Influence of Initial Water Content on Synthesis of Silicalite-1 Zeolite
2018-01-19ZhangHonghanZhangShanheWangHaorenLiChunyi
Zhang Honghan; Zhang Shanhe; Wang Haoren; Li Chunyi
(State Key Laboratory of Heavy Oil Processing, China University of Petroleum, Qingdao 266580)
1 Introduction
Water, one of the most influential factors in the synthesis of ZSM-5 zeolite, can powerfully affect the physicochemical properties of the zeolite[1]. A higher water content of the reaction mixture usually can lead to larger crystals and yield spherical monocrystalline product[2]. Petrik, et al.[3]showed clearly that the water content can significantly affect the acidity and catalytic performance of ZSM-5 zeolite. As the Al-free MFI-type zeolite, silicalite-1 zeolite has weak water affinity, but Turov, et al.[4]indicated that the existence of defect sites,such as hydroxyl groups, may significantly influence the adsorption of water molecules. In addition, it was found that pre-adsorption of water on silicalite-1 zeolite changed the intracrystalline diffusivity of small molecules[5].However, there is little literature talking about the in fl uence of initial water content, especially about the pore size distribution or the generation of hierarchical pores.
Hierarchical pores zeolites, especially the micro/mesoporous zeolitic composites, have attracted increasing attention because of its good shape selectivity and mass transfer ability[6]. There are many strategies relating to synthesizing the hierarchical silicalite-1 zeolites,which can be divided into two groups, viz.: the bottomup methods and the top-down strategies[7]. The bottom-
up approaches often unlock new types of synthetic possibilities and yield zeolites with exceptional features,but these methods involve expensive organics, such as tetra-alkylammonium cations, and the cost concerns make them less attractive[8]. The top-down strategies cover dealumination by acid treatment and desilication by base treatment, which have the intrinsic advantage of nonorganic fingerprint. Nevertheless, the top-down strategies have many unfavorable features. Verboekend, et al.[9]used aqueous NaOH on ZSM-22 zeolite to gain mesoporous structure, but it lost much solid. It reported that the mesostructured zeolite can be obtained by NH4OH treatment, but a pore-directing agent is necessary[10].Such methods for synthesizing the ordered or disordered hierarchical pores zeolites have respectively relative merits, however, the cheap and simple strategies to realize the zeolite synthesis are always in the spotlight[11-17].
As a sort of traditional catalyst applied in MTP reaction,HZSM-5 zeolite catalysts have the uniquely excellent properties because of its uniform channel structure, and superior thermal and hydrothermal stability[18]. Besides,the influence of Si/Al ratio on its catalytic performance isalways the research hotspot as well. It was reported that the massive decrease of Al content in HZSM-5 zeolite could effectively reduce the acid density, which notably enhanced the propylene selectivity and the resistance to coke formation on HZSM-5 zeolite in MTP reaction[18-19].In recent years, the silicalite-1 zeolite possesses a more and more important position in the field of shape selectivity catalytic materials. Although the silicalite-1 zeolite is oleophylic and hydrophobic, its surface can generate a certain amount of defects at a certain temperature, and ulteriorly these defects can yield a plentiful of silicon hydroxyl groups with BrØnsted acid sites[20]. It was reported that the silicon hydroxyl nest could function as the active center of Beckmann rearrangement reaction[21-22]. Meng, et al.[20]indicated that Si-ZSM-11 (silicalite-2) could catalyze the transformation of methanol to propene and the author stated that silanol groups were the active sites. Silicalite-1 has the similar skeleton structure and acid property of silicalite-2. So applying silicalite-1 to the MTP reaction is promising. Besides, we have used Na2SiO3as the silica source to synthesize Ce-silicalite-1 and applied it to the MTP reaction, resulting in promising results[23].
Up to now, to the best of our knowledge, few studies reported in the literature are related with the influence of initial water content on the synthesis of silicalite-1 zeolite, especially the synthesis of the hierarchical silicalite-1 zeolite by regulating the initial water content.In this work, a series of hierarchical silicalite-1 zeolites were synthesized only through changing the initial water content and studied in the MTP reaction, leading to a highest propene mass yield of 26.81% ( on the CH2basis)at a P/E ratio of 6.28 in 2 h.
2 Experimental
2.1 Reagents
Silica sol (SiO2, 40%), tetrapropylammonium bromide(TPABr, 99.0%), and sodium hydroxide (NaOH, 99.0%)were purchased from the Sinopharm Chemical Reagent Co., Ltd. (China) and used without further purification.
2.2 Samples preparation
In a typical run, the zeolite samples were prepared through three steps: firstly, tetrapropylammonium bromide (TPABr)was dissolved in colloidal silica under stirring for 20—30 min to get a mixture A. Then the mixture A was added dropwise to a series of certain amount of deionized water(initial water content, as shown in Table. 1) under stirring for 20—30 min to get a mixture B, to which finally the sodium hydroxide solution and the rest of deionized water were added drop by drop. After vigorous stirring the white homogeneous gel for 3—5 h at room temperature, a mixture with a molar composition of SiO2:0.3TPA+:0.1Na2O:100H2O was obtained and transferred into a static Teflon-lined stainless-steel autoclave for hydrothermal treatment at 170°C under autogenous pressure for 8 h. The obtained solid was washed, filtered and dried at 120 °C overnight. The template was removed by calcination in air at 550 °C for 6 h, and afterwards the obtained Na-ZSM-5 zeolite was turned into the H-form by three consecutive ion-exchange procedures with 1 mol/L NH4NO3solution at 80 °C for 2 h,and the resulting samples were dried at 140 °C for 6 h and calcined again at 550 °C for 3 h to yield the H-silicalite-1 zeolite catalysts.
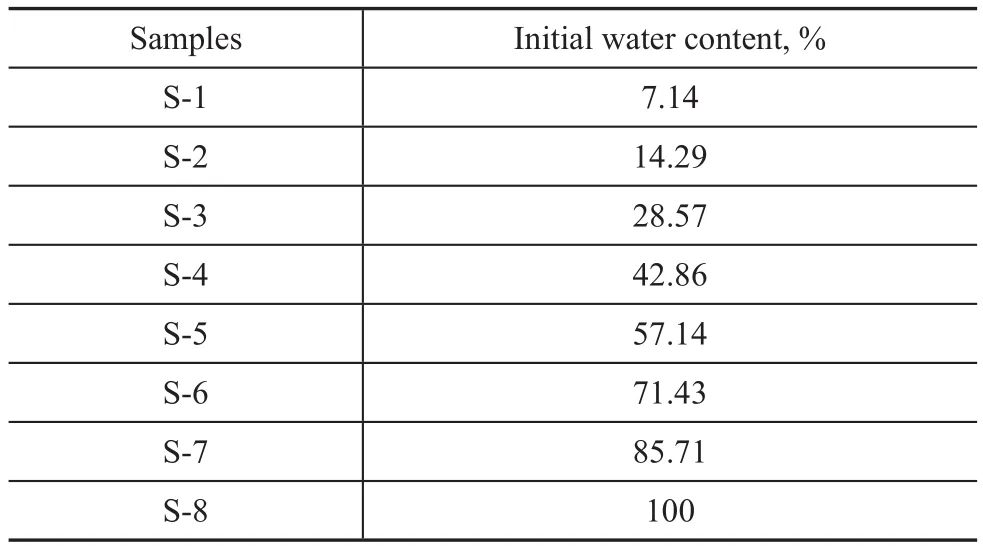
Table 1 Initial water content of synthesized silicalite-1 zeolites
2.3 Samples characterization
The XRD patterns of the samples were recorded on a Philips X’Pert PRO MPD diffractometer operating at 40 kV and 40 mA, using Cu Kαradiation with a scanning speed of 10(°)/min. The particle size and apparent morphology of the samples were measured by a scanning electron microscope S-4800. The parameters of specific surface area and pore structure of the catalyst samples were measured by nitrogen adsorption–desorption isotherms at 77 K on a Quadrasorb SI apparatus. Prior to the measurements, the samples were degassed at 300 °C under vacuum for 12 h to remove the adsorbed moisture.Pyridine Fourier transform infrared spectroscopy (Py-FTIR) measurements were conducted on a Tensor 27
FTIR spectrometer. The spectra were recorded with a resolution of 4 cm−1and 64 scans.
2.4 Catalysis test
The MTP reaction was carried out at 450 °C in a fixedbed tubular reactor under atmospheric pressure. For each test, 1.0 g of catalyst with a particle size of 40—60 mesh was loaded into the center of the reactor. The mass balance was above 95 %.
The methanol conversion is calculated through Eq. 1
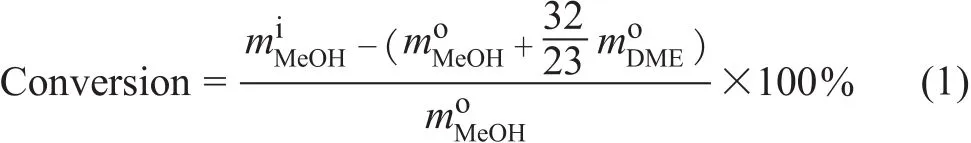
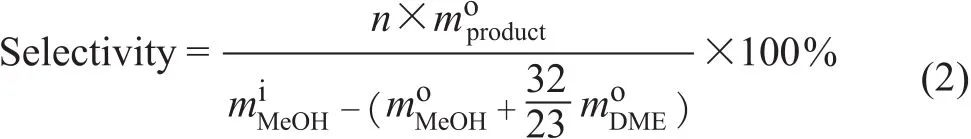
The product selectivity on the carbon basis is defined as:where m is the weight and n is the number of carbon atoms. The superscripts i and o refer to the components at the inlet and outlet of the reactor, respectively. It should be noticed that the major by-product water is not considered in this way as it does not contain carbon atoms.
3 Results and Discussion
3.1 Effect of initial water content on physicochemical properties of silicalite-1 zeolite
Figure 1a shows the XRD patterns of silicalite-1 zeolites with different initial water content. Evidently, each sample has an obvious MFI topological structure, because the peaks at 2θ= 7.9°, 8.79°, 23.03°, 23.24°, and 23.87° are strong without obvious impurity peak, but the peak at 2θ=8.79° is erratic. The results indicate that different initial water content can impact insignificantly on the skeleton structure of silicalit-1 zeolite, but it can influence the crystal face growth orientation of silicalite-1 zeolite (assigned to (200))[24]. The relative crystallinity, as assessed by XRD techniques, is exhibited in Figure 1b, which reveals that there is no apparent connection between the initial water content and the matching rate of the as-synthesized silicalite-1 zeolite samples and the standard sample.

Figure 1 XRD patterns (a) and relative crystallinity (b) of all the samples
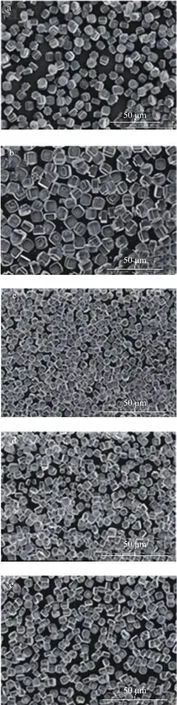
Figure 2 shows the SEM images of S-1, S-2, S-4, S-6,and S-8 samples. Obviously, the crystal size of silicalite-1 zeolites synthesized at a lower initial water content is bigger than that synthesized at a higher water content,but their morphology shows little difference, since all samples are smooth-faced cuboid monolith. On the contrary, Suzuki, et al.[2]made a point that the bigger crystal size of zeolite was synthesized at higher water content. As a result, the suppositional idea is as follows.The less initial water percentage means that more water was added to the reaction mixture in the later process, and consequentially it could change the original concentration of the composition. Upon taking into consideration the initial water content, the concentration change would be increasingly smaller until it disappeared. Perhaps this is the bigger change of concentration that can lead to the change in crystal growth.
The difference of the pore structure in S-1, S-2, S-4,S-6 and S-8 samples is evidenced by the N2adsorptiondesorption isotherms presented in Figure 3, in which S-1 and S-2 show a type I isotherm and the other three samples show a type IV isotherm but with different hysteresis loops, revealing different pore structure. The steep increase of the five samples at p/p0< 0.1 illustrates their perfect microporous structure. At a high relative pressure of p/p0=0.4-1.0, the S-1 and S-2 samples show a typical characteristic of microporous adsorption without a hysteresis loop and the other three samples show the type-H4hysteresis loops with a jump on the desorption branch around p/p0=0.42. The special hysteresis loops are attributed to the cavitation effect of an ink-bottle type mesopores, in which desorption of the N2is suddenly removed from the pore body after emptying its neck (with either micropores or entrances < 4 nm).
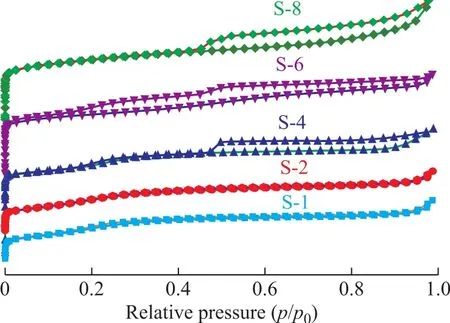
Figure 3 N2 adsorption–desorption isotherms of the assynthesized silicalite-1 catalysts
By combining with the SEM images, we can disclose that after adding the initial water content beyond 15%, the particle size become smaller and appearing mesoporous.In addition, these mesopores are intracrystalline pores with its pore diameter equating to 3.2 nm (Figure 4). Li,et al.[25]used the gel including a type of structure directing agent as the seed to synthesize the hierarchical silicalite-1 zeolite. Fernandez, et al.[26]obtained the target zeolite via the NaOH or HCl treatment. Indeed, these approaches avoided the import of expensive mesoporous template agent, but the secondary operation process is quite complicated.
The detailed information about pore textural properties of the five samples was calculated, with the results listed in Table 2. Notably, after adding initial water content to15%, the mesoporous volume (Vmeso) increased but the particle size and BET surface area (SBET) decreased. As regards the relationship between the particle size and the surface area, it is widely accepted that the crystal size is negatively correlated to the surface area[27-28]. However,there is another appearance that the external surface area gradually increased with the reduction of particle size, and the microporous surface area is lessening.The addition of external surface area is attributed to particle size reduction, and the emergence of mesopores is accomplished at the expense of micropores with the exception of the sample S-6, because it is speculated that the mesopores in S-6 may emanate from self-assembly.
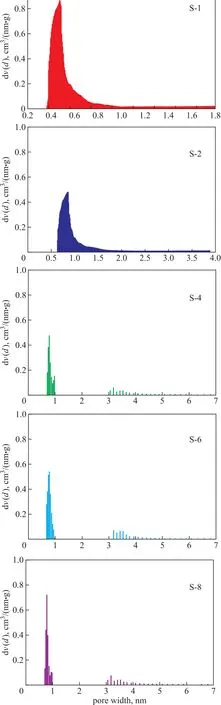
Figure 4 Pore size distribution of the five silicalite-1 samples

Table 2 Textural properties of the five silicalite-1 samples
If the initial water content is increased, the rest of water addition should be accordingly reduced, which means that the concentration difference is smaller. In other words, the lower initial water amount means the larger concentration difference. Upon combining Figure 2 and Table 2, we can speculate that larger concentration difference is good for crystal size growth, and furthermore, in a fairly homogeneous system, the pore size of silicalite-1 zeolite is easier to expand.
3.2 Effect of silicalite-1 on catalytic performance of MTP reaction
The nature of hydroxyls of silicalite-1 has been widely studied by IR spectroscopy[29-31]. Table 3 lists the frequencies and tentative assignments of hydroxyls bands(3800—3400 cm-1). The literature[22]pointed out the isolated silanols at the outer silicalite-1 zeolite surface including the terminal H-bonded silanols and silanols inside the chains and hydrogen nests (Figure 5), and the latter one functioned as weak Brønsted acid sites, which were the active sites of methanol conversion[20,23].
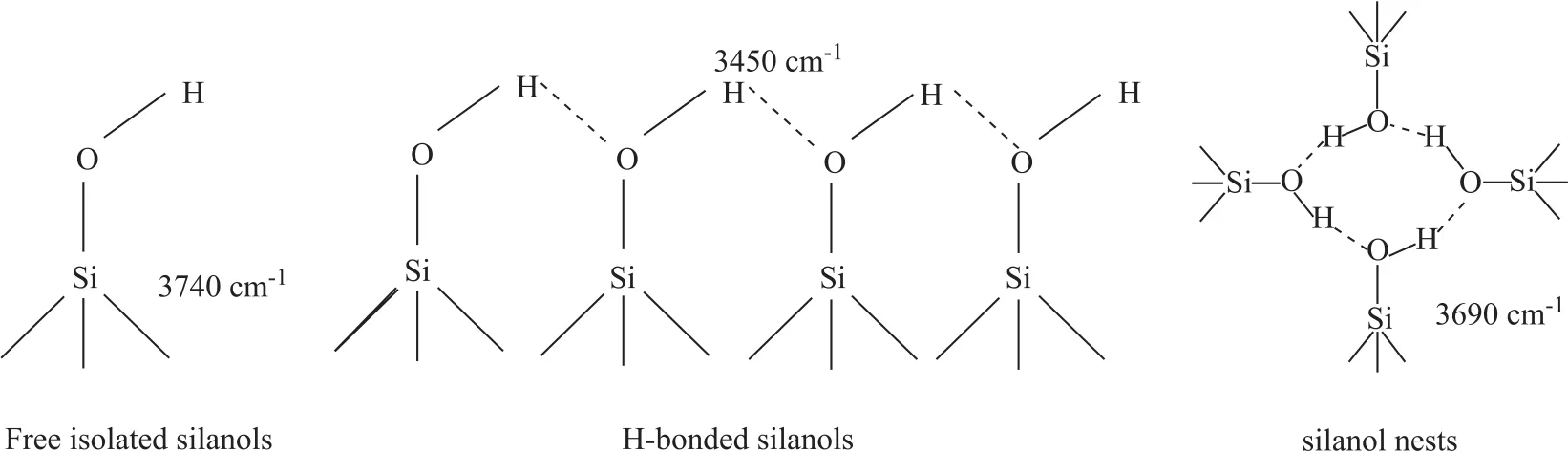
Figure 6 shows the FTIR spectra in the OH-stretching region (a) and pyridine adsorption (b) of the five silicalite-1 samples, which are compared with those of the standard H-ZSM-5 sample (Si/Al=100, purchased from the Nankai Catalysts Company). Obviously, the wavenumber of 3740 cm-1is vested in the isolated sillanols that can indwell in every specimen, but the silanol nests (at 3500 cm-1) are not evident in samples S-8 and S-4, and only H-ZSM-5 has distinct terminal silanols (at 3690 cm-1). Upon combining Figure 6b, it is known that samples S-4 and S-8 have nearly no Brønsted acid (1540 cm-1), whereas the other samples have weak Brønsted acid, and furthermore, the Lewis acid and strong Lewis acid (SL) are distributed irregularly, while the weak Lewis acid (WL) amount is almost constant in all samples. This result is consistent with the relative crystallinity (RC) data (Figure 1b). In other words, the samples S-1, S-2 and S-6 have relatively low RC, which is in agreement with the SEM images (showing all samples to be well crystallized without the amorphous form), and it can be inferred that the above-mentioned three samples are rich in defects. So these three samples have a definite amount of silanol nests and possess acid sites.
To investigate the effect of initial water content addition on the catalytic performance in the MTP reaction, a series of silicalite-1 catalysts with varying initial water content ranging from 7.14% to 100% were prepared.Figure 7 displays the conversion of methanol with the time on stream (TOS) over silicalite-1 catalysts in the MTP reaction. Based on the RC-related discussion,it can be preliminary concluded that a high methanol conversion is attributed to the major defects. Besides,the samples S-4 and S-8 have less microporous surfacearea, denoting to the fewer active sites loaded, and the active sites of MTP reaction are concentrated mainly on the internal surface area. So the methanol conversion over these two samples S-4 and S-8 is not as high as other samples. Furthermore, carbon deposition can cause deactivation of the catalyst, leading to low methanol conversion in MTP reaction, and carbon is deposited on the external surface of catalyst[34-36]. Besides, the mesopores ensure an optimal accessibility and transport of reactants and products[37-38], so the methanol conversion over samples S-1 and S-2 decreases rapidly, and as the reaction proceeds, the methanol conversion over samples S-4, S-6 and S-8 gradually levels off.

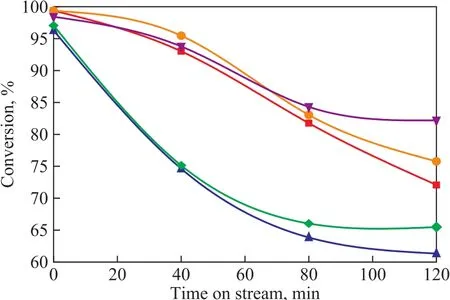
Figure 7 Methanol conversion with time on stream of thefive silicalite-1 catalysts
More meaningfully, the selectivity to light olefins (C2=–C4=), especially propylene, is greatly enhanced by the reduction of initial water content (Figure 8). Propylene selectivity decreases with different proportion of initial water content ranging from 7.14% (S-1) to 100% (S-8) within 2 h of reaction. Likewise, the ethylene and butene selectivity decreases with higher initial water adjunction. There is a general agreement that zeolite can only be accessible to molecules, the size of which is approximately equal to the pore size of the zeolite. In other words, micropore can only sieve small molecules,but mesopore or macropore allows macromolecular substance passing through[37]. Besides, it is widely believed that weak acid strength or low acid density is good to enhance the propylene selectivity[38], but the acidity of silicalite-1 is so weak and the presence of mesopores reduces the acid density of silicalite-1 catalyst.Upon combining the acid property and channel structure,it can be concluded that silicalite-1 zeolite catalysts synthesized by a range of initial water content have a great influence on product distribution. The lesser water can result in more light olefins and fewer macromolecular product, but the conversion drops quickly. However,this is nearly opposite to the zeolite catalysts, the initial water content of which is beyond 15%. Upon taking into consideration the methanol conversion and propylene selectivity, the sample S-6 (prepared at an initial water content of 71.43%) is the best fit catalyst.

Figure 8 The light olefins selectivity and P/E ratio versus TOS over silicalite-1 zeolite catalysts■—S-1; ●—S-2; ▲—S-4; ▼—S-6; ◆—S-8
4 Conclusions
The effect of initial water content on the catalytic performance of silicalite-1 zeolite catalysts in MTP reaction was studied. The main changes induced by initial water addition are the reduction of the particle size and the BET surface area, albeit with a rise of pore diameter.The S-6 catalyst prepared with an initial water content of 71.43% has the most excellent catalytic performance in the MTP reaction, while excess initial water content(>71.43%) causes lower catalytic activity because of the loss of acid density, while insufficient initial water content(<42.86%) leads to lower catalyst stability. The influence of BET surface area and intracrystalline mesopores on the catalyst used in the MTP reaction deduces that the hierarchical silicalite-1 zeolite with nano-sized intergranular accumulation is the fittest catalyst thanks to its huge BET surface area, tiny intracrystalline pores, and major intergranular pores.
[1]Grieken R V, Sotelo J L, Menéndez J M, et al.Anomalous crystallization mechanism in the synthesis of nanocrystalline ZSM-5[J]. Microporous & Mesoporous Materials, 2000, 39(1/2): 135-147
[2]Suzuki K, Kiyozumi Y, Matsuzaki K, et al. Influence of the synthesis conditions of H-ZSM-5 on its physical properties and catalytic activity in methanol conversion: The water content of the reaction mixture[J]. Applied Catalysis, 1987,35(2): 401-409
[3]Petrik L. The influence of cation, anion and water content on the rate of formation and pore size distribution of zeolite ZSM-5[J]. South African Journal of Science. 2010,105(7/8): 251-257
[4]Turov V V, Brei V V, Khomenko K N, et al.1H NMR studies of the adsorption of water on silicalite[J]. Microporous &Mesoporous Materials, 1998, 23(3/4): 189-196
[5]Bussai C, Vasenkov S, Liu H, et al. On the diffusion of water in silicalite-1: MD simulations using ab initio fitted potential and PFG NMR measurements[J]. Applied Catalysis A: General, 2002, 232(1/2): 59-66
[6]Xuan V, Armbruster U, Martin A. Micro/mesoporous zeolitic composites: Recent developments in synthesis and catalytic applications[J]. Catalysts, 2016, 6(12):183
[7]Verboekend D, Nuttens N, Locus R, et al. Synthesis,characterization, and catalytic evaluation of hierarchical faujasite zeolites: Milestones, challenges, and future directions[J]. Chem Soc Rev, 2016, 45(12): 3331-3352
[8]Inayat A, Knoke I, Spiecker E, et al. Assemblies of mesoporous FAU-type zeolite nanosheets.[J]. Angewandte Chemie, 2012, 51(8):1962-1965
[9]Verboekend D, Chabaneix A M, Thomas K, et al.Mesoporous ZSM-22 zeolite obtained by desilication:Peculiarities associated with crystal morphology and aluminium distribution[J]. Cryst Eng Comm, 2011, 13(10):3408-3416
[10]Garcíamartínez J, Johnson M, Valla J, et al. Mesostructured zeolite Y—high hydrothermal stability and superior FCC catalytic performance[J]. Catalysis Science & Technology,2012, 2(5): 987-994
[11]Chen H, Wydra J, Zhang X, et al. Hydrothermal synthesis of zeolites with three-dimensionally ordered mesoporousimprinted structure[J]. Journal of the American Chemical Society, 2011, 133(32):12390-12393
[12]Choi M, Na K, Kim J, et al. Stable single-unit-cell nanosheets of zeolite MFI as active and long-lived catalysts[J]. Nature, 2009, 461(7261): 246-249
[13]Fan W, Snyder M A, Kumar S, et al. Hierarchical nanofabrication of microporous crystals with ordered mesoporosity[J]. Nature Materials, 2008, 7(12): 984-991
[14]Lopezorozco S, Inayat A, Schwab A, et al. Zeolitic materials with hierarchical porous structures[J]. Advanced Materials, 2011, 23(22/23): 2602-2615
[15]Na K, Choi M, Ryoo R. Recent advances in the synthesis of hierarchically nanoporous zeolites[J]. Microporous &Mesoporous Materials, 2013, 166(2): 3-19
[16]Na K, Jo C, Kim J, et al. Directing zeolite structures into hierarchically nanoporous architectures.[J]. Science, 2011,333(6040): 328-32
[17]Pérezramírez J, Christensen C H, Egeblad K, et al.Hierarchical zeolites: Enhanced utilisation of microporous crystals in catalysis by advances in materials design[J].Chem Soc Rev, 2008, 37(11): 2530-2542
[18]Stöcker M. Methanol-to-hydrocarbons: Catalytic materials and their behavior 1[J]. Microporous & Mesoporous Materials, 1999, 29(1/2): 3-48
[19]Liu J, Zhang C, Shen Z, et al. Methanol to propylene:Effect of phosphorus on a high silica HZSM-5 catalyst[J].Catalysis Communications, 2009, 10(11): 1506-1509
[20]Meng X, Yu Q, Gao Y, et al. Enhanced propene ethene selectivity for methanol conversion over pure silica zeolite:Role of hydrogen-bonded silanol groups[J]. Catalysis Communications, 2015, 61: 67-71
[21]Heitmann G P, Dahlhoff G, Hölderich W F. Catalytically active sites for the Beckmann rearrangement of cyclohexanone oxime to ε-caprolactam[J]. Journal of Catalysis, 1999, 186(1): 12-19
[22]Bonelli B, Forni L, Aloise A, et al. Beckmann rearrangement reaction: About the role of defect groups in high silica zeolite catalysts[J]. Microporous & Mesoporous Materials, 2007, 101(1): 153-160
[23]Huang H W, Zhang M X, Chen C, et al. Catalytic performance of cerium modified silicalite-1 molecular sieves in the conversion of methanol to propene[J]. Journal of Fuel Chemistry & Technology, 2016, 44(12): 1485-1493[24]Ishii R, Kiyozumi Y, Mizukami F. Re-crystallization of silicalite-1 crystals from a crystalline layered silicate using nanosized seeds[J]. Materials Letters, 2008, 62(19): 3465-3467
[25]Chen L, Wang Y M, He M Y. Hydrothermal synthesis of hierarchical titanium silicalite-1 using single template[J].Materials Research Bulletin, 2011, 46(5): 698-701
[26]Fernandez C, Stan I, Gilson J P, et al. Hierarchical ZSM-5 zeolites in shape-selective xylene isomerization: role of mesoporosity and acid site speciation[J]. Chemistry(Weinheim an der Bergstrasse, Germany), 2010, 16(21):6224-6233
[27]Colombo F, Alberti G D. Conversion of linear butenes to propylene on H-ZSM-5 zeolites: Effect of reaction parameters and zeolite morphology on catalytic activity[J].Studies in Surface Science & Catalysis, 1984, 18: 233-240[28]Firoozi M, Baghalha M, Asadi M. The effect of micro and nano particle sizes of H-ZSM-5 on the selectivity of MTP reaction[J]. Catalysis Communications, 2009, 10(12):1582-1585
[29]Astorino E, Peri J B, Willey R J, et al. Spectroscopic characterization of silicalite-1 and titanium silicalite-1[J].Journal of Catalysis, 1995, 157(2): 482-500
[30]Vikulov K, Martra G, Coluccia S, et al. FTIR spectroscopic investigation of the active sites on different types of silica catalysts for methane partial oxidation to formaldehyde[J].Catalysis Letters, 1996, 37(3): 235-239
[31]Wovchko E A, Camp J C, Jr. Glass J A, et al. Active sites on SiO2: Role in CH3OH decomposition[J]. Langmuir,1995, 11(7): 2592-2599
[32]Bolis V, Busco C, Bordiga S, et al. Calorimetric and IR spectroscopic study of the interaction of NH3with variously prepared defective silicalites: Comparison with ab initio computational data[J]. Applied Surface Science,2002, 196(1–4): 56-70
[33]Bordiga S, Ugliengo P, Damin A, et al. Hydroxyls nests in defective silicalites and strained structures derived upon dehydroxylation: Vibrational properties and theoretical modelling[J]. Topics in Catalysis, 2001, 15(1): 43-52
[34]Kim J, Choi M, Ryoo R. Effect of mesoporosity against the deactivation of MFI zeolite catalyst during the methanolto-hydrocarbon conversion process[J]. Journal of Catalysis,2010, 269(1): 219-228
[35]Bjørgen M, Svelle S, Joensen F, et al. Conversion of methanol to hydrocarbons over zeolite H-ZSM-5: On the origin of the ole fi nic species[J]. Journal of Catalysis, 2007,249(2): 195-207
[36]Gilson J P, Derouane E G. On the external and intracrystalline surface catalytic activity of pentasil zeolites[J]. Journal of Catalysis, 1984, 88(2): 538-541
[37]Janssen A H. Generation, characterization, and impact of mesopores in zeolite catalysts[J]. Catalysis Reviews, 2003,45(2): 297-319
[38]Schneider D M C, Burgfels D G C, Buchold H C. Process for preparing lower olefins: EP, EP0448000[P]. 1994-05-25
杂志排行
中国炼油与石油化工的其它文章
- Preparation of Novel Dechlorination Adsorbent and Study on Its Adsorption Mechanism
- Research Progress in Catalytic Cracking Reaction of Tetralin and Decalin
- Preparation of AgCeY Zeolite Using Microwave Irradiation and Its Adsorptive Desulfurization Performance
- The Function of β Zeolite for Enhancing the Propylene Yield in FCC Process
- Synthesis and Tribological Properties of Graphene-Copper Nanoparticle Composites as Lithium Grease Additive
- Study on Catalytic Alkylation of Benzene with Methanol over ZSM-22 and ZSM-35
