lnfluence of drought hardening on the resistance physiology of potato seedlings under drought stress
2018-02-05ZHANGShuhanXUXuefengSUNYeminZHANGJunlianLlChaozhou
ZHANG Shu-han, XU Xue-feng, SUN Ye-min, ZHANG Jun-lian,, Ll Chao-zhou
1 Gansu Key Laboratory of Crop Genetics & Germplasm Enhancement, College of Life Sciences and Technology, Gansu Agricultural University, Lanzhou 730070, P.R.China
2 College of Horticulture, Gansu Agricultural University, Lanzhou 730070, P.R.China
1. lntroduction
There are four major mechanisms of plant drought resistance: drought avoidance, drought tolerance, drought escape and drought recovery (Fang and Xiong 2015).Of these, drought avoidance and drought tolerance are the two primary mechanisms for survival under drought conditions. Drought avoidance refers to the ability of plants to sustain high water status by increasing water uptake or reducing water loss in dry conditions (Yueet al. 2005).For example, increasing the root/shoot ratio improves the ability to uptake water, and closing stomata reduces water loss from transpiration. The main drought avoidance traits include root morphological traits and physiological traits(such as stomatal conductance and leaf relative water content (RWC)). Drought tolerance is defined as the ability of plants to maintain a certain level of physiological activity. This is accomplished through the regulation of numerous genes (for example, those related to stress signal transduction) (Zhanget al. 2014) and a series of metabolic pathways that reduce or repair the resulting stress damage.Drought tolerance is usually associated with physiological parameters related to osmotic adjustment (such as proline(Pro), soluble sugar and abscisic acid (ABA) content) and the alleviation of drought damage (such as the activities of protective enzymes and chlorophyll content) (Luo 2010;Fang and Xiong 2015).
In order to reduce the adverse impact of drought stress on crop production, several methods and technologies have been put to use to enhance the drought resistance of crops.Drought hardening is a convenient and feasible method and involves exposing plants to arid conditions such as reduced irrigation or partial drought during the seedling stage so as to improve the ability to adapt to subsequent serious drought(Thomas 2009; Huanget al. 2013). The effectiveness of drought hardening lies in the fact that young plants are malleable and are usually more able to survive under arid conditions if they have undergone a previous period of low moisture stress. It has been found that drought hardening applied in the nursery before planting improved the seedling survival rate under extreme xeric conditions (Driessche 1991). In addition, seedling hardening can improve the growth adaptability and drought resistance of mulberry(Huanget al. 2013). This strategy has been widely adopted in wheat, rice and other plants (Villar-Salvadoret al. 2004;Yanget al. 2015).
Potato is the fourth most important food crop in the world,with an annual production exceeding 300 million tons, and is pivotal to agricultural production and people’s livelihood(http://faostat.fao.org/). Compared to other crops, potato is considered to be more sensitive to drought, and even a short period of stress may cause significant reduction in tuber yield (Loon 1981).
To date, numerous studies on potato drought resistance have been reported (Zhanget al. 2014; Baniket al. 2016;Ciolocaet al. 2016; Kabira and Muthoni 2016). Most of them have focused on the physiological and biochemical responses to drought stress and the signal transduction pathways involved. In this study, we investigated drought resistance in both contrast seedlings and drought-hardened potato seedlings in terms of resistance physiology, growth status and leaf anatomical structure, and in this paper we discuss the connection between certain indexes with the aim of gaining a more comprehensive understanding of the mechanisms of drought resistance in potato seedlings after drought hardening.
2. Materials and methods
2.1. Plant culture and stress treatments
Potato (Solanum tuberosumL.cv. cultivar Atlantic) tubers with one apical bud were germinated in pots (diameter in 35 cm, height in 40 cm) in a greenhouse at a temperature of(25±1)°C with a 13 h photoperiod and a photon flux density of approximately 400 μmol m-2s-1. All potato samples were divided randomly into two equal-sized groups. One group(regarded as the contrast) was watered normally, and the soil water content was maintained at approximately 15.0%by weighting before imposing drought stress. Another group was treated by the same method as the contrast group before germination and at the beginning 24 days after germination, then drought hardening was carried out for 25 days by withholding water and maintaining the soil water content at approximately 12.5%. On the 26th day the soil water content of drought hardening groups was increased to 15%, the same water content as the contrast, for 2 days.The soil water content was measured by weighing, and a moderate amount of water was added to maintain the desired soil water content. On the 28th day drought stress was imposed by withholding water from both groups. After 7 and 14 days of the drought treatment (the first day of no watering was regarded as 0 day), the second leaves from the base and white new roots of the potato seedlings were harvested for the experiments described below.
2.2. Determination of RWC, relative electric conductivity, malondialdehyde (MDA) content and root vigor
RWC of the potato seedling leaves was calculated as follows: RWC (%)=100×(FW–DW)/FW, where, FW is the fresh weight and DW is the dry weight (Barrs and Weatherly 1962). Relative electric conductivity of the potato seedling leaves was measured according to the method of Liuet al.(2014): thirty plant leaf wafers were placed into test tubes,10 mL distilled water were added, and tubes were sealed with plastic caps and shaken for a while so as to let the leaves immersed in distilled water. The tubes were uncapped and placed under vacuum for 10 min, and then kept for 1 h at room temperature before measuring the electrical conductance value (S1). The tubes were then placed in boiling water for 20 min, then cooled and the electrical conductance value was measured (S2). The electrical conductance value of distilled water was also measured (S3). The relative electric conductivity of the leaves was calculated using the formula: Relative electric conductivity (%)=(S1–S3)/(S2–S3)×100. MDA content was determined following the method of Heath and Packer(1968) with slight modifications. One gram of experimental material was ground in a mortar, and after adding 2 mL 10% chilled trichloroacetic acid (TCA) solution and a little quartz sand, the sample was ground further. The homogenate was centrifuged for 10 min at 4 000 r min-1,then 2 mL supernatant was placed into a test tube, and 2 mL distilled water was added to another test tube to serve as a standard for OD determination. To both tubes 2 mL 0.6% thiobarbituric acid (TBA) solution was added, and the tubes were placed in boiling water for 15 min. After cooling,the samples were centrifuged again. The supernatant was used for the determination of OD value at 532, 600, and 450 nm, respectively. The MDA concentration was calculated according to the formula: MDA concentration(μmol L-1)=6.45×(OD532–OD600)–0.56×OD450. Root vigor was determined according to the method of Liuet al. (2014).
卡夫卡与父亲站在了对立的两方面,这种对立和反抗甚至于从未从卡夫卡的人生中消失。尽管卡夫卡本身很清楚这场抗战本身没有任何意义,可是却忍不住还是像孩子一般地把这种反抗当成了自己一生的事业。
2.3. Determination of chlorophyll content, net photosynthetic rate (Pn), transpiration rate (Tr) and water utilization efficiency (WUE)
Chlorophyll content was measured by the method of Arnon(1949).PnandTrwere measured between 10:30 and 11:30 in the morning using a LI-6400 portable photosynthesis system (LI-Cor, USA). WUE was calculated according to the formula: WUE (%)=Pn/Tr×100
2.4. Determination of superoxide dismutase (SOD),catalase (CAT) and peroxidase (POD) activity
SOD activity was determined spectrophotometrically according to the method of Spychalla and Desborough(1990). CAT activity was determined according to the method described by Lin and Wang (2002). CAT activity was measured by following the consumption of H2O2(extinction coefficient 39.4 mmol L-1cm-1) at 240 nm for 2 min. POD activity was measured following the method of Tang and Newton (2005).
2.5. Determination of polyamines (PAs), ABA, Pro and soluble sugar content
PAs extraction and HPLC analysis were conducted following the method of Flores and Galston (1982), and authentic standards of putrescine (Put), spermidine (Spd)and spermine (Spm) were benzoylated according to the procedure described by Flores and Galston (1982).Programmable liquid chromatography (Model Waters 600E,Waters Inc., USA) was applied to measure the concentration of these PAs. The solvent system consisted of methanol and water (65% v/v methanol) at a flow rate of 1 mL min-1.The benzoylated extracts were eluted at room temperature through a reverse-phase column (Waters Symmetry C18,3.9 mm×150 mm, 5 μm in particle size), and the absorbance at 254 nm was measured with a UV detector (Liet al. 2004).ABA content was measured according to the method of Rosset al. (2004). Pro was measured according to the method described by Bateset al. (1973). Soluble sugars were assayed by the method of Zhanget al. (2006).
2.6. Measurement of growth and development indexes
The main stalk height was regarded as the plant height. The stem diameter was measured at the junction between the plant and soil surface with a vernier caliper. Fibrous root number was counted visually. Root length was determined by taking the average of the length of the five longest roots.Leaf area was determined using a leaf area meter (LI-3000,LI-Cor, Inc., USA). Biomass was determined by measuring the dry weight of the whole seedling after drying in an oven at 80°C for 24 h.
2.7. Measurement of stomatal density, stomatal size and stomatal aperture
Stomatal measurements was done using the method of Chen and Gallie (2004) with slight modifications. Stomatal density was calculated from the average of 25 field of views in microscope per sample. Stomatal size was calculated by examining at least 50 closed stomatas per sample. The stomatal aperture was calculated from the width and length of at least 50 open stomatas per sample.
2.8. Measurement of anatomical structure
Anatomical structures were measured from paraffin sections:the 3rd leaf of each seedling was removed, washed with distilled water and cut into 5 mm×10 mm pieces near the midrib of the leaf. Leaf pieces were then fixed with formalinacetic acid-alcohol (FAA) fixative for 24 h, and dyed with safranin and fast green stain. Sections were observed with an Olympus microscope (CX22LED, Olympus, USA) and photographed. Indexes including leaf thickness, palisade tissue thickness and spongy parenchyma thickness were measured, and the thickness ratio of palisade tissue to spongy parenchyma was calculated.
2.9. Statistical analysis
All data were obtained from three or more independent replicates and were subjected to one-way analysis of variance (ANOVA). The mean differences were compared with Duncan’s multiple range test (DMRT) using SPSS statistical software (ver. 17.0 SPSS, Chicago, USA).Differences atP<0.05 were considered significant.
3. Results
3.1. lnfluences of drought hardening on RWC, relative electric conductivity, MDA content and root vigor
A sharp decrease in the RWC of the contrast potato seedling leaves was observed when the drought stress lasted 14 days,but the reduction in the RWC of drought-hardened seedlings was lower relative to contrast seedlings (Fig. 1-A). Leaf relative electric conductivity and MDA content increased with drought stress, yet less of an increase was observed in the drought-hardened seedlings. For instance, the relative electric conductivity of the seedling leaves treated with drought hardening was 24.7% lower than the contrast when drought stress lasted 7 days and 6.0% lower at the 14th day(Fig. 1-B). MDA content of the drought-hardened seedling leaves was 10.4 and 9.2% lower than the contrast when the stress lasted 7 and 14 days, respectively (Fig. 1-C). Root vigor of drought-hardened seedlings increased significantly and was 13.6 and 9.5% higher than the contrast when the stress lasted 7 and 14 days, respectively (P<0.05) (Fig. 1-D).
3.2. lnfluences of drought hardening on chlorophyll content, Pn, Tr and WUE

Fig. 1 Influence of drought hardening on the relative water content, relative electric conductivity and malondialdehyde (MDA)content of potato seedling leaves and potato seedling root vigor under drought stress. DW, dry weight. Bar means standand error.Different lowercase letters indicate significant differences between groups at the P<0.05 level.
Chlorophyll content decreased with prolonged drought, but there was less of a decrease observed in drought-hardened seedlings, chlorophyll content in drought-hardened seedlings was 5.3 and 29.7% higher than the contrast when drought stress lasted 7 and 14 days, respectively (P>0.05)(Fig. 2-A).Pnwas significantly reduced when the drought stress lasted 7 and 14 days, but drought hardening treatment alleviated the reduction;Pnwas 41.7 and 154.2% higher than the contrast when drought stress lasted 7 and 14 days,respectively (Fig. 2-B).
With prolonged drought stress, a significant decrease in theTrwas observed in both drought-hardened and contrast potato seedling leaves. When drought stress lasted 14 days, theTrof the drought-hardened potato seedling leaves was 3.9% lower than the contrast. WUE of the drought-hardened potato leaves was much higher than the contrast when the drought stress lasted 14 days(P<0.05) (Fig. 2-C and D).
3.3. lnfluences of drought hardening on SOD, CAT and POD activity
The activities of SOD, POD and CAT in potato seedling leaves increased when the drought stress lasted 7 days,then decreased at the 14th day in the contrast (Fig. 3-AC). Prior to drought stress (0 day), drought hardening significantly increased the activities of POD and CAT(P<0.05) (Fig. 3-B and-C). SOD activity also increased, but this increase was not significant (P>0.05) (Fig. 3-A). The activities of SOD, POD and CAT in the drought-hardened potato seedlings were lower than the contrast when the stress lasted 7 days, but higher than the contrast when the stress lasted 14 days (P>0.05) (Fig. 3-A-C).
3.4. lnfluences of drought hardening on PAs and ABA content
Put, Spm and Spd are the three most important PAs in plants. In the contrast, the levels of Put, Spm and Spd increased significantly when drought stress lasted 7 days and then decreased significantly when the stress lasted 14 days (P<0.05) (Fig. 4-A-C). Drought hardening treatment increased the levels of Put, Spm and Spd significantly in the leaves when the drought stress lasted 14 days (P<0.05);levels were 116.2, 69.2 and 183.6% higher than the contrast,respectively. Drought hardening increased the ABA content before drought stress (0 day), and when the stress lasted 14 days, the ABA content in the drought hardening-treated seedling leaves was still significantly higher than the contrast(P<0.05) (Fig. 4-D).
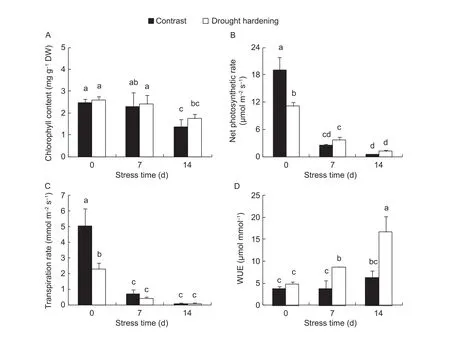
Fig. 2 Influence of drought hardening on the chlorophyll content, net photosynthetic rate (Pn), transpiration rate (Tr) and water utilization efficiency (WUE) of potato seedling leaves under drought stress. Bar means standard error. Different lowercase letters indicate significant differences between groups at the P<0.05 level.
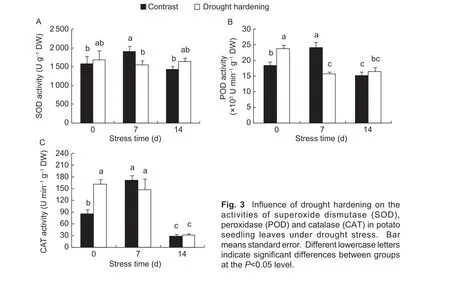
Fig. 3 Influence of drought hardening on the activities of superoxide dismutase (SOD),peroxidase (POD) and catalase (CAT) in potato seedling leaves under drought stress. Bar means standard error. Different lowercase letters indicate significant differences between groups at the P<0.05 level.
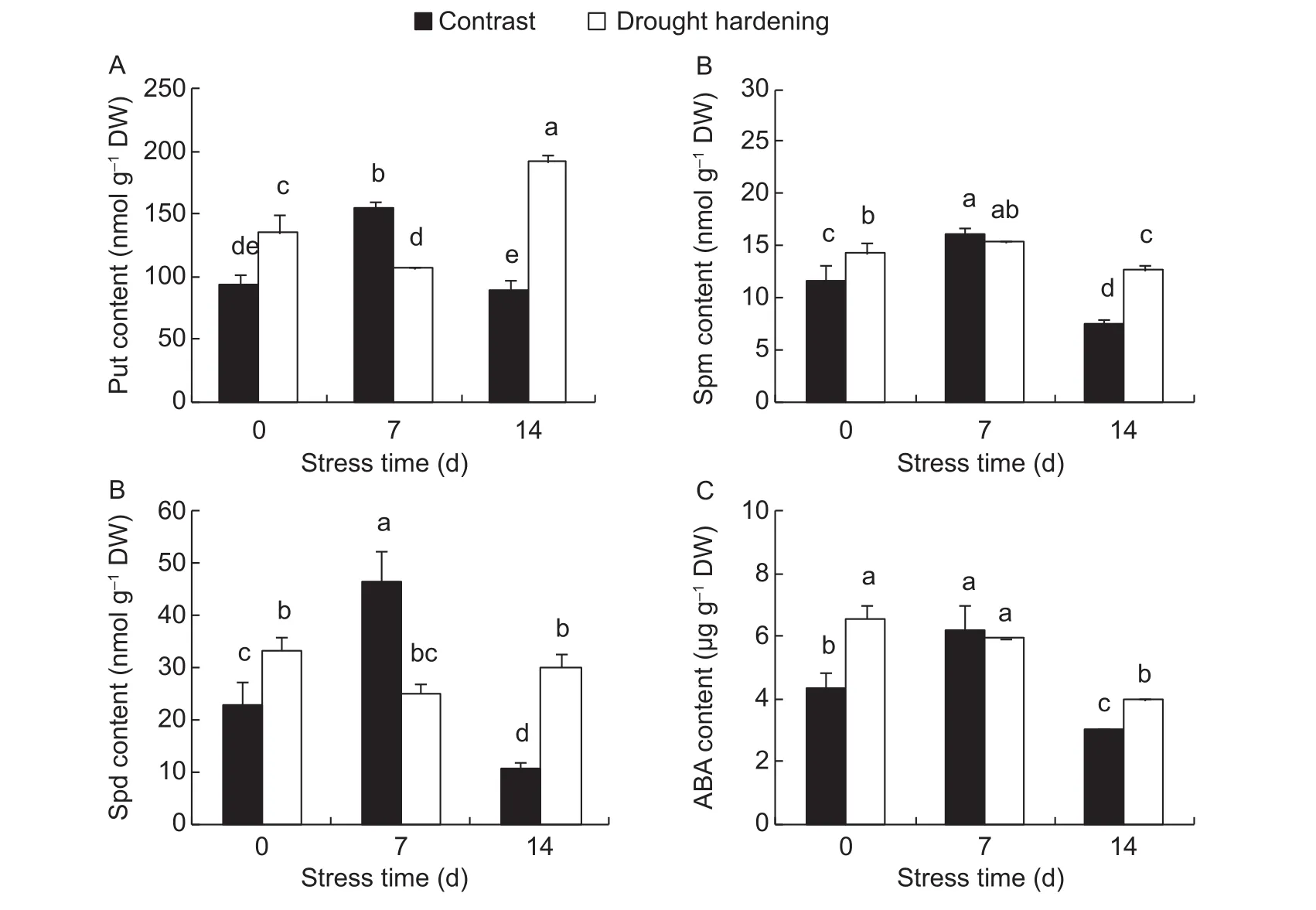
Fig. 4 Influence of drought hardening on the levels of polyamines (PAs) and abscisic acid (ABA) in potato seedling leaves under drought stress. Put, putrescine; Spm, spermine; Spd, spermidine. Bar means standard error. Different lowercase letters indicate significant differences between groups at the P<0.05 level.
3.5. lnfluences of drought hardening on Pro and soluble sugar content
There was a significant increase in the levels of Pro and soluble sugars with prolonged drought stress, and the drought hardening treatment markedly enhanced the levels of Pro and soluble sugars compared to the contrast (P<0.05).For instance, when the stress lasted 14 days, the levels of Pro and soluble sugars in the drought-hardened potato seedling leaves were 24.4 and 11.7% higher, respectively,than the contrast (Fig. 5-A and B).
3.6. lnfluences of drought hardening on growth and development indexes
Plant height, stem diameter, fibrous root number, root length and leaf area in both the contrast and drought-hardened potato seedlings increased gradually over time. Drought hardening increased the stem diameter, fibrous root number and root length relative to the contrast when the stress lasted 7 and 14 days, whereas the plant height and leaf area indices were reduced. For instance, fibrous root number and root length were 13.0 and 13.7% higher than the contrast,respectively, when the stress lasted 14 days, while leaf area was 14.7% lower than the contrast. When the drought stress lasted 14 days, the biomass and the root-shoot ratio of the drought hardening treated potato seedlings were significantly higher than the contrast (P<0.05) (Table 1).
3.7. lnfluences of drought hardening on stomatal density, stomatal size and stomatal aperture
The stomatal density of potato seedling leaves increased while stomatal size and stomatal aperture decreased significantly during drought stress. Drought hardening treatment reinforced this trend. For instance, when the stress lasted 0, 7 and 14 days, the stomatal aperture width of drought-hardened seedlings was reduced by 2.2, 9.9 and 22.0%, respectively, compared with the contrast (Table 2).
3.8. lnfluences of drought hardening on anatomical structure
In the contrast seedling leaves, there was a slight decrease in leaf thickness as the drought stress progressed, and there was a significant decrease in the thickness of the spongy parenchyma and a significant increase in the thickness of the palisade tissue and the palisade tissue to spongy parenchyma ratio (P<0.05). Drought hardening treatment also significantly increased the thickness of palisade tissue(P<0.05) compared with the contrast. The palisade tissue to spongy parenchyma ratio also increased compared with the contrast, but this increase was not significant (P>0.05)(Table 3).
4. Discussion


Fig. 5 Influence of drought hardening on the content of proline and soluble sugars in potato seedling leaves under drought stress.Bar means standard error. Different lowercase letters indicate significant differences between groups at the P<0.05 level.
MDA is a by-product of lipid peroxidation and is often used as an index of oxidative stress (Oralet al. 2006; Göbelet al. 2009). In this paper, we showed that the change in relative electric conductivity and MDA content followed similar trends under drought stress and both indices were comparatively lower in drought-hardened than contrast samples (Fig. 1-B and C). Consistent with this, antioxidant enzyme activity in drought-hardened potato seedling leaves was markedly higher than contrast seedlings prior to drought stress (Fig. 3-A-C). After 7 to 14 days of drought stress, drought hardening reduced the decline in the activity of three antioxidant enzymes (SOD, POD,CAT) compared to the contrast. These results indicate that drought-hardened potato seedlings have a higher ability to reduce ROS accumulation and thus maintain the stability of the membrane system and alleviate the damage induced by drought stress. We concluded that drought hardening enhanced drought resistance in potato seedling leavesviaimproving the activity of the antioxidant enzymes.
PAs, primarily consisting of Spd, Spm and their diamine precursor, Put, are low-molecular-weight aliphatic polycations that are widespread and found in almost all living organisms (Bohraet al. 2015). Being positively charged at physiological pH, they can interact with various cellular macromolecules, such as nucleic acids,proteins, membrane phospholipids and thus regulate many fundamental cellular processes (Martin-Tanguy 2001; Shiet al. 2010; Fiscalettiet al. 2013). It has been reportedthat PAs frequently accumulate in response to biotic and abiotic stresses (Chattopadhayayet al. 2002; Kubiś 2008;Kusanoet al. 2008; Alcázaret al. 2010) and could act as signaling molecules, resulting in the alleviation of negative drought effects (Kubiśet al. 2014). When encountering environmental stresses, such as water deficit, plants accumulate a large number of amine substances to promote the activities of scavenging enzymes, slow down the process of lipid peroxidation, and maintain plasma membrane integrity (Shenet al. 2000; Liuet al. 2007). In this study we found that the levels of Put, Spm and Spd increased after 7 days drought stress then decreased after 14 days in the contrast seedlings (Fig. 4-A-C). Drought hardening treatment prevented the decrease in PAs content and markedly increased PAs content compared to the contrast at the 14th day (P<0.05) (Fig. 4-A-C), and thus improved the drought resistance of the potato seedlings.

Table 1 Influence of drought hardening on the growth and development of potato seedlings under drought stress
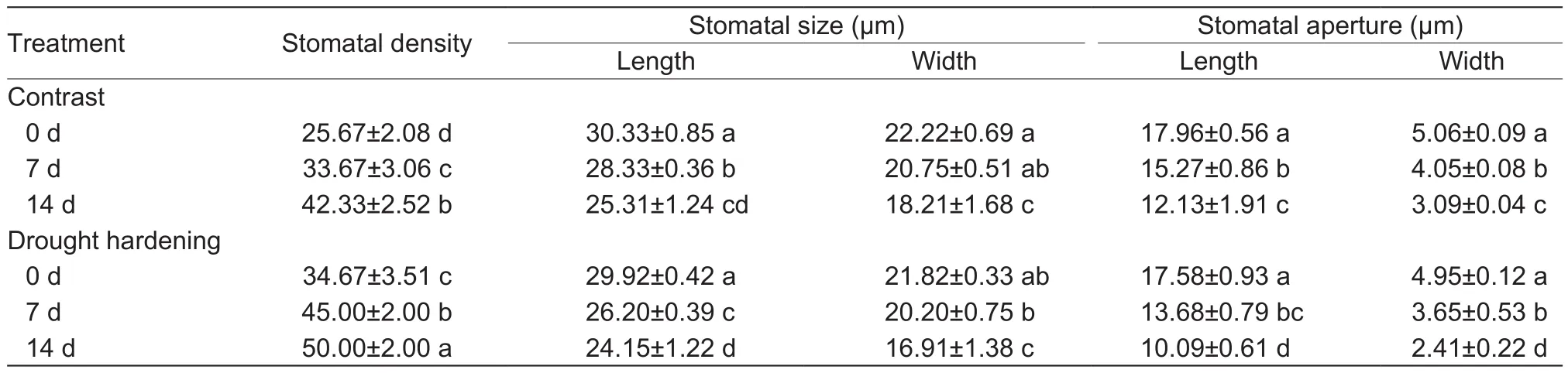
Table 2 Influence of drought hardening on the stomatal density, stomatal size and stomatal aperture of potato seedling leaves under drought stress

Table 3 Influence of drought hardening on the anatomical structure of potato seedling leaves under drought stress
Among the phytohormones, ABA plays a major role in the response of plants to environmental stresses, such as water stress and extreme temperature stress (Sauteret al.2001; Bray 2002; Finkelsteinet al. 2002), and it is one of the key factors regulating stomatal behavior. ABA curtails transpirational water loss by promoting stomatal closure,which limits gas and water exchange between the interior of leaves and the atmosphere, and thus prevents water loss (Bhargava and Sawant 2013; Hernándezet al. 2013;Koffleret al. 2014). Thus higher ABA concentrations during drought stress help to maintain water status in plants (Duanet al. 2008; Zhanget al. 2010). Endogenous ABA content increases rapidly even up to 30-fold during drought stress and improves drought tolerance by enhancing osmotic adjustment and inducing stomatal closure (Acharya and Assmann 2009; Penget al. 2012; Bhargava and Sawant 2013). We showed that drought hardening increased ABA content in the potato seedling leaves before drought stress,and after 14 days of drought stress the ABA content in the leaves of drought-hardened plants was still 30.6% higher than the contrast (P<0.05) (Fig. 4-D). Similarly, theTrwas sharply reduced at the 14th day (Fig. 2-C). The result suggests that, the increase in ABA content after drought hardening enhance the ability of potato seedlings to adapt to drought stress by inducing stomatal closure to reduce water lossviatranspiration.
Smaller stomata can open and close more rapidly and their general association with high stomatal densities provides the capacity for rapid increase in leaf stomatal conductance and maximizes CO2diffusion into the leaf(Aasamaaet al. 2001). In this paper, drought hardening treatment increased stomatal density and reduced stomatal size and aperture (Table 2), and thus reduced leaf transpiration rate and improved WUE (Fig. 2-C and D). This should have a positive effect on potato seedlings growth and development. Consistent with this, drought hardening increased biomass (Table 1) and root vigor (Fig. 1-D) relative to the contrast.
Leaves are the action centers of photosynthesis in higher plants and have the largest area exposed to the environment among plant organs (Liuet al. 2016 ). The long-term influence of arid environments alters leaf morphology (Gaoet al. 2003). Plants have evolved many leaf structural adaptations to protect against and minimize water loss under limited moisture conditions (Hameedet al. 2012).Thicker plant leaves result in a higher water storage capacity,which prevents excessive transpiration and guarantees a higher WUE. Palisade tissue provides mechanical support for leaves and can prevent moisture loss, whereas spongy parenchyma can store a lot of water and efficiently avoid leaf blight caused by drought. To survive under long-term water shortages, the thickness of palisade tissue increased and the thickness of spongy parenchyma decreased(Chartzoulakiset al. 2002). We found a significant increase in the thickness of the palisade tissue and in the ratio of palisade tissue to spongy parenchyma during drought stress, and these increases were even higher after drought hardening treatment (Table 3). These results indicate that better-developed potato leaf palisade tissue might have less mechanical damage under drought stress conditions.
Plants have evolved several mechanisms to manage the damaging effects of abiotic stresses, and there exists a positive compensation mechanism in plants under drought stress. Photosynthetically active radiation is absorbed by chlorophyll, and a higher chlorophyll content under water stress conditions is believed to result in more efficient use of light energy (Guoet al. 2008). We observed that, compared with contrast seedlings, drought hardening increased the RWC, chlorophyll content andPnof potato seedling leaves under drought stress (Fig. 1-A and Fig. 2-A and B). High leaf WUE is a water-saving strategy allowing plants to maintain strong drought tolerance (Wu and Bao 2012). It is generally believed that reduced transpiration and increased photosynthesis jointly lead to improvement in leaf WUE. In our experiment, when the stress lasted 14 days, theTrof the drought-hardened potato seedling leaves was 3.9% lower than the contrast, while thePnwas about 154.2% higher than the contrast on the same day (Fig. 2-B and C), thus resulting in an evident increase in leaf WUE (Fig. 2-D).
5. Conclusion
With drought hardening treatment, leaf area, stomatal size,stomatal aperture as well as theTrwere reduced, so the reduction of water content caused by drought stress was alleviated; bothPnand WUE were improved; these changes may be beneficial for plant growth. Furthermore, drought hardening treatment enhanced the levels of PAs, ABA, Pro and soluble sugars and the activities of antioxidant enzymes in seedling leaves, thus improving the resistance physiology of potato seedlings. In a word, drought hardening improves the drought resistance of potato seedlings by impacting aspects of leaf microstructure and anatomical structure, the levels of endogenous hormones, osmotic adjustment and antioxidant enzyme activity.
Acknowledgements
The work was supported by the China Agricultural Research System (CARS-09-P14), the Science and Technology Support Project of Gansu Provincial Sci. & Tech.Department, China (1604NKCAa52-3).
Aasamaa K, Sober A, Rahi M. 2001. Leaf anatomical characteristics associated with shoot hydraulic conductance,stomatal conductance and stomatal sensitivity to changes of leaf water status in temperate deciduous trees.Australian Journal of Plant Physiology, 28, 765–774.
Acharya B R, Assmann S M. 2009. Hormone interactions in stomatal function.Plant Molecular Biology, 69, 451–462.
Adams H D, Guardiola-Claramonte M, Barron-Gafford G A,Villegas J C, Huxman T E. 2009. Temperature sensitivity of drought-induced tree mortality portends increased regional die-off under global-change-type drought.Proceedings of the National Academy of Sciences of the United States of America, 106, 7063–7066.
Alcázar R, Altabella T, Marco F, Bortolotti C, Reymond M,Tiburcio A F. 2010. Polyamines: Molecules with regulatory functions in plant abiotic stress tolerance.Planta, 231,1237–1249.
Anirban G, Debashree S, Girish K R, Attipalli R R. 2010. An integrated diagnostic approach to understand drought tolerance in mulberry (Morus indicaL.).Flora, 205, 144–151.
Apel K, Hirt H. 2004. Reactive oxygen species: Metabolism,oxidative stress, and signal transduction.Annual Review of Plant Biology, 55, 373–399.
Arnon D T. 1949. Copper enzymes in isolated chloroplasts polyphenaloxidase in Beta vulgaris.Plant Physiology, 24,1–15.
Banik P, Zeng W, Tai H, Bizimungu B, Tanino K. 2016. Effects of drought acclimation on drought stress resistance in potato (Solanum tuberosumL.) genotypes.Environmental& Experimental Botany, 126, 76–89.
Barrs H D, Weatherly P E. 1962. A re-examination of relative turgidity for estimating water deficits in leaves.Australian Journal of Biological Sciences, 15, 413–428.
Bartels D, Sunkar R. 2005. Drought and salt tolerance in plants.Critical Reviews in Plant Sciences, 24, 23–58.
Bates L S, Waldren R P, Teare I D. 1973. Rapid determination of free proline for water stress studies.Plant Soil, 39, 205–207.
Benjamin J G, Nielsen D C. 2006. Water deficit effects on root distribution of soybean, field pea and chickpea.Field Crops Research, 97, 248–253.
Bhargava S, Sawant K. 2013. Drought stress adaptation:Metabolic adjustment and regulation of gene expression.Plant Breeding, 132, 21–32.
Bohra A, Sanadhya D, Bhatia D S. 2015. Polyamines:Metabolism and role in abiotic stress amelioration.Journal of Plant Science Research, 31, 183–195.
Bray E A. 2002. Abscisic acid regulation of gene expression during water-deficit stress in the era of theArabidopsisgenome.Plant Cell & Environment, 25, 153–161.
Budak H, Kantar M, Kurtoglu K Y. 2013. Drought tolerance in modern and wild wheat.The Scientific World Journal,2013, 548246.
Chartzoulakis K, Patakas A, Kofidis G, Bosabalidis A, Nastou A. 2002. Water stress affects leaf anatomy, gas exchange,water relations and growth of two avocado cultivars.Scientia Horticulturae, 95, 39–50.
Chattopadhayay M K, Tiwari B S, Chattopadhayay G, Bose A,Sengupta D N, Ghosh B. 2002. Protective role of exogenous polyamines on salinity-stressed rice (Oriza sativa) plants.Physiology Plant, 116, 192–199.
Chen Z, Gallie D R. 2004. The ascorbic acid redox state contrasts guard cell signaling and stomatal movement.The Plant Cell, 116, 1143–1162.
Chutipaijit S. 2016. Changes in physiological and antioxidant activity of indica rice seedlings in response to mannitolinduced osmotic stress.Chilean Journal of Agricultural Research, 76, 455–462.
Cioloca M A, Tican A M, Ianoşi M, Bădărău C L. 2016. The growth response of several potato genotypes (Solanum tuberosumL.) to induced water stress using sorbitol and polyethylene glycol.Notulae Scientia Biologicae, 8,511–519.
Van den Driessche R. 1991. Influence of container nursery regimes on drought resistance of seedlings following planting. I. Survival and growth.Canadian Journal of Forest Research, 21, 555–565.
Duan B, Xuan Z, Zhang X, Korpelainen H, Li C. 2008.Interactions between drought, ABA application and supplemental UV-B inPopulus yunnanensis.Physiology Plant, 134, 257–269.
Fang Y J, Xiong L Z. 2015. General mechanisms of drought response and their application in drought resistance improvement in plants.Cellular & Molecular Life Sciences Cmls, 72, 673–689.
Finkelstein R R, Gampala S S, Rock C D. 2002. Abscisic acid signaling in seeds and seedlings.The Plant Cell,14(Suppl.14), S15.
Fiscaletti D, Angeli D, Tarozzi L, Barozzi G S. 2013. Plant polyamines in abiotic stress responses.Acta Physiologiae Plantarum, 35, 2015–2036.
Flores H E, Galston A W. 1982. Analysis of polymines in higher plants by high performance liquid chromatography.PlantPhysiology, 69, 701–706.
Foyer C H, Noctor G. 2005. Oxidant and antioxidant signalling in plants: A re-evaluation of the concept of oxidative stress in a physiological context.Plant Cell & Environment, 28,1056–1071.
Gao J P, Wang Y H, Chen D F. 2003. Anatomical characteristics of leaf epidermis and vessel elements ofSchisandra sphenantherafrom different districts and their relationships to environmental factors.Acta Botanica Boreali-occidentalia Sinica, 23, 715–723.
Gill S S, Tuteja N. 2010. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants.Plant Physiology & Biochemistry, 48, 909–930.
Göbel C, Feussner I, Hause B, Wasternack C, Strack D.2009. Methods for the analysis of oxylipins in plants.Phytochemistry, 70, 1485–1503.
Guo P, Michael B, Rajeevk V, Andreas G, Stefania G, Salvatore C. 2008. QTLs for chlorophyll and chlorophyll fluorescence parameters in barley under post-flowering drought.Euphytica, 163, 203–214.
Hameed M, Batool S, Naz N, Nawaz T, Ashraf M. 2012. Leaf structural modifications for drought tolerance in some differentially adapted ecotypes of blue panic (Panicum antidotaleretz.).Acta Physiologiae Plantarum, 34,1479–1491.
Heath R L, Packer L. 1968. Photoperoxidation in isolated chloroplast. I. Kinetics and stoichiometry of fatty acid peroxidation.Archives of Biochemistry & Biophysics, 125,189–198.
Henricks P A, Nijkamp F P. 2001. Reactive oxygen species as mediators in asthma.Pulmonary Pharmacology &Therapeutics, 14, 409–420.
Hernández I, Cela J, Alegre L, Munnébosch S. 2013. Antioxidant defenses against drought stress. In: Aroca R, ed.,Plant Responses to Drought Stress. Springer-Verlag, Berlin,Heidelberg. pp. 231–258.
Huang X H, Liu Y, Li J X, Xiong X Z, Chen Y, Yin X H, Feng D L. 2013. The response of mulberry trees after seedling hardening to summer drought in the hydro-fluctuation belt of three gorges reservoir areas.Environmental Science and Pollution Research, 20, 7103–7111.
Kabira J N, Muthoni J. 2016. Potato production under drought conditions: Identification of adaptive traits.International Journal of Horticulture, 6, 1–9.
Koffler B E, Luschin-Ebengreuth N, Stabentheiner E, Müller M, Zechmann B. 2014. Compartment specific response of antioxidants to drought stress inArabidopsis.Plant Science,227, 133–144.
Kubiś J. 2005. The effect of exogenous spermidine on superoxide dismutase activity, H2O2and superoxide radical level in barley leaves under water deficit conditions.Acta Physiologiae Plantarum, 27, 289–295.
Kubiś J. 2008. Exogenous spermidine alters in different ways activities of some scavenging system enzymes, H2O2and superoxide radical levels in water stressed cucumber leaves.Journal of Plant Physiology, 165, 397–406.
Kubiś J, Floryszak-Wieczorek J, Arasimowicz-Jelonek M. 2014.Polyamines induce adaptive responses in water deficit stressed cucumber roots.Journal of Plant Research, 127,151–158.
Kusano T, Berberich T, Tateda C, Takahashi Y. 2008.Polyamines: Essential factors for growth and survival.Planta, 228, 367–381.
Langebartels C, Wohlgemuth H, Kschieschan S, Grün S,Sandermann H. 2002. Oxidative burst and cell death in ozone-exposed plants.Plant Physiology & Biochemistry,40, 567-575.
Li C Z, Jiao J, Wang G X. 2004. The important roles of reactive oxygen species in the relationship between ethylene and polyamines in leaves of spring wheat seedlings under root osmotic stress.Plant Science, 166, 303–315.
Lin J S, Wang G X. 2002. Double CO2could improve the drought tolerance better in sensitive cultivars than in tolerant cultivars in spring wheat.Plant Science, 163, 627–637.
Liu J H, Kitashiba H, Wang J, Ban Y, Moriguchi T. 2007.Polyamines and their ability to provide environmental stress tolerance.Plant Biotechnology, 24, 117–126.
Liu J J, Wei Z, Li J H. 2014. Effects of copper on leaf membrane structure and root activity of maize seedling.Botanical Studies, 55, 47.
Liu Q, Li Z H, Wu J Y. 2016. Research progress on leaf anatomical structures of plants under drought stress.Agricultural Science & Technology, 17, 4–7, 14.
Loon C D V. 1981. The effect of water stress on potato growth,development, and yield.American Journal of Potato Research, 58, 51–69.
Luo L J. 2010. Breeding for water-saving and droughtresistancerice (WDR) in China.Journal of Experimental Botany, 61, 3509–3517.
Martin-Tanguy J. 2001. Metabolism and function of polyamines in plants: Recent development (new approaches).Plant Growth Regulation, 34, 135–148.
Oral O, Kutlu T, Aksoy E, Fıçıcıoğlu C, Uslu H, Tuğrul S. 2006.The effects of oxidative stress on outcomes of assisted reproductive techniques.Journal of Assisted Reproduction and Genetics, 2, 81–85.
Peng S, Jiang H, Zhang S, Chen L H, Li X G, Korpelainen H, Li C Y. 2012. Transcriptional profiling reveals sexual differences of the leaf transcriptomes in response to drought stress inPopulus yunnanensis.Tree Physiology, 32, 1541–1555.
Ravikumar G, Manimaran P, Voleti S R, Balachandran S M. 2014. Stress-inducible expression of AtDREB1A transcription factor greatly improves drought stress tolerance in transgenic indica rice.Transgenic Research,23, 421–439.
Ross A R, Ambrose S J, Cutler A J, Feurtado J A, Kermode A R, Nelson K, Zhou R, Abrams S R. 2004. Determination of endogenous and supplied deuterated abscisic acid in plant tissues by high-performance liquid chromatographyelectrospray ionization tandem mass spectrometry with multiple reaction monitoring.Analytical Biochemistry, 329,324–333.
Sato Y, Yokoya S. 2008. Enhanced tolerance to drought stress in transgenic rice plants overexpressing a small heat-shock protein, shsp17.7.Plant Cell Reports, 27, 329–334.
Sauter A, Davies W J, Hart W. 2001. The long-distance abscisic acid signal in the droughted plant: The fate of the hormone on its way from root to shoot.Journal of Experimental Botany, 52, 1991–1997.
Serraj R, Kumar A, Mcnally K L, Slametloedin I, Bruskiewich R, Mauleon R, Cairns J, Hijmans R J. 2009. Improvement of drought resistance in rice.Advances in Agronomy, 103,41–99.
Shen W, Nada K, Tachibana S. 2000. Involvement of polyamines in the chilling tolerance of cucumber cultivars.Plant Physiology, 124, 431–439.
Shi J, Fu X Z, Peng T, Huang X S, Fan Q J, Liu J H. 2010.Spermine pretreatment cinfers dehydration tolerance of citrusin vitroplantsviamodulation of antioxidative capacity and stomatal response.Tree Physiology, 30, 914–922.
Siedlinski M, van Diemen C C, Postma D S, Vonk J M, Boezen H M. 2009. Superoxide dismutases, lung function and bronchial responsiveness in a general population.European Respiratory Journal, 33, 986–992.
Spychalla J P, Desborough S L. 1990. Superoxide dismutase,catalase and alpha tocopherol content of stored potato tubers.Plant Physiology, 94, 1214–1218.
Tang W, Newton R J. 2005. Peroxidase and catalase activities are involved in direct adventitious shoot formation induced by thidiazuron in eastern white pine (Pinus strobusL.)zygotic embryos.Plant Physiology Biochemistry, 43,760–769.
Thomas D S. 2009. Survival and growth of drought hardenedEucalyptus pilularisSm. seedlings and vegetative cuttings.New Forests, 38, 245–259.
Uzilday B I, Turkan, A H, Sekmen R, Ozgur, Karakaya H C. 2012.Comparison of ROS formation and antioxidantenzymes inCleome gynandra(C4) andCleome spinosa(C3) under drought stress.Plant Science, 182, 59–70.
Villar-Salvador P, Planelles R, Peňuelar-Rubira J. 2004.Drought tolerance and transplanting performance of holm oak (Quercus ilex) seedlings after drought hardening in the nursery.Tree Physiology, 24, 1147–1155.
Wang W, Vinocur B, Altman A. 2003. Plant responses to drought, salinity and extreme temperatures: Towards genetic engineering for stress tolerance.Planta, 218, 1–14.
Wu X L, Bao W K. 2012. Statistical analysis of leaf water use efficiency and physiology traits of winter wheat under drought condition.Journal of Integrative Agriculture, 11,82–89.
Yang S L, Chen K, Wang S S, Gong M. 2015. Osmoregulation as a key factor in drought hardening-induced drought tolerance inJatropha curcas.Biologia Plantarum, 59,529–536.
Yue B, Xiong L, Xue W, Xing Y, Luo L, Xu C. 2005. Genetic analysis for drought resistance of rice at reproductive stage in field with different types of soil.Theoretical and Applied Genetics, 111, 1127–1136.
Zhang N, Liu B L, Ma C Y, Zhang G D, Chang J, Si H J, Wang D. 2014. Transcriptome characterization and sequencingbased identification of drought-responsive genes in potato.Molecular Biology Reports, 41, 505–517.
Zhang S, Chen F, Peng S, Ma W, Korpelainen H, Li C. 2010.Comparative physiological, ultrastructural and proteomic analyses reveal sexual differences in the responses ofPopulus cathayanaunder drought stress.Proteomics, 10,2661–2677.
Zhang Z J, Li H Z, Zhou W J, Takeuchi Y, Yoneyama K. 2006.Effect of 5-aminolevulinic acid on development and salt tolerance of potato (Solanum tuberosumL.) microtubersin vitro.Plant Growth Regulation, 49, 27–34.
猜你喜欢
杂志排行
Journal of Integrative Agriculture的其它文章
- Experimental infectivity of Theilerialuwenshuni and Theileria uilenbergi in Chinese Kunming mice
- Development of a sensitive and reliable droplet digital PCR assay for the detection of ‘Candidatus Liberibacter asiaticus’
- One size fits all? Contract farming among broiler producers in China
- Designing price-contingent vegetable rotation schedules using agent-based simulation
- The effects of aeration and irrigation regimes on soil CO2 and N2O emissions in a greenhouse tomato production system
- The efficiency of long-term straw return to sequester organic carbon in Northeast China’s cropland
