mice were weighed weekly and monitored for outward signs of health over the course of the treatment period.
Extraction of CL from tissues
Previously frozen heart,skeletal muscle,and liver tissues were homogenized using a FastPrep FP120 Cell Disruptor and Lysing Matrix M(MP Biomedicals,Santa Ana,CA,USA),according to the manufacturer's instructions.Homogenates were further disrupted by sonication and extracted as previously described[16].Tetramyristoyl CL[(14:0)4-CL]was used as internal standard(heart=10µg,muscle/liver=1µg)
Liquid chromatography/mass spectrometry(LC/MS)analysis
Negative ion electrospray ionization LC/MS analysis of lipid extracts was performed on a Thermo scientific(San Jose,CA,USA)Vantage TSQ mass spectrometer with Thermo Accela UPLC operated by Xcalibur software.Lipids were separated on a Restek 150×2.1 mm(5µm particle size)Viva C4column under established conditions.The tetramyristoyl CL internal standard(m/z 1,240,[M-H]-)elutes at 13.6 minutes while tetralinoleoyl CL(m/z 1,448,[M-H]-)elutes at 14.4 minutes.Complete details of CL analysis by LC/MS are provided elsewhere[16,21-24].
Statistical analysis
Statistical analyses were performed using the Student's t-test and data are shown as mean±SEM where P≤0.05 is considered significant.
Results
Single dose CL-ND toxicity study
To identify a safe,nontoxic dose of CL-ND,WT mice were subjected to a single injection protocol.Groups of 5 mice each were injected with 0,30,60,90 and 150 mg/kg CL as CL-ND.After 24 h,plasma was obtained and analyzed for evidence of kidney and liver toxicity(Fig.1).Plasma levels of the liver enzymes,ALT and AST,were within the normal range at all CL concentrations tested.However,at the highest dose administered(150 mg/kg CL),a trend toward increased activity for both enzymes was noted,suggesting a liver toxicity threshold.At the same time,no CL concentration-dependent differences were observed in the levels of creatinine or BUN and all values were within the normal range,consistent with normal kidney function.Based on these findings,a dose of 90 mg/kg CL,in the form of ND,was employed in subsequent studies.
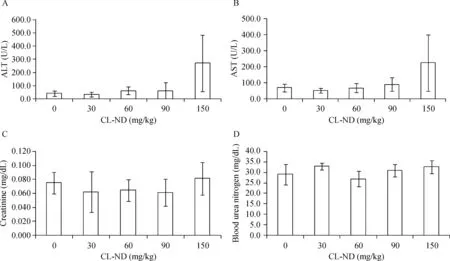
Fig.1 Effect of CL-ND administration on liver and kidney toxicity.WT C57BL/6J mice were injected with escalating doses of CL-ND.Twenty-four hours after CL-ND administration,blood was collected and plasma analyzed for liver enzyme(ALT and AST)activity.Kidney toxicity was evaluated by measuring plasma levels of creatinine,and blood urea nitrogen.Results are reported as mean±SEM(n=5).CL:cardiolipin;ND:nanodisk.
CL-ND administration to taz shRNA KD mice
Two approaches to CL-ND administration were employed.In the first,a single weekly dose of 90 mg/kg was injected IP,while the second approach employed 5 daily injections of 18 mg/kg CL-ND IP.Groups of taz shRNA KD mice received a)daily or b)weekly injections of CL-ND for 10 weeks while control taz shRNA KD mice received weekly injections of PBS.WT mice were administered either PBS or CL-ND.Over the 10-week treatment period,mice were monitored for activity and outward signs of ill health.No differences in activity between groups were detected by visual comparison,and for the first 8 weeks of the treatment phase,all mice appeared healthy.During the final 2 weeks of treatment,however,three taz shRNA KD mice in the 90 mg/kg CL-ND weekly bolus treatment group died,two at week 9 and the third at week 10.Thus,in this treatment group,only 50%of the mice survived the 10-week treatment phase.On the other hand,all mice in the daily injection group(18 mg CL/kg)survived the treatment phase and appeared healthy throughout.Based on this discrepancy,it may be concluded that daily dosing is better tolerated than a single weekly bolus injection.
From the outset,taz shRNA KD mice were smaller and weighed less than control WT C57BL/6J mice and this difference in weight persisted throughout the treatment phase of the experiment(Fig.2).On average,taz shRNA KD mice weighed 16%less than their WT littermates(P<0.0005),a result that is consistent with the findings of Cole et al.[25]Although no mortality was observed in WT C57BL/6J mice administered a 90 mg/kg weekly bolus of CL-ND,a trend toward reduced weight gain over time was observed.In the case of WT mice,the only CL-ND injection group was 90 mg/kg per week.Thus,it remains unclear if a daily injection protocol would have the same effect on weight gain in WT mice.
CL levels in key tissues
After 10 weeks of treatment,mice were sacrificed,tissues harvested, flash frozen and stored at-80°C until extraction and analysis by LC/MS.Consistent with known effects of taz KD,heart,muscle,and liver tissue of taz shRNA KD mice had a significantly lower ratio of CL/MLCL,as compared to the same tissues from WT mice(Fig.3A-C).CL-ND treatment,either as a weekly bolus injection or daily injection,failed to induce a change in the CL/MLCL ratio in tissues from WTor taz shRNA KD mice.Absolute levels of tetralinoleoyl CL followed a similar pattern(Fig.3D-E).
Discussion
ND technology has been exploited for numerous applications including:packaging transmembrane proteins in a native-like membrane environment,solubilization of hydrophobic biomolecules,and as a transport vehicle for contrast agents used in magnetic resonance imaging of atherosclerotic lesions[15].Recently,ND have been shown to be an effective means to solubilize CL[16].Negative stain electron microscopy revealed that tetralinoleoyl CL-ND are discoidal particles with a diameter in the range of 18-31 nm.Thus,stable and water-soluble CL-ND can be generated via a facile onestep formulation process.
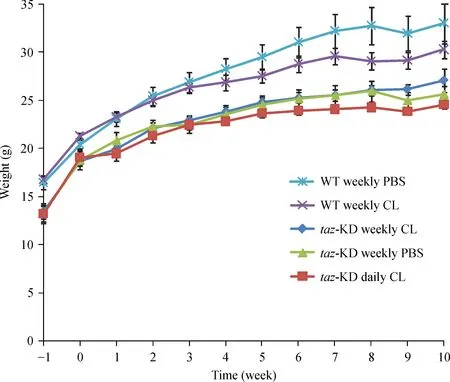
Fig.2 Effect of CL-ND administration on mouse weight gain.Mice were weighed upon arrival(week-1)and every week thereafter until the conclusion of the experiment.Results are reported as mean±SEM.CL:cardiolipin;KD:knockdown.

Fig.3 Cardiolipin analysis.At the end of the treatment period,organs were harvested from both taz shRNA KD mice(TAZ)and wild type littermates(WT),homogenized,and subject to lipid extraction.The ratio of tetralinoleoyl CL to trilinoleoyl MLCL(CL/MLCL)was quantified by LC/MS(A-C).The absolute amount of tetralinoleoyl CL was quantified relative to a tetramyristoyl CL internal standard and presented as tetralinoleoyl CL relative to protein(D-F).Data are presented as mean±SEM.CL:cardiolipin;ND:nanodisk;TAZ:taffazin.
Makaryan et al.[13]reported that shRNA-mediated KD of TAZ in HL60 myeloid progenitor cells leads to induction of apoptosis.Ikon et al.[16]con firmed this effect but also found that incubation of these cells with exogenous tetralinoleoyl CL-ND attenuates the apoptotic response.When HL60 cells were incubated with CLND harboring trace amounts of a fluorescent CL analog,it was determined that exogenous CL localizes to mitochondria.These results suggest that exogenous tetralinoleoyl CL,solubilized in ND,is taken up by HL60 cells and utilized in lieu of de novo synthesized and/or remodeled cardiolipin.The cell culture studies also suggested that CL-ND have the capability to deliver CL to cells in vivo,and thus may have therapeutic potential.
The goal of the present study was to extend these in vitro findings to an in vivo setting,with a view to CL replacement therapy as a treatment strategy for BTHS.Studies were performed using the dox-inducible taz shRNA KD mouse[17],which manifests BTHS-associated CL abnormalities within 8 weeks.Following identification of an optimal CL-ND dose,a 10 week study was conducted to assess the effect of CL-ND administration on:(1)the ratio of CL/MLCL and(2)the abundance of CL in key tissues.The results of LC/MS analysis indicate CL-ND administration does not alter the aberrant CL pro file of taz shRNA KD mice.Given the relatively high dose of CL administered(90 mg/kg per week),it is unlikely that increasing CL-ND concentration,frequency of administration,or duration of treatment will overcome the transport impediment that prevents exogenously administered CL from reaching mitochondria in vivo.These results highlight the difficulties inherent in extrapolation of results from cell culture studies to intact animals.Based on the results obtained in this mouse model of BTHS,we conclude that CL-ND administration is not a viable therapy option for treatment of this rare mitochondrial disorder.
Supported by grants from the Barth Syndrome Foundation to TF and the National Institutes of Health(R37 HL-64159)to RR.Mass spectrometry facility of Washington University is supported by NIH grants P41GM103422,P30DK020579,P30DK056341,R21HL120760.NI acknowledges receipt of a Barth Syndrome Foundation Travel Award.Parts of this work were previously presented at the 2016 Barth Syndrome Foundation Conference.
[1]Clarke SL,Bowron A,Gonzalez IL,et al.Barth syndrome[J].Orphanet J Rare Dis,2013,8:23.
[2]Bione S,D’Adamo P,Maestrini E,et al.A novel X-linked gene,G4.5.is responsible for Barth syndrome[J].Nat Genet,1996,12(4):385-389.
[3]Whited K,Baile MG,Currier P,et al.Seven functional classes of Barth syndrome mutation[J].Hum Mol Genet,2013,22(3):483-492.
[4]Xu Y,Malhotra A,Ren M,et al.The enzymatic function of tafazzin[J].J Biol Chem,2006,281(51):39217-39224.
[5]Valianpour F,Mitsakos V,Schlemmer D,et al.Monolysocardiolipins accumulate in Barth syndrome but do not lead to enhanced apoptosis[J].J Lipid Res,2005,46(6):1182-1195.
[6]Ikon,N and Ryan RO.Cardiolipin and mitochondrial cristae organization[J].BBA Biomembranes,2017,1859:1156-1163.
[7]Bissler JJ,Tsoras M,Göring HH,et al.Infantile dilated X-linked cardiomyopathy,G4.5 mutations,altered lipids,and ultrastructural malformations of mitochondria in heart,liver,and skeletal muscle[J].Lab Invest,2002,82(3):335-344.
[8]Ferri L,Donati MA,Funghini S,et al.New clinical and molecular insights on Barth syndrome[J].Orphanet J Rare Dis,2013,8:27.
[9]Ikon N,Ryan RO.Barth syndrome:connecting cardiolipin to cardiomyopathy[J].Lipids,2017,52(2):99-108.
[10]Houtkooper RH,Vaz FM.Cardiolipin,the heart of mitochondrial metabolism[J].Cell Mol Life Sci,2008,65(16):2493-2506.
[11]Schlame M,Ren M.Barth syndrome,a human disorder of cardiolipin metabolism[J].FEBS Lett,2006,580(23):5450-5455.
[12]Valianpour F,Wanders RJ,Overmars H,et al.Linoleic acid supplementation of Barth syndrome fibroblasts restores cardiolipin levels:implications for treatment[J].J Lipid Res,2003,44(3):560-566.
[13]Makaryan V,Kulik W,Vaz FM,et al.The cellular and molecular mechanisms for neutropenia in Barth syndrome[J].Eur J Haematol,2012,88(3):195-209.
[14]Ryan RO.Nanodisks:hydrophobic drug delivery vehicles[J].Expert Opin Drug Deliv,2008,5(3):343-351.
[15]Ryan RO.Nanobiotechnology applications of reconstituted high density lipoprotein[J].J Nanobiotechnology,2010,8:28.
[16]Ikon N,Su B,Hsu FF,et al.Exogenous cardiolipin localizes to mitochondria and prevents TAZ knockdown-induced apoptosis in myeloid progenitor cells[J].Biochem Biophys Res Commun,2015,464(2):580-585.
[17]Acehan D,Vaz F,Houtkooper RH,et al.Cardiac and skeletal muscle defects in a mouse model of human Barth syndrome[J].J Biol Chem,2011,286(2):899-908.
[18]Soustek MS,Falk DJ,Mah CS,et al.Characterization of a transgenic short hairpin RNA-induced murine model of Tafazzin deficiency[J].Hum Gene Ther,2011,22(7):865-871.
[19]Phoon CK,Acehan D,Schlame M,et al.Tafazzin knockdown in mice leads to a developmental cardiomyopathy with early diastolic dysfunction preceding myocardial noncompaction[J].J Am Heart Assoc,2012,1(2):e000455.
[20]Ryan RO,Forte TM,Oda MN.Optimized bacterial expression of human apolipoprotein A-I[J].Protein Expr Purif,2003,27(1):98-103.
[21]Hsu FF,Turk J,Rhoades ER,et al.Structural characterization of cardiolipin by tandem quadrupole and multiple-stage quadrupole ion-trap mass spectrometry with electrospray ionization[J].J Am Soc Mass Spectrom,2005,16(4):491-504.
[22]Hsu FF,Turk J.Characterization of cardiolipin as the sodiated ions by positive-ion electrospray ionization with multiple stage quadrupole ion-trap mass spectrometry[J].J Am Soc Mass Spectrom,2006,17(8):1146-1157.
[23]Hsu FF,Turk J.Characterization of cardiolipin from Escherichia coli by electrospray ionization with multiple stage quadrupole ion-trap mass spectrometric analysis of[M-2H+Na]-ions[J].J Am Soc Mass Spectrom,2006,17(3):420-429.
[24]Hsu FF,Turk J.Toward total structural analysis of cardiolipins:multiple-stage linear ion-trap mass spectrometry on the[M-2H+3Li]+ions[J].J Am Soc Mass Spectrom,2010,21(11):1863-1869.
[25]Cole LK,Mejia EM,Vandel M,et al.Impaired cardiolipin biosynthesis heaptic steatosis and diet-induced obesity[J].Diabetes,2016,65(11):3289-3300.
杂志排行
THE JOURNAL OF BIOMEDICAL RESEARCH的其它文章
- Legal protection of the rights of clinical trial subjects in China
- Re-evaluating the role of epithelial-mesenchymal-transition in cancer progression
- Multifunctional quantum dots and liposome complexes in drug delivery
- Pathological significance and regulatory mechanism of lymphotoxin β receptor overexpression in T cells of patients with systemic lupus erythematosus
- A novel entry point for pedicle screw placement in the thoracic spine
- Effects of leptin on femoral fracture in rats
