Effects of a soybean milk product on feto-neonatal development in rats
2018-03-27EunSukAnDongsunParkYoungHwanBanJieunChoiDaWoomSeoYoonBokLeeMiYaeShonEhnKyoungChoiYunBaeKim
Eun Suk An,Dongsun Park,Young-Hwan Ban,Jieun Choi,Da Woom Seo,Yoon Bok Lee,Mi Yae Shon,Ehn-Kyoung Choi,✉,Yun-Bae Kim,✉
1College of Veterinary Medicine and Veterinary Medical Center,Chungbuk National University,Cheongju 28644,Republic of Korea;
2Central Research Institute,Dr.Chung's Food Co.Ltd.,Cheongju 28446,Republic of Korea;
3International Ginseng and Herb Research Institute,Geumsan 312804,Republic of Korea.
Introduction
Since phytoestrogens were found to exert bene ficial effects on the improvement of menopausal symptoms and reduction of cancer incidence[1–3],studies on flavonoids rich in beans have been carried out for a long time.The estrogenic iso flavones including genistein,daidzein,and glycitein display their effects through binding to estrogen receptors(ER)[4].
By contrast,estrogenic chemicals as endocrine disruptors are well known to cause regression and dysfunction of reproductive organs,growth failure,cancers,and so on,in ecosystem and human bodies[5–6].Although phytoestrogens are believed to be relatively safe as compared to long-lasting estrogenic chemicals and innate 17β-estradiol[7–8],over-dosage and longterm use of iso flavones can also in fluence the endocrine system[9–10].Thus,the adverse effects of iso flavones remain controversial[11].
Indeed,it was demonstrated that iso flavones adversely affect the reproductive organ development and cause bleeding in male animals[12–13].Chavarro et al.explained that ingestion of soybean foods renders the iso flavones to interrupt the activity of body hormones stimulating spermatogenesis,leading to a decrease in sperm number[14].It was also suggested that abnormal reproductive organs could be developed in male offspring of dam taking foods containing genistein during the gestational and lactational periods and that disorders could be discovered as the male pups grow[15].In another study,decreased testis weights and delayed pubertal spermatogenesis in male rats were observed by exposing to genistein during the neonatal period[16].In addition,repeated treatment of genistein to male mice during the juvenile period caused Leydig cell hyperplasia and decreased sperm counts,although the motility of sperms increased[7].
However,the effects of soybean ingestion on the male reproductive system and development of pubs remain unclear in many aspects,including the concentration of iso flavones in diet,exposure route(ingestion via meals or direct injection),adaptation of embryos,fetuses,and offsprings,exposure time and duration(during the gestational,lactational or juvenile periods),and so on[7–8].For instance,soybean milks are routinely prescribed for pre-term infants in pediatric clinics,as well as for babies as infant formula.Since soybean milks contain estrogenic ingredients(phytoestrogens)higher than mother's and cow's milks,it is necessary to check their safety for post-generational development.In the present study,we investigated the physical and sexual developments of offspring following feeding of Vegemil®,a soybean milk,from the embryonic to developmental stages.
Materials and methods
Animals
Twelve-week-old male and female Sprague-Dawley rats were purchased from Orient-Bio(Seongnam,Korea)and maintained at the constant temperature(22±1°C),relative humidity(55±10%),and photoperiod(12-hours light/dark cycle).The animals were fed standard rodent chow and puri fied water ad libitum.After the male and female rats were mated,the day when a vaginal plug was observed from females was de fined as gestational day(GD)0.All experimental procedures were approved and carried out in accordance with the Institutional Animal Care and Use Committee of the Laboratory Animal Research Center at Chungbuk National University,Korea.
Soybean milk and treatment
Vegemil®,a soybean milk,was obtained from Dr.Chung's Food Co.,Ltd.(Cheongju,Korea).The soybean milk(/g)contained 30 mg proteins,35 mg fats,40 mg carbohydrates(including 2.7µg soybean oligosaccharides and 7 mg dietary fibers),86.20µg vitamins,8.25 µg niacin,and 13.55 µg ions and electrolytes.The contents of iso flavones and their derivatives amounted to 162 ppm,in which glycoside form of iso flavones was predominant:i.e.,the ratio of aglycone(such as genistein,daidzein,and glycitein)to glycoside(including genistin,daidzin,glycitin,etc.)iso flavones was 1 to 87.More speci fically,the ratio of total iso flavones in Vegemil®was 1:84:0.3:3 for aglycones:glycosides:acetylglucosides:malonylglucosides.
The pregnant female animals were randomly divided into four groups(n=8/group).Control group animals(Group 1)were given normal puri fied water.In Group 2(GD5%),the rats were fed water containing 5%soybean milk(Vegemil®)from GD6(implantation date)to parturition date.Group 3(GD-PND5%)and Group 4(GD-PND100%)were given 5%and 100%of soybean milk from GD6 to PND56,respectively.The waters containing soybean milk were freshly prepared every day.
Developmental parameters
During the entire experimental period,bodyweights of dams and offspring were recorded.Duration of pregnancy,as well as stillbirth and survival rates of offspring,was investigated.In addition,physical and sexual development parameters,including the dates of abdominal hair growth,incisor teeth eruption,eyelid opening,testicular descent,and vaginal opening,were examined.At PND57,the offspring were sacri ficed and the weights of reproductive organs(testes,epididymides,prostates,seminal vesicles,uterus,and ovaries)were weighed.
Microscopic examination
At PND57,the animals were sacri ficed under deep anesthesia with diethyl ether,the male reproductive organs,including the testes,epididymides,prostates,and seminal vesicles,were removed.After a formal tissue processing,paraf fin-embedded sections were stained with hematoxylin-eosin and examined under a light microscope.
Sperm counts and motility
The right testis and epididymis of each rat were homogenized at 8,000 rpm for 2 minutes and sonicated at 4°C for 3 minutes to obtain homogenization-resistant sperm heads.The number of sperm heads was counted with a hemacytometer[17–18].
The left epididymis was dissected into a Petri dish containing M-199 media and incubated at 37°C for 10 minutes.After removing tissue debris,an aliquot of sperm was loaded on a slide glass and sperm motility was examined under a light microscope[17–18].Separately,an aliquot of sperm was stained with the same volume of 1%eosin,smeared on a slide glass,and examined for deformity of sperm.
Statistical analysis
The results are expressed as means±SD.Tests of signi ficance were performed using Duncan's multiplerange test after one-way analysis of variance(ANOVA),with P<0.05 as the criterion of signi ficance.
Results
In all animals in the control and soybean milktreatment groups(GD5%:5%soybean milk during gestational days,GD-PND5%:5%soybean milk during gestational and post-natal days,GD-PND100%:100%soybean milk during the gestational and post-natal days),no speci fic symptoms in dams and offspring were observed during the entire experimental period.The bodyweight gains of soybean milk-treated dams and offspring also displayed patterns similar to those of the control group(Fig.1).
No signi ficant differences in delivery indices,such as duration of pregnancy,stillbirth rate,and sex ratio in male to female neonates,were observed(Table 1).Furthermore,developmental parameters including the times of abdominal hair growth,incisor teeth eruption,eyelid opening,testicular descent,and vaginal opening in male and female offspring were not signi ficantly different between control and soybean milk-treated rats.
In the reproductive organ weight,the weights of testes and epididymides at PND57 tended to increase following soybean milk feeding:i.e.,a long-term treatment(GD-PND)of 100%soybean milk,instead of drinking water,signi ficantly increased both organs,although 5%soybean milk did not signi ficantly increase their weights(Table 2).Soybean milk did not affect the weights of the prostates and seminal vesicles in males,nor the uterus and ovaries in females.By comparison,feeding soybean milk only during GD was ineffective in increasing both the male and female reproductive organ weights.In microscopic findings,no abnormal features were observed in the testes,epididymides,prostates,and seminal vesicles of male offpring exposed to soybean milk during GD-PND periods(Fig.2).
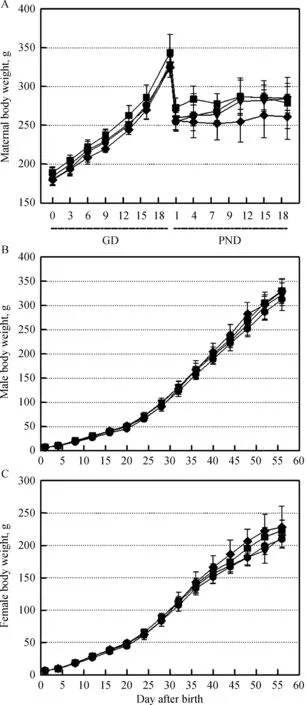
Fig.1 Changes in bodyweights of maternal rats and their offspring administered with 5%soybean milk(GD5%)from gestational day 6(GD6)to parturition or 5%(GD-PND5%)and 100%(GD-PND100%)soybean milk from GD6 to postnatal day 56(PND56),respectively.●,water control;▼,GD5%;■,GDPND5%;◆,GD-PND100%.
Notably,the sperm count signi ficantly increased(by 23%)following feeding 100%soybean milk during both the GD and PND periods(GD-PND100%,Fig.3A).In addition,sperm motility also enhanced to 76.6%in the GD-PND100%group from 54.1%in the control animals(Fig.3B).A low concentration(5%)of soybean milk also enhanced sperm motility,although no statistical signi ficance after feeding only during the GD period was reached.
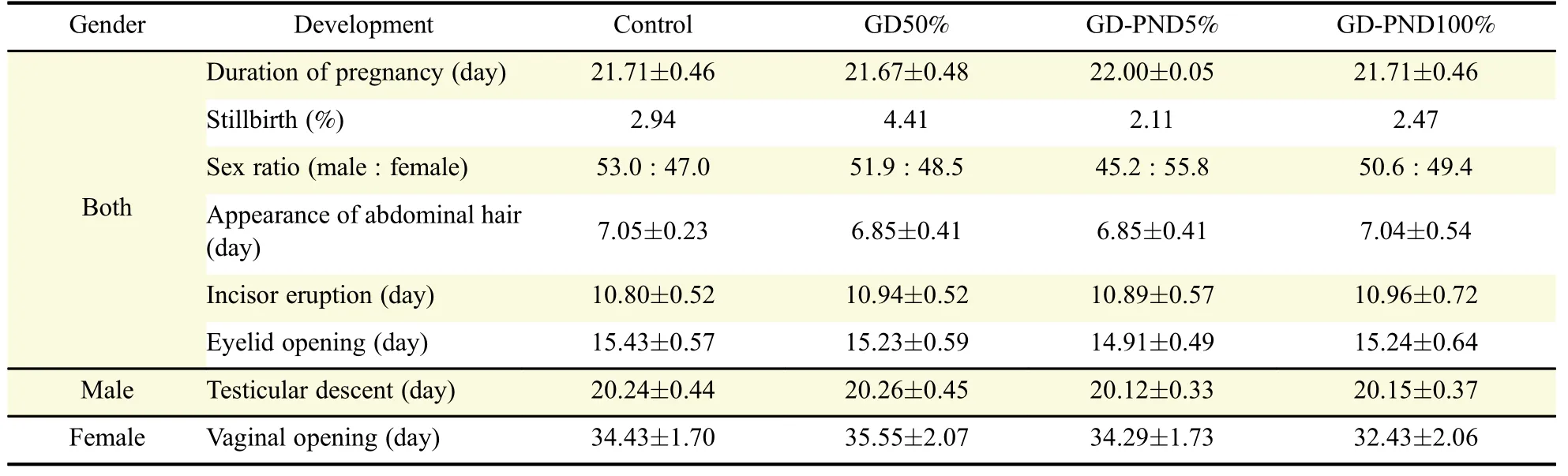
Table 1 Birth and physical development of offsprings born of maternal rats fed soybean milk
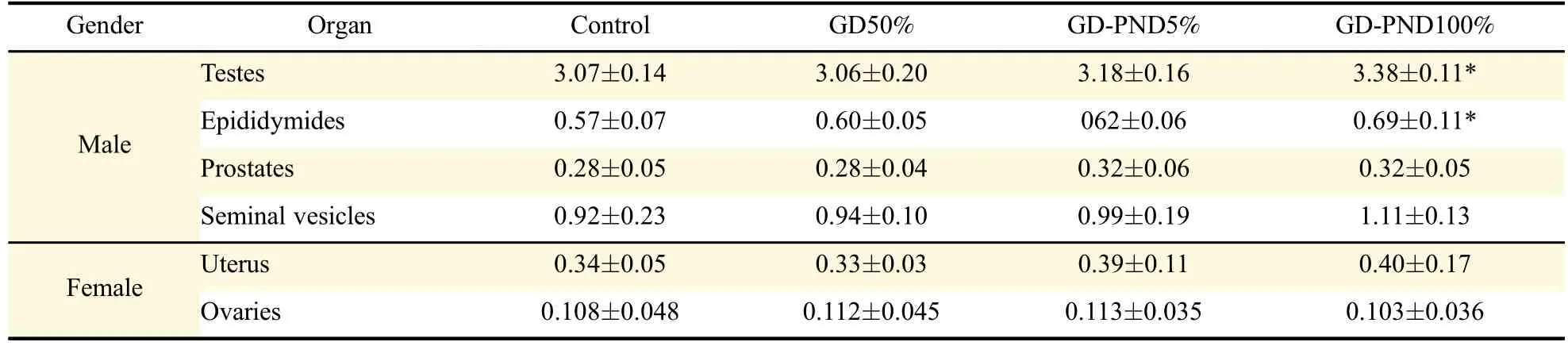
Table 2 Reproductive organ weights(g)of offsprings born of maternal rats fed soybean milk
Discussion
In the present study,ingestion of a soybean milk containing phytoestrogens during gestational and postnatal days(from GD6 to PND56)remarkably improved male reproductive function,in which weights of the testes and epididymides,as well as sperm count and motility,signi ficantly increased.Indeed,based on the 10%and 21%increases in the testes and epididymides weights,respectively,the total sperm number in the GDPND100%group increased by 26%,in addition to the increase in sperm motility.Therefore,it is of interest to note that a long-term feeding of soybean milk exerts bene ficial effects on the post-generational male reproductive function.
In spite of the bene ficial ef ficacies of soy iso flavones for menopausal symptoms[3],there have been controversial results on the endocrine system due to the estrogenic activity of aglycones including genistein,daidzein,and glycitein.However,it is clear that there are different effects of iso flavones on the male reproductive system according to the exposure time during various developmental periods.High doses of iso flavones(up to 2,000 ppm in diet)and genistein(up to 1,250 ppm in diet)did not cause any adverse effects on adult and neonatal male rats[19–20].By contrast,genistein(higher than 1,250 ppm)decreased the birthweight and delayed development of rat offspring with aberrant spermatogenesis following exposure during the GD-PND periods[21–22]and increased the developmental toxicity of phthalate[23],implying that gonadal development during the gestational period may be affected by iso flavones.Furthermore,it is widely assumed that the adverse effects of iso flavones on the spermatogenesis are prevented by blood-testis barrier which may be incomplete for permeability during the GD and neonatal periods.Indeed,it was reported that genistein and genistin were cytotoxic to cultured myogenic cells,wherein inhibition of protein synthesis and cell proliferation was observed as low as 1 μmol/L[24].It was also demonstrated that soy iso flavones altered transcription levels of important proteins in Sertoli cell culture[25],and that phytoestrogens kudzu and puerarin reduced motility and acrosome reaction of boar spermatozoa via ERβ in vitro without barrier[26].In addition,genistein inhibited steroidogenesis via ERα and thereby impaired testosterone secretion from E12.5 fetal testis culture,but not from E18.5 testis[27],suggesting that there is a susceptible period in embryo-fetal stages as well.Otherwise,glycosides including genistin,daidzin,and glycitin were major iso flavones in Vegemil®,in comparison with relativelylow contents of estrogenic aglycone iso flavones.Therefore,it is assumed that Vegemil®did not exert adverse effects found from genistein exposure.
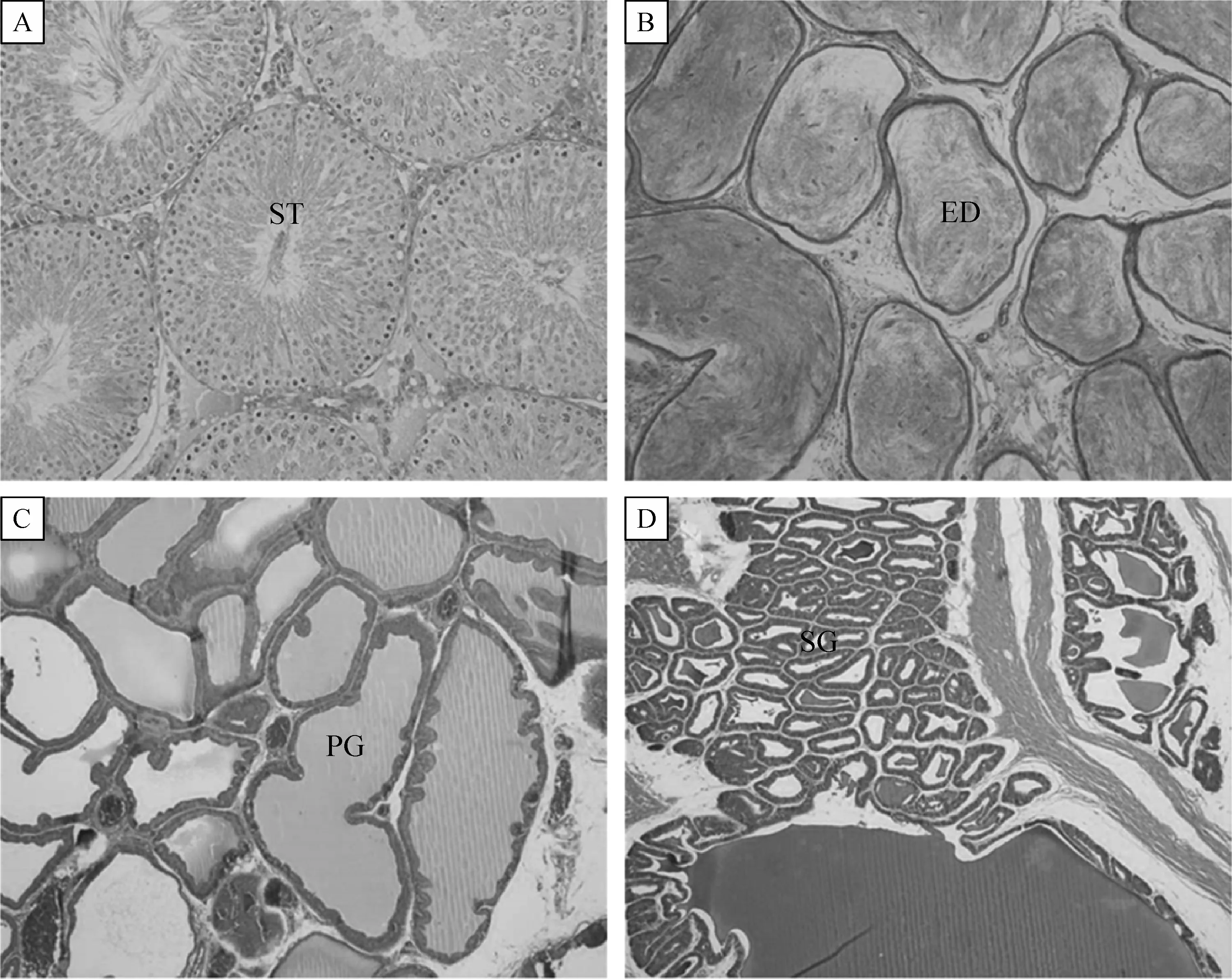
Fig.2 Microscopic findings of the reproductive organs of male offspring administered with 100%(GD-PND100%)soybean milk from gestational day 6(GD6)to postnatal day 56(PND56).A:testis(ST:seminiferous tubule),B:epididymis(ED:efferent ductule),C:prostates(PG:prostate gland),D:seminal vesicle(SG:seminal glands).
It is of interest to note that active aromatase and ER are found in immature germ cells and ejaculated spermatozoa,as well as in Leydig cells and Sertoli cells.Aromatase,a converting enzyme of testosterone to estrogen,is upregulated in motile spermatozoa,and estrogen produced locally is required for spermatogenesis,spermiogenesis,and sperm motility[28–31].In comparison with predominant expression of androgen receptors(AR)in somatic cells,ER was localized in testicular cells,indicative of the role of estrogen in the regulation of spermatogenesis(proliferation,apoptosis,survival,and maturation)and acquisition of motility[31–32].Interestingly,it was demonstrated that high concentrations(up to 1,000 ppm)of genistein did not inhibit aromatase,in contrast to a strong inhibition by diethylstilbestrol(DES),and that soy meal protected germ cells in aromatase-knockout mice displaying spermatogenic impairment[33].Actually,another phytoestrogen trans-resveratrol increased gonadotrophins and sperm counts in rats[34–35].Recently,Eumkeb et al.reported that daidzein and genistein,extracted from Butea superb Roxb.,which has been used as an aphrodisiac to improve erectile dysfunction in humans,increased testosterone level,as well as sperm number and motility in mice[36].In line with the above reports,our results also demonstrate that soybean milk containing iso flavonesenhance sperm count andmotility in rats.
Collectively,the dose-response relationship in the bene ficial andadverse effects of phytoestrogenson male reproductive function might be dependent on their accessibility to germ cells in the seminiferous tubules,although there may be species-speci fic differences[37]:i.e.,exposure to high concentrations of iso flavones during the embryo-fetal stages may affect the spermatogenic process.In mice,soy-based products(340 ppm iso flavones)administered from conception to adulthood(GD-PND)impaired spermatogenesis,resulting in reduced epididymal sperm counts and litter size of offspring[38].In addition,administration of a low dose(5 mg/kg≒62.5 ppm)of genistein to gestational and lactational mouse dams slightly altered sperm counts and motility of offspring[7].On the contrary,a high concentration(600 ppm)of soy/iso flavone-rich diet fed during the GD-PND periods enhanced testicular and prostate health in rats[39].These findings are consistent with our observations that feeding rats with soybean milk(containing 8.1-162 ppm iso flavones)increased sperm counts and motility.Actually,the critical dose of genistein for embryotoxicity was suggested to be 100 mg/kg(1,250 ppm)in rats[21–22,40].

Fig.3 Changes in sperm counts(A)and motility(B)of male offspring administered with 5%soybean milk(GD5%)from gestational day 6(GD6)to parturition or 5%(GD-PND5%)and 100%(GD-PND100%)soybean milk from GD6 to postnatal day 56(PND56),respectively.*Signi ficantly different from water control,P<0.05.
Estrogens play a signi ficant role in sperm development by affecting the transcriptional process of target genes through ERα or ERβ[30–32].In animals and humans de ficient in ER or aromatase,the number and motility of sperms decreased,leading to infertility[33,41].In the present and previous studies,it was demonstrated that feeding soybean milk during the GD-PND periods improved the male reproductive function,and that soy meal protected germ cells in mice with spermatogenic dysfunction[33].In human studies,soy-based infant formula did not lead to considerable differences in the developmental,endocrinological and reproductive outcomes in young adulthood[42–43].
The results indicate that soybean milk containing relatively-low concentrations of glycoside iso flavones is safe for dams,embryos,fetuses,and offspring,as well as improves the post-generational development of the male reproductive function.Although optimal concentrations for the bene ficial effects of phytoestrogens remain to be clari fied,it is also suggested that formulas containing phytoestrogens,such as iso flavones and trans-resveratrol,could be good strategies for the improvement of male reproductive function of infants and/or infertile men.
[1] Setchell KD.Phytoestrogens:the biochemistry,physiology,and implications for human health of soy iso flavones[J].Am J Clin Nutr,1998,68(6 Suppl):1333S–1346S.
[2] Barnes S.Phytoestrogens and breast cancer[J].Baillieres Clin Endocrinol Metab,1998,12(4):559–579.
[3] Song WO,Chun OK,Hwang I,et al.Soy iso flavones as safe functional ingredients[J].J Med Food,2007,10(4):571–580.
[4] Turner JV,Agatonovic-Kustrin S,Glass BD.Molecular aspects of phytoestrogen selective binding at estrogen receptors[J].J Pharm Sci,2007,96(8):1879–1885.
[5] Sun Y,Huang H,Sun Y,et al.Ecological risk of estrogenic endocrine disrupting chemicals in sewage plant ef fluent and reclaimed water[J].Environ Pollut,2013,180:339–344.
[6] Kavlock RJ,Daston GP,DeRosa C,et al.Research needs for the risk assessment of health and environmental effects of endocrine disruptors:a report of the U.S.EPA-sponsored workshop[J].Environ Health Perspect,1996,104(Suppl 4):715–740.
[7] Lee BJ,Jung EY,Yun YW,et al.Effects of exposure to genistein during pubertal development on the reproductive system of male mice[J].J Reprod Dev,2004,50(4):399–409.
[8] Jung EY,Lee BJ,Yun YW,et al.Effects of exposure to genistein and estradiol on reproductive development in immature male mice weaned from dams adapted to a soybased commercial diet[J].J Vet Med Sci,2004,66(11):1347–1354.
[9] Santti R,Mäkelä S,Strauss L,et al.Phytoestrogens:potential endocrine disruptors in males[J].Toxicol Ind Health,1998,14(1-2):223–237.
[10]Jefferson WN,Padilla-Banks E,Newbold RR.Disruption of the female reproductive system by the phytoestrogen genistein[J].Reprod Toxicol,2007,23(3):308–316.
[11]Cederroth CR,Nef S.Soy,phytoestrogens and metabolism:A review[J].Mol Cell Endocrinol,2009,304(1-2):30–42.
[12]Strauss L,Mäkelä S,Joshi S,et al.Genistein exerts estrogenlike effects in male mouse reproductive tract[J].Mol CellEndocrinol,1998,144(1-2):83–93.
[13]West MC.The impact of dietary oestrogens on male and female fertility[J].Curr Opin Obstet Gynecol,2007,19(3):215–221.
[14]Chavarro JE,Toth TL,Sadio SM,et al.Soy food and iso flavone intake in relation to semen quality parameters among men from an infertility clinic[J].Hum Reprod,2008,23(11):2584–2590.
[15]Wisniewski AB,Klein SL,Lakshmanan Y,et al.Exposure to genistein during gestation and lactation demasculinizes the reproductive system in rats[J].J Urol,2003,169(4):1582–1586.
[16]Atanassova N,McKinnell C,Turner KJ,et al.Comparative effects of neonatal exposure of male rats to potent and weak(environmental)estrogens on spermatogenesis at puberty and the relationship to adult testis size and fertility:evidence for stimulatory effects of low estrogen levels[J].Endocrinology,2000,141(10):3898–3907.
[17]Jeon JH,Shin S,Park D,et al.Fermentation filtrates ofRubus coreanusrelax the corpus cavernosum and increase sperm count and motility[J].J Med Food,2008,11(3):474–478.
[18]Shin S,Jeon JH,Park D,et al.trans-Resveratrol relaxes the corpus cavernosum ex vivo and enhances testosterone levels and sperm quality in vivo[J].Arch Pharm Res,2008,31(1):83–87.
[19]Faqi AS,Johnson WD,Morrissey RL,et al.Reproductive toxicity assessment of chronic dietary exposure to soy iso flavones in male rats[J].Reprod Toxicol,2004,18(4):605–611.
[20]Nagao T,Yoshimura S,Saito Y,et al.Reproductive effects in male and female rats of neonatal exposure to genistein[J].Reprod Toxicol,2001,15(4):399–411.
[21]Flynn KM,Ferguson SA,Delclos KB,et al.Effects of genistein exposure on sexually dimorphic behaviors in rats[J].Toxicol Sci,2000,55(2):311–319.
[22]Delclos KB,Bucci TJ,Lomax LG,et al.Effects of dietary genistein exposure during development on male and female CD(Sprague-Dawley)rats[J].Reprod Toxicol,2001,15(6):647–663.
[23]Zhang LD,Deng Q,Wang ZM,et al.Disruption of reproductive development in male rat offspring following gestational and lactational exposure to di-(2-ethylhexyl)phthalate and genistein[J].Biol Res,2013,46(2):139–146.
[24]Ji S,Willis GM,Frank GR,et al.Soybean iso flavones,genistein and genistin,inhibit rat myoblast proliferation,fusion and myotube protein synthesis[J].J Nutr,1999,129(7):1291–1297.
[25]Yin D,Zhu Y,Liu L,et al.[Potential detrimental effect of soy iso flavones on testis sertoli cells][J].Zhong Nan Da Xue Xue Bao Yi Xue Ban,2014,39(6):598–604.
[26]Gray SL,Lackey BR,Boone WR.Impact of kudzu and puerarin on sperm function[J].Reprod Toxicol,2015,53:54–62.
[27]Lehraiki A,Chamaillard C,Krust A,et al.Genistein impairs early testosterone production in fetal mouse testis via estrogen receptor alpha[J].Toxicol In Vitro,2011,25(8):1542–1547.
[28]Carreau S,Silandre D,Bourguiba S,et al.Estrogens and male reproduction:a new concept[J].Braz J Med Biol Res,2007,40(6):761–768.
[29]Carreau S,Hess RA.Oestrogens and spermatogenesis[J].Philos Trans R Soc Lond B Biol Sci,2010,365(1546):1517–1535.
[30]Carreau S,Bouraima-Lelong H,Delalande C.Estrogens in male germ cells[J].Spermatogenesis,2011,1(2):90–94.
[31]Carreau S,Bouraima-Lelong H,Delalande C.Estrogen,a female hormone involved in spermatogenesis[J].Adv Med Sci,2012,57(1):31–36.
[32]Carreau S,Bois C,Zanatta L,et al.Estrogen signaling in testicular cells[J].Life Sci,2011,89(15-16):584–587.
[33]Robertson KM,O’Donnell L,Simpson ER,et al.The phenotype of the aromatase knockout mouse reveals dietary phytoestrogens impact signi ficantly on testis function[J].Endocrinology,2002,143(8):2913–2921.
[34]Juan ME,González-Pons E,Munuera T,et al.trans-Resveratrol,a natural antioxidant from grapes,increases sperm output in healthy rats[J].J Nutr,2005,135(4):757–760.
[35]Turner RT,Evans GL,Zhang M,et al.Is resveratrol an estrogen agonist in growing rats[J]?Endocrinology,1999,140(1):50–54.
[36]Eumkeb G,Tanphonkrang S,Sirichaiwetchakoon K,et al.The synergy effect of daidzein and genistein isolated fromButea superbaRoxb.on the reproductive system of male mice[J].Nat Prod Res,2017,31(6):672–675.
[37]Hart JE.Endocrine pathology of estrogens:species differences[J].Pharmacol Ther,1990,47(2):203–218.
[38]Cederroth CR,Zimmermann C,Beny JL,et al.Potential detrimental effects of a phytoestrogen-rich diet on male fertility in mice[J].Mol Cell Endocrinol,2010,321(2):152–160.
[39]Blake C,Hansen T,Simmons TC,et al.Long time exposure to soy/iso flavone-rich diet enhances testicular and prostate health in Long-Evans rats[J].J Funct Foods,2013,5:1494–1501.
[40]McClain RM,Wolz E,Davidovich A,et al.Reproductive safety studies with genistein in rats[J].Food Chem Toxicol,2007,45(8):1319–1332.
[41]Korach KS.Insights from the study of animals lacking functional estrogen receptor[J].Science,1994,266(5190):1524–1527.
[42]Strom BL,Schinnar R,Ziegler EE,et al.Exposure to soy-based formula in infancy and endocrinological and reproductive outcomes in young adulthood[J].JAMA,2001,286(7):807–814.
[43]Merritt RJ,Jenks BH.Safety of soy-based infant formulas containing iso flavones:the clinical evidence[J].J Nutr,2004,134(5):1220S–1224S.
杂志排行
THE JOURNAL OF BIOMEDICAL RESEARCH的其它文章
- Interference with the C-terminal structure of MARF1 causes defective oocyte meiotic division and female infertility in mice
- Cervical spine fractures in osteopetrosis:a case report and review of the literature
- Dietary crocin reverses melanoma metastasis
- Safety of axitinib and sorafenib monotherapy for patients with renal cell carcinoma:a meta-analysis
- Sample size re-estimation without un-blinding for time-to-event outcomes in oncology clinical trials
- Oxidized phospholipids and lipoprotein-associated phospholipase A2as important determinants of Lp(a)functionality and pathophysiological role
