An Analysis on Leaf Traits of 22 Helianthus tuberosus Germplasm Resources Introduced from Abroad
2018-03-21,,,,,*
, ,, , ,*
1.Qinghai Academy of Agriculture and Forestry Sciences, Qinghai University/ Qinghai Key Laboratory of Vegetable Genetics and Physiology, Xining 810016, China; 2.College of Horticulture, Northwest A&F University, Yangling 712100, China; 3.National Engineering Research Center for Vegetables, Beijing 100097, China; 4.State Key Laboratory of Plateau Ecology and Agriculture, Qinghai University, Xining 810016, China
1 Introduction
HelianthustuberosusLinn. is a perennial herbaceous plant belonging to the Compositae family, and the tubers ofH.tuberosusLinn. are rich in synanthrin. The aboveground part has large biomass, and the stems and leaves can be used as feed. In the flourishing season ofH.tuberosusLinn., the ground stems and leaves can be cut and used as green fodder, and can be used as dry feed after grinding in autumn[1]for rabbits, pigs, sheep, cattle, horses and the like.H.tuberosusLinn. can be also used as functional nutritional food[2], and its leaves are used as the raw materials for tea[3].
In recent years, many domestic scholars have made great progress in the studies of the plant leaves. Cao Jingjingetal.[4]study the essential oil components of Michelia maudiae and its biological activity. Sun Luanzietal.[5]study the biological activity of different extracts from chicory leaves to armyworm. Zhang Haijuanetal.[6]study the extraction process of chlorogenic acid fromH.tuberosusLinn.leaves. Yue Huilanetal.[7]study the changes in the content of chlorogenic acid fromH.tuberosusLinn. leaves in Tsaidam Basin at different growth stages. Fan Junhuaetal.[8]conduct principal component analysis and cluster analysis on the physiological properties of leaves of 52H.tuberosusLinn. cultivars introduced from southern Xinjiang.
Currently, the studies onH.tuberosusLinn. germplasm resources are mainly focused on genetic diversity analysis[9-11], resistance identification[12], breeding[13]and so on, while the study ofH.tuberosusLinn. leaf traits is simple and not deep enough. The aboveground stem and leaf yield ofH.tuberosusLinn. can reach 2 t/667 m2, and the aboveground part ofH.tuberosusLinn. after harvest is idle or simply as livestock feed, and its value is far from being exploited. To fully and effectively give play to the value ofH.tuberosusLinn. leaves, it is necessary to determine the leaf component content differences betweenH.tuberosusLinn. resources of different provenances.
Qinghai Academy of Agriculture and Forestry Sciences of Qinghai University has collected and stored 350H.tuberosusLinn. germplasm resources, and is carrying out research on its genetic background and functional use. By selecting 22H.tuberosusLinn. resources introduced from abroad, this paper measured its leaf characteristics, and determined the mineral element and nutrient content.
Using principal component analysis and cluster analysis, with two domesticH.tuberosusLinn. varieties as reference, this paper analyzed the leaf traits of 22H.tuberosusLinn. germplasm resources introduced from abroad, in order to provide a scientific basis for the future collection, evaluation, classification, identification and breeding ofH.tuberosusLinn. germplasm resources, and provide theoretical support for the full use and theoretical support ofH.tuberosusLinn.
2 Materials and methods
2.1MaterialsThe 24H.tuberosusLinn. germplasm resources for test were stored inH.tuberosusLinn. Research and Development Center of Qinghai Academy of Agriculture and Forestry Sciences of Qinghai University, of which 11 were from Denmark, 11 were from France and 2 were theH.tuberosusLinn. varieties validated in China.
On August 25, 2016, the leaves were sampled, and 3 plants were select from eachH.tuberosusLinn. germplasm resource. 10 to 25 middle leaves were collected from the plants, respectively, frozen in liquid nitrogen after being mixed, and stored at -80℃ after lyophilization.
The test site is in 36°43′35.20″ N, 101°45′1.07″ E, the area is the irrigated area in the Huangshui watershed, and the soil is chestnut soil, with organic matter content of 20.28 g/kg, pH of 8.12, total nitrogen content of 1.17 g/kg, total phosphorus content of 2.18 g/kg, total potassium content of 22.5 g/kg, available nitrogen content of 0.069 g/kg, available phosphorus content of 0.065 g/kg, and available potassium content of 229.0 mg/kg.
2.2IndexdeterminationBased on the grading standards of reference[14], the shape and size ofH.tuberosusLinn. leaves were graded, and the water content of leaves was determined using the freeze-drying method.
The content of mineral elements was determined using atomic absorption spectroscopy[15]. The pre-treated lyophilized tuber samples ofH.tuberosusLinn. were precisely weighed, and put into clean dried flask according to the sample preparation procedures, and 2.5 ml of nitric acid-perchloric acid solution was added followed by digestion on the hot plate.
After the digesting liquid was removed and cooled, the volume was set to 100 mL with distilled water and it was shaken for test. It was determined using AA-800 atomic absorption spectrophotometer (PE Corporation, USA) and 6400A flame photometer (The Third Analytical Instrument Factory in Shanghai).
The chlorogenic acid and flavone content was determined using UV spectrophotometry; the cellulose was determined using the sulfuric acid and potassium dichromate oxidation method[16-17]. Each sample was repeated 3 times and averaged.
2.3DataanalysisExcel and DPS7.05 were employed for statistical analysis of data.
3 Results and analysis
3.1LeafcharacteristicsofH.tuberosusLinnFrom Table 1, it was found that in the 24H.tuberosusLinn. germplasm resources, Qingyu 4 had the highest water content in leaves, reaching 30.05%, while F16 had the lowest water content in leaves, only 19.02%; there was little difference in the average leaf water content ofH.tuberosusLinn. germplasm resources from Denmark and France, less than that of twoH.tuberosusLinn. cultivars from China, and F16 could be a candidate for breeding ofH.tuberosusLinn. cultivars resistant to drought.
The leaf length was 16.10-23.60 cm, the leaf width is 9.48-17.22 cm, and the ratio of leaf length to leaf width was 1.24-1.88. In accordance with the length-width ratio and relevant standards[14], the leaf shape of 24H.tuberosusLinn. germplasm resources was divided into three types: orbicular; oval; long oval. There were 4 orbicular resources, accounting for 16.7% of total germplasm resources; there were 19 oval resources, accounting for 79.2% of total germplasm resources; there was 1 long oval resource, accounting for 4.2% of total germplasm resources (Fig. 1).

Fig.1Classificationofleafshape
Table1Leafcharacteristicsof24Helianthustuberosusgermplasmresources

GermplasmresourcecodeSourcesWatercontent∥%Length∥cmWidth∥cmLength⁃widthratioLeafarea∥cm2LeafshapeD1Denmark21.7620.5016.531.24339.84±20.64OrbicularD3Denmark20.8822.6713.101.73297.67±84.74OvalD4Denmark22.6520.1013.221.52267.12±9.69OvalD5Denmark23.1220.5715.241.35315.37±52.13OrbicularD7Denmark20.4918.3312.641.45232.67±24.83OvalD8Denmark21.4419.4713.241.47259.36±33.12OvalD10Denmark26.7018.3311.681.57214.00±15.10OvalD11Denmark25.6817.839.481.88171.65±24.25LongovalD12Denmark26.3619.0011.381.67216.00±33.06OvalD13Denmark23.3716.1010.061.60163.21±16.95OvalD14Denmark27.0417.4011.681.49206.15±30.76OvalF6France24.4521.5714.771.46325.58±59.19OvalF7France23.6421.3315.021.42320.00±34.64Oval
(To be continued)
(Continued)

GermplasmresourcecodeSourcesWatercontent∥%Length∥cmWidth∥cmLength⁃widthratioLeafarea∥cm2LeafshapeF8France21.4218.0012.081.49217.99±15.88OvalF9France28.1422.6014.391.57325.81±32.29OvalF10France25.5022.0314.691.50325.14±49.74OvalF12France27.5821.7014.761.47322.54±35.84OvalF14France19.6319.6014.311.37282.20±60.19OrbicularF16France19.0221.7715.441.41336.68±11.19OvalF17France21.6919.5711.931.64234.12±16.22OvalF19France26.1220.0012.051.66234.07±55.19OvalF20France21.7623.6015.731.50373.23±43.15OvalQingyu3China29.7121.5213.121.64282.34±45.77OvalQingyu4China30.0523.4217.221.36403.29±31.90Orbicular
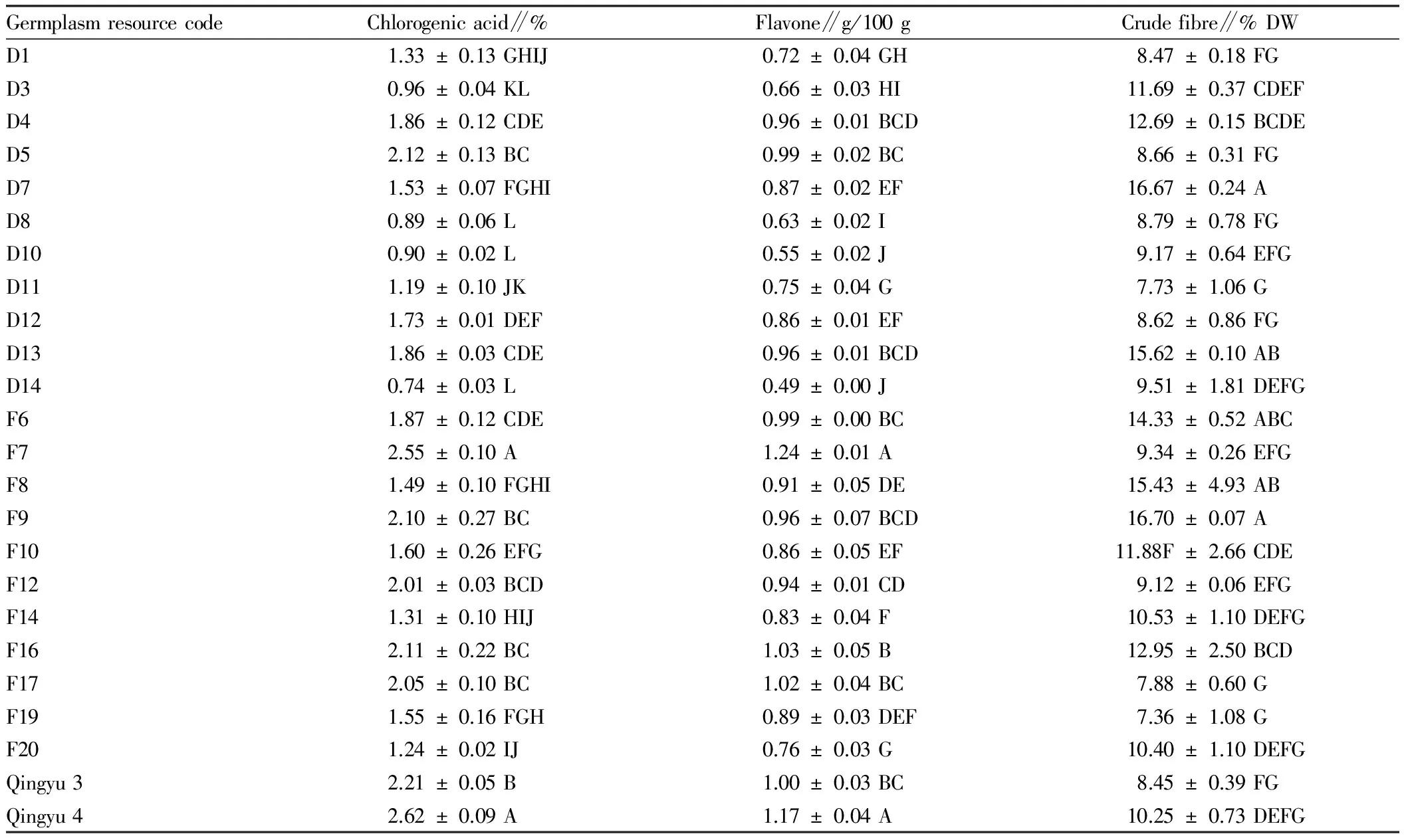
3.2ThecontentofthemainbiologicallyactivesubstanceinH.tuberosusLinn.leavesFrom Table 2, it was found that Qingyu 4 had the highest content of chlorogenic acid, reaching 2.62%, while D14 had the lowest content of chlorogenic acid, reaching 0.74%, with a difference of 3.5 times; F7 had the highest content of flavone, reaching 1.24 g/100 g, while D14 had the lowest content of flavone, reaching 0.49 g/100 g, with a difference of 2.5 times.
The analysis results of difference significance showed that there were significant differences in the chlorogenic acid and flavone content among differentH.tuberosusLinn. germplasm resources. Taking D1 from Denmark for example, there was an extremely significant difference in the chlorogenic acid content between D1 and some germplasm resources (D3, D4, D5, D8, D10, D12, D13, D14); there was an extremely significant difference in the flavone content between D1 and some germplasm resources (D4, D5, D7, D8, D10, D12, D13, D14).
Table2ThecontentofthemainbiologicallyactivesubstanceinHelianthustuberosusLinn.leaves
GermplasmresourcecodeChlorogenicacid∥%Flavone∥g/100gCrudefibre∥%DWD11.33±0.13GHIJ0.72±0.04GH8.47±0.18FGD30.96±0.04KL0.66±0.03HI11.69±0.37CDEFD41.86±0.12CDE0.96±0.01BCD12.69±0.15BCDED52.12±0.13BC0.99±0.02BC8.66±0.31FGD71.53±0.07FGHI0.87±0.02EF16.67±0.24AD80.89±0.06L0.63±0.02I8.79±0.78FGD100.90±0.02L0.55±0.02J9.17±0.64EFGD111.19±0.10JK0.75±0.04G7.73±1.06GD121.73±0.01DEF0.86±0.01EF8.62±0.86FGD131.86±0.03CDE0.96±0.01BCD15.62±0.10ABD140.74±0.03L0.49±0.00J9.51±1.81DEFGF61.87±0.12CDE0.99±0.00BC14.33±0.52ABCF72.55±0.10A1.24±0.01A9.34±0.26EFGF81.49±0.10FGHI0.91±0.05DE15.43±4.93ABF92.10±0.27BC0.96±0.07BCD16.70±0.07AF101.60±0.26EFG0.86±0.05EF11.88F±2.66CDEF122.01±0.03BCD0.94±0.01CD9.12±0.06EFGF141.31±0.10HIJ0.83±0.04F10.53±1.10DEFGF162.11±0.22BC1.03±0.05B12.95±2.50BCDF172.05±0.10BC1.02±0.04BC7.88±0.60GF191.55±0.16FGH0.89±0.03DEF7.36±1.08GF201.24±0.02IJ0.76±0.03G10.40±1.10DEFGQingyu32.21±0.05B1.00±0.03BC8.45±0.39FGQingyu42.62±0.09A1.17±0.04A10.25±0.73DEFG
Note: The crude fiber content is on a dry mass basis; uppercase letters represent<0.01.
There was also a significant difference in the leaf crude fiber content among 24H.tuberosusLinn. germplasm resources. F9 had the highest crude fiber content, reaching 16.70%, while F19 had the lowest crude fiber content, reaching 7.36%, with a difference of 2.3 times. 7H.tuberosusLinn. germplasm resources (D4, D7, D13, F6, F8, F9, F16) with high content of crude fiber can be used as raw materials for the development of crude fiber food additive; the germplasm resources (D11, F17, F19) with low content of crude fiber can be used as raw materials for the development of livestock feed.
The crude fiber content of D1 was significantly lower than the crude fiber content of the germplasm resources (D4, D7, D13, F6, F8, F9, F16). There was a highly significant difference in the crude fiber content between D3 and some germplasm resources (D7, D11, D13, F8, F9, F17, F19).
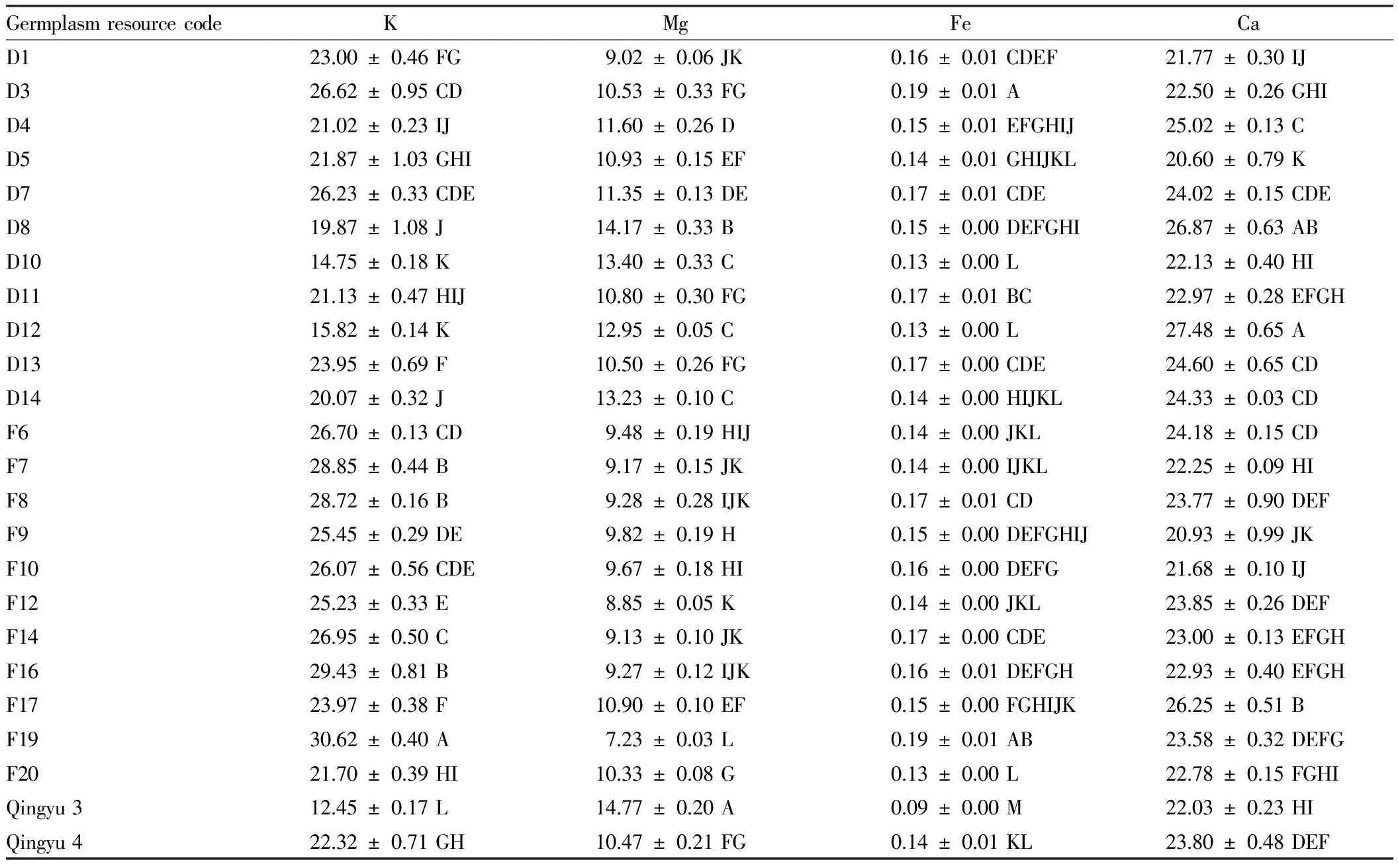
3.3ThecontentofthemineralelementsinH.tuberosusLinn.leavesIn the leaves of 24H.tuberosusLinn. germplasm resources, F19 had the highest content of K, reaching 30.62 mg/g, while Qingyu 3 had the lowest content of K, only 12.45 mg/g, with a difference of 2.5 times; Qingyu 3 had the highest content of Mg, reaching 14.77 mg/g, while F19 had the lowest content of Mg, only 7.23 mg/g; there was little difference in the content of Fe, and the content was not high, and F19 and D3 had the highest content, reaching 0.19 mg/g; D12 had the highest content of Ca, reaching 27.48 mg/g while D5 had the lowest content of Ca, reaching 20.60 mg/g.
The analysis results of difference significance showed that there was a difference in the content of leaf mineral elements among differentH.tuberosusLinn. germplasm resources. Similarly, taking D1 from Denmark for example, there was a significant difference in the content of K between D1 and some germplasm resources (D3, D4, D7, D10, D11, D12); there was also a significant difference in the content of Mg between D1 and some germplasm resources (D3, D4, D5, D7, D8); there was an extremely significant difference in the content of Fe between D1 and some germplasm resources (D3, D5, D10, D12); there was an extremely significant difference in the content of Ca between D1 and some germplasm resources (D3, D4, D5, D7) (Table 3).
Table3ThecontentofthemineralelementsinHelianthustuberosusLinn.leavesUnit: mg/g
Note: The crude fiber content is on a dry mass basis; uppercase letters represent<0.01.
3.4ClusteranalysisAfter data normalization of the test indices aboutH.tuberosusLinn. germplasm resources, DPS 7.05 was used for cluster analysis of 24H.tuberosusLinn. germplasm resources. The results (Fig. 1) showed that at the genetic distance of 0.65, the 24H.tuberosusLinn. germplasm resources could be divided into three categories.
The first category comprised D1, D3, F10, F14, F20, D4, D13, F17, D7, F8, F19, D5, F7, F12, Qingyu 4, F6, F9 and F16, and the leaf was mostly oval and orbicular; the water content was 19.02%-30.05%, the chlorogenic acid content was 0.96%-2.62%, the flavone content was 0.66-1.24 g/100 g, and the crude fiber content was 7.36%-16.7%; in the mineral elements, the K content was 21.02-30.62 mg/g, the Mg content was 7.23-11.60 mg/g, and the Ca content was 20.60-26.25 mg/g.
The second category only included one resource, namely D11, whose leaves were long oval, different from the leaf shape of the remaining 23H.tuberosusLinn. germplasm resources.
The third category comprised D8, D14, D12, D10 and Qingyu 3, and the leaf was all oval; the water content was 21.44%-29.71%, the chlorogenic acid content was 0.74%-2.21%, the flavone content was 0.49%-1.0%, and the crude fiber content was 8.45%-9.51%; in the mineral elements, the K content was 14.75-20.07 mg/g, the Fe content was 0.09-0.15 mg/g, and the Ca content was 22.03-26.78 mg/g.
At the genetic distance of 0.49, the 24H.tuberosusLinn. germplasm resources could be divided into five sub-categories. The first sub-category included D1, D3, F10, F14 and F20; the second sub-category included D4, D13, F17, D7, F8, F19, D5, F7, F12, Qingyu 4, F6, F9 and F16; the third sub-category only included D11; the fourth sub-category included D8, D14, D12 and D10; the fifth sub-category only included Qingyu 3.
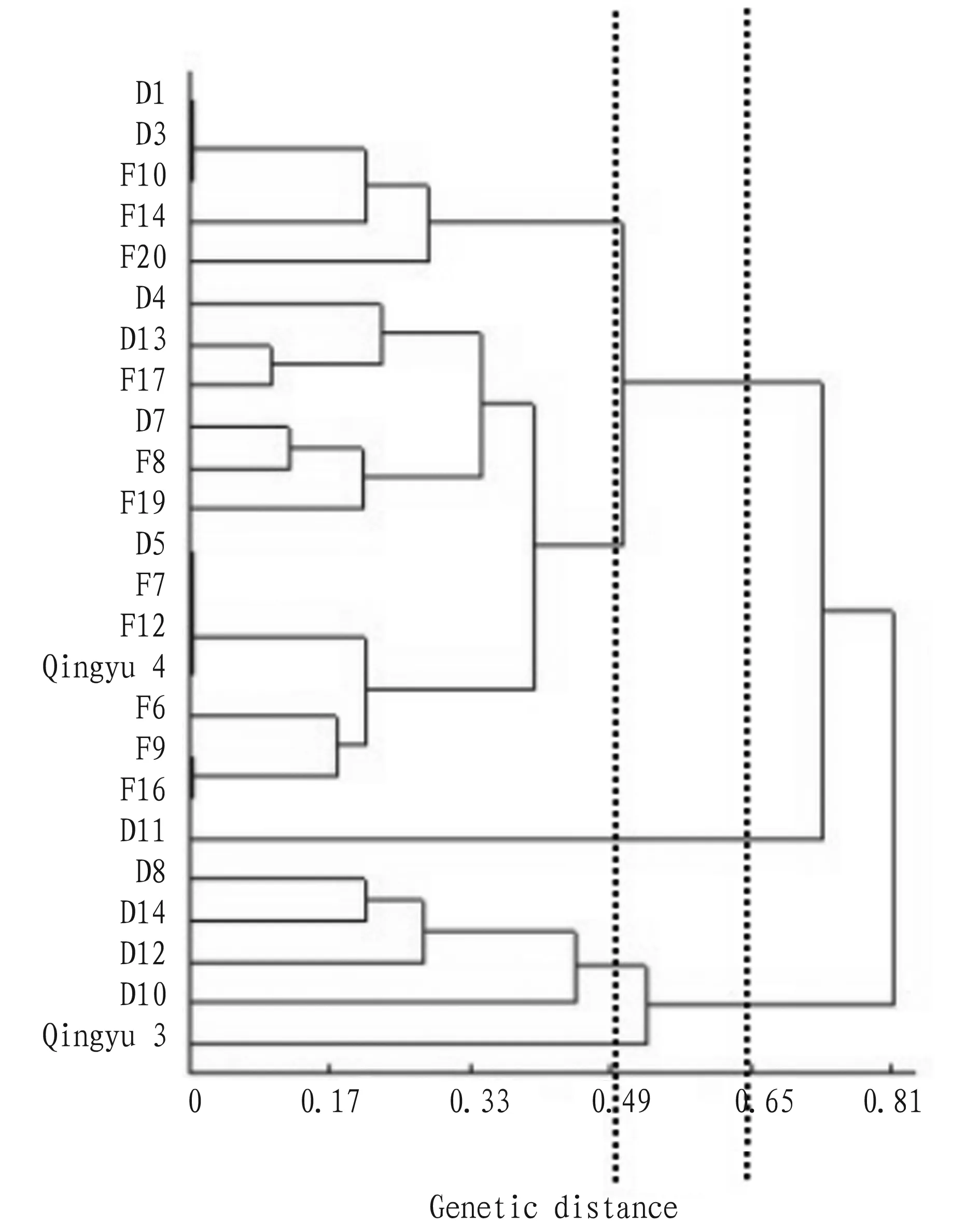
Fig.1Clusteranalysisof24Helianthustuberosusresourcesbasedontheleaftraits
4 Conclusions and discussions
The plant drought resistance is related to the plant moisture conditions, and the water loss rate or water retention capability of plant leaves reflects the condition of free water or irreducible water within the plant cells. Reganetal.[18]generally believe that the varieties with low water loss rate and strong water retention capability in the leaves are resistant to drought, and the leaf water content is a reliable indicator of drought tolerance in plants.
The foreignH.tuberosusLinn. germplasm resource F9 can be used as the material for the future breeding of drought-resistant varieties. There were significant differences in the leaf mineral elements and biologically active substance among differentH.tuberosusLinn. germplasm resources, the chlorogenic acid content was 0.74%-2.62%, and the flavone content was 0.49-1.24 g/100 g. Qingyu 4 had the chlorogenic acid content of 2.62%, and it was the germplasm resource with the highest content among the test materials, much higher than the chlorogenic acid content of theH.tuberosusLinn. germplasm resources introduced from abroad.
Crude fiber is the human gastrointestinal scavenger, and can promote gastrointestinal digestion, and effectively prevent and treat bad breath. The leaf crude fiber content of 24H.tuberosusLinn. germplasm resources was 7.36 %-16.7%. From the viewpoint of food development, 7H.tuberosusLinn. germplasm resources (D4, D7, D13, F6, F8, F9, F16) with high content of crude fiber can be used as raw materials for the development of crude fiber food additive; the germplasm resources (D11, F17, F19) with low content of crude fiber can be used as raw materials for the development of livestock feed.
In the 24H.tuberosusLinn. germplasm resources, F19 had the highest content of K in leaves (30.62 mg/g), and could be used as material for future development of K-rich foods. Qingyu 3 had the highest content of Mg (14.77 mg/g) while F19 had the lowest content of Mg, only 7.23 mg/g; there was little difference in the content of Fe, and the content was not high, and F19 and D3 had the highest content, reaching 0.19 mg/g; D12 had the highest content of Ca, reaching 27.48 mg/g while D5 had the lowest content of Ca, reaching 20.60 mg/g.
After the principal component analysis of leaf test indices, the cluster analysis was done. The results showed that at the genetic distance of 0.65, the 24H.tuberosusLinn. germplasm resources could be divided into three categories, and at the genetic distance of 0.49, the 24H.tuberosusLinn. germplasm resources could be divided into five sub-categories. From the point of view of kinship, someH.tuberosusLinn. germplasm resources from France and Denmark in the first category were clustered, indicating that theH.tuberosusLinn. germplasm resources from France and Denmark shared close sources to a certain extent, and there was a need to conduct further study in the future.
Compared with the 22H.tuberosusLinn. germplasm resources introduced from abroad, the 2H.tuberosusLinn. varieties in China (Qingyu 3, Qingyu 4) occupied obvious advantage in terms of biologically active substance content, and in the future, we can increase the development and utilization of the twoH.tuberosusLinn. varieties.
By analyzing the leaf mineral elements and biologically active substance in differentH.tuberosusLinn. germplasm resources, we selected 6 specificH.tuberosusLinn. germplasm resources: F19 with the highest content of K, reaching 30.62 mg/g; D8 with the highest content of Mg, Ca, reaching 14.17 mg/g, 26.87 mg/g, respectively; F9 with the highest content of crude fiber, reaching 16.7 %; F7 with the highest content of chlorogenic acid, flavone, reaching 2.55%, 1.24 g/100g, respectively; D14 with the lowest content of chlorogenic acid, flavone, reaching 0.74% and 0.49, respectively; D11 with long oval leaves. It is necessary to conduct in-depth study of these resources in the future.
[1] WANG SW, HAN XM. Effects of different proportion ofHelianthustuberosusdiets on growth and rumen environment of weaning lamb[J]. Journal of Gansu Agricultural University, 2014, 49(2): 48-54. (in Chinese).
[2] LIN Y, LU RD. Jerusalem artichoke series of functional food research[J]. Guangdong Agricultural Science, 1998(5): 38-39. (in Chinese).
[3] SUO JL, ZHU H, LIU HY,etal. The study on tea beverage processing ofHelianthustuberosusandChrysanthemum[J]. Beverage Industry, 2015, 18(2): 66-68. (in Chinese).
[4] CAO JJ, CHEN FM, GONG YX,etal. Study on constituents and biological activity of volatileoil from leaves of Micheliamaudiae[J]. Journal of Plant Resources and the Environment, 2007, 16(3): 27-30. (in Chinese).
[5] SUN YZ, HU TM,WANG QZ,etal. Bioactivity of different solvent extracts fromCichoriumin tybus leaf onMythimnaseparata[J].Journal of Plant Resources and the Environment,2010, 19(4):31-36. (in Chinese).
[6] ZHANG HJ, LIU L, ZHENG XT,etal. Study on optimization of extraction technology of chlorogenic acid inHelianthustuberosusleaves[J]. Science and Technology of Food Industry, 2011, 32(5): 261-265. (in Chinese).
[7] YUE HL, BI HT, YU RT,etal. Research of the contents variation of chlorogenic acid in the leaves ofHelianthustuberosusL. during different growth stages in Chaidamu[J]. Science and Technology of Food Industry, 2014, 35(1): 283-285. (in Chinese).
[8] FAN JH, LIU M, WU QZ,etal. Principal component and cluster analysis of leaf physiological traits of 52Helianthustuberosusgermplasm resources in Southern Xinjiang[J].Journal of Agriculture, 2016, 6(1):66-72. (in Chinese).
[9] ZHAO ML, HAN R, LI L. ISSR marker analysis on genetic diversity of twenty-four cultivars ( lines) ofHelianthustuberosus[J]. Journal of Plant Resources and Environment,2013,22(4):44-49. (in Chinese).
[10] ZHAO ML, SUN XM, WANG LH,etal. ISSR bases genetic diversity of 43HelianthustuberosusL.[J].Journal of Northwest A&F University(Nat.Sci.Ed.), 2015, 9(43): 1-8. (in Chinese).
[11] MA SC. Analysis of genetic diversity of 41HelianthustuberosusL. resources by SRAP markers[D]. Qinghai xining,Qinghai University College of Agriculture and Animal Husbandry, 2014. (in Chinese).
[12] ZHAO JX, REN CM, WU FZ,etal. Comprehensive identification of saline-alkaline tolerance of 16 Jerusalem artichoke accessions at seedling stage[J]. Chinese Journal of Eco-Agriculture, 2015, 23(5): 620-627. (in Chinese).
[13] LI Y, SUN XM, ZHONG QW,etal. A new processing variety of jerusalem artichoke-‘Qingyu No.3’[J]. China Vegetables, 2011(10): 100-102. (in Chinese).
[14] LI L. Jerusalem artichoke[M]. Qinghai: Qinghai People’s Publishing House, 2012. (in Chinese).
[15] BAO SD. Soil analysis (version 3)[M]. Beijing: China Agriculture Press, 2000: 56-58, 79-83, 106-107, 133-140, 191, 237-239. (in Chinese).
[16] XIONG SM, ZUO XF, ZHU YY. Determination of cellulose, hemi-cellulose and liginin rice hull[J]. Cereal&Feed Industry, 2005, 8(20): 40-41. (in Chinese).
[17] FAN PC, TIAN J, HUANG JM,etal. On the determination of cellulose and lignin of peanut shells[J]. Journal of Chongqing Institute of Technology: Natural Science, 2008, 10(5): 64-65, 67. (in Chinese).
[18] REGAN KL, WHAN BR, TURNER NC. Evaluation of chemical desiccation as a selection technigne for droughtresistance in dryland wheat breeding program[J]. Australian Journal of Agricultural Research, 1993, 44(8):1683-1691.
杂志排行
Asian Agricultural Research的其它文章
- System Factors for Land Corruption in China
- Value Appeal of Harmonious Moral Life of Chinese College Students
- Interaction between Hosts and Guests of Rural Tourism in Northeast China Based on Symbolic Interaction Theory
- Rules for Access of Foreign Capitals to Agricultural Field in Countries along the "Belt and Road"
- Promoting Cotton Green Production in Shandong through Accelerating Simplified Cultivation Technique
- Comprehensive Evaluation of Main Traits of Sugarcane Germplasm Resources with High Sucrose Content
