烷氧基桥联四香豆素的简便合成
2018-01-16姚荣
姚 荣
(扬州大学 化学化工学院, 江苏 扬州 225002)
香豆素是广泛存在于自然界中的内酯类化合物,在对人体具有抗高血压、抗凝血等药理作用和一定的生理毒性[1-3]。
4-羟基香豆素类衍生物是很多合成工业产品的中间体[4]。因其具有非线性的光学特性,是很好的荧光增白剂、激光染料、荧光探针及非线性光学材料[5]。这些应用都引起了人们的极大兴趣[6-7]。
1 实验仪器
红外光谱分析:Bruker Tensor27红外光谱仪,KBr压片法,检测范围为4000~400cm-1;1H-NMR分析:Bruker AV600型核磁共振仪,CDCl3或DMSO-d6为溶剂,TMS为内标; LWMC-205型可调功率微波反应器(南京陵江科技开发公司);X-4显微熔点测定仪(北京泰克公司),数据未经校正。
2 实验试剂
所有试剂均为分析纯试剂。2,2'-烷氧基双苯甲醛参照文献[8-9]方法合成。
3 烷氧基桥联双香豆素的合成
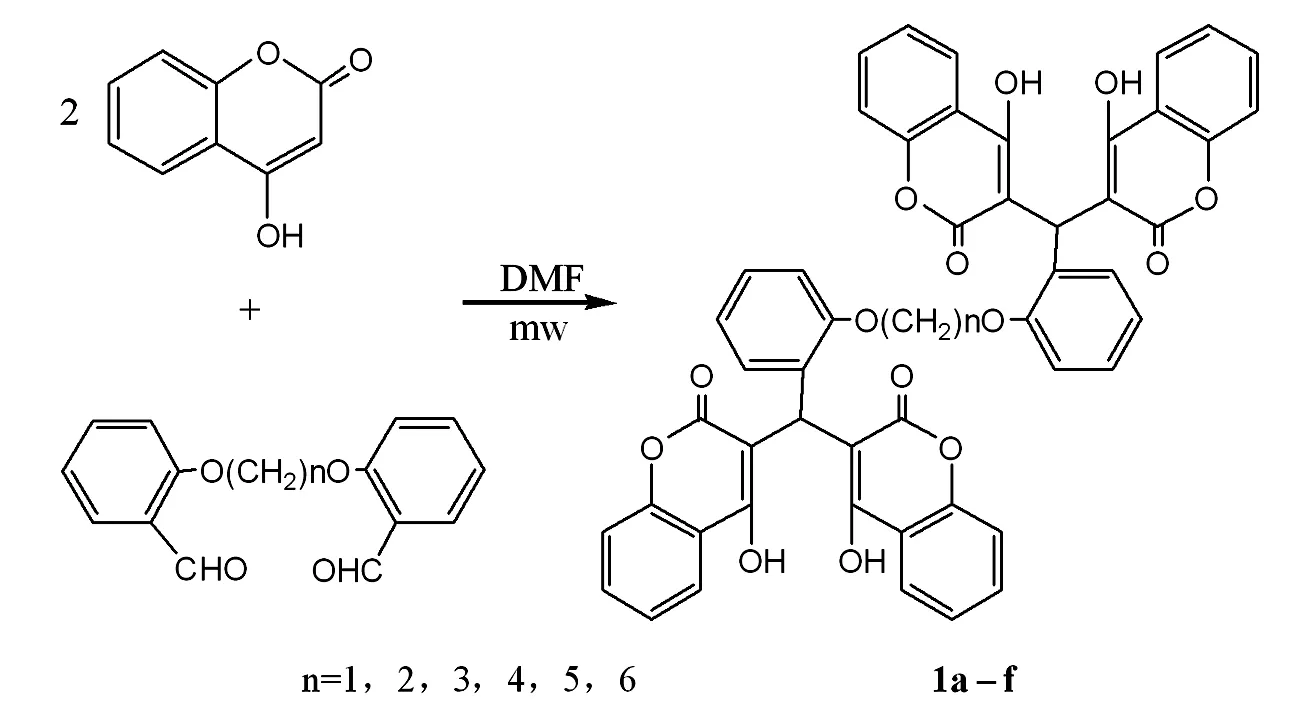
取1 mmol双醛, 与4 mmol 4-羟基香豆素于100 mL的烧瓶中,加入 4 mL DMF,稍加热使固体溶解,放入微波炉中,功率打到0.5 kW,每10 s作为一个加热循环,总加热时间控制在10 min左右。加热过程中可以补入少量溶剂防止溶剂蒸干烤焦产品。反应结束后在烧瓶中加 20~30 mL水,析出白色固体 (若固体较少可加入NaCl进行盐析)。抽滤出固体并晾干,最后用氯仿-乙醇重结晶。得到对应白色固体1a-f。
1a, 产率18%,m.p.234~237℃,IR (KBr,νcm-1):3002 (w),1698 (vs),1611 (vs),1567(s),1489 (m),1452(w),1308(s),1218 (m),1159(m),1105(m),1066 (m),902(m),805(vs);1H NMR (CDCl3, 600 MHz)δ:11.33(s,4H,OH),8.00(d,J=7.8 Hz,4H,ArH),7.59(t,J=7.2 Hz,4H,ArH),7.36~7.39(m,8H,ArH),7.29~7.32(m,4H,ArH),7.20(d,J=7.8 Hz,2H,ArH),7.12~7.15(br,2H,ArH),5.37(s,2H,OCH2);MS(m/s,%):336.13(63), 608.60(48),868.60(100),707.13(21)。
1b, 产率30%, m.p.235~233℃,IR (KBr, ν cm-1) : 2933 (w), 2724 (w), 2598 (w),1659 (vs), 1609 (s),1563 (s),1491 (m), 1448 (m),1348 (m), 1238(m), 1114 (w), 1054 (w), 755 (vs);1H NMR (CDCl3,600 MHz)δ: 11.54 (s, 2H, OH), 11.16 (s, 2H,OH),8.00(br,4H,ArH),7.50(br, 4H, ArH),7.38(br,8H,ArH),7.32(br,4H,ArH), 6.94 (br, 2H, ArH),6.84 (m,2H,ArH),6.03 (s, 2H, CH), 3.91 (br, 4H, OCH2);MS (m/s, %) : 335.80 (33),622.67(100),721.6(17),792.6(17),881.40 (15)。
1c, 产率35%, m.p. 220~224℃,IR (KBr, νcm-1) :3001 (w), 1608 (s), 1643 (s),1612 (s),1567(m),1489 (m),1462 (m),1391 (w),1342 (m), 1218 (m), 1160 (m),1067 (m), 755(vs);1H NMR (CDCl3,600 MHz)δ:11.73 (s, 2H, OH),10.87 (s, 2H, OH),7.99(m,4H,ArH),7.60(d,J=7.2 Hz, 2H, ArH),7.37(m,8H, ArH),7.25 (d, J=7.8 Hz, 2H, ArH), 7.21 (t, J=7.8 Hz, 2H, ArH), 6.93 (t, J=7.8 Hz, 2H, ArH), 6.45 (d, J=7.8 Hz, 2H, ArH), 5.97 (s, 2H, CH), 3.44 (br, 4H, OCH2),1.25 (br, 2H,CH2); MS (m/s, %) : 336.13 (90),636.53 (100),895.73 (63)。
1d, 产率38%, m.p. 240~243℃,IR (KBr, ν cm-1) : 3553 (s), 3478 (s), 3415 (vs), 3237 (w), 1660 (vs), 1615 (vs), 1504 (s), 1493 (m),1450 (m), 1347 (w),1308 (w), 1241 (w),1112 (w),1047 (w), 757 (s);1H NMR (CDCl3,600 MHz)δ: 11.57 (s, 2H, OH),10.94 (s, 2H, OH), 8.00 (br, 4H, ArH), 7.57 (br, 4H, ArH), 7.38 (br, 8H, ArH), 7.26 (br, 4H, ArH), 6.94 (br, 2H, ArH), 6.84 (m, 2H, ArH), 6.02(s,2H,CH),3.85(br,2H,OCH2), 3.24 (br,2H,OCH2),0.91 (br, 4H,CH2);MS(m/s,%):336.20(100),650.67 (70),821.6 (35), 910.33 (36)。
1e, 产率36%, m.p. 178~180℃,IR (KBr,ν cm-1) : 3553 (w), 3411 (w), 2890 (w), 1669 (s),160 (m), 1563 (m), 1495 (w), 1451 (w), 1347 (w), 1247 (w), 950 (w), 757 (s);1H NMR (CDCl3, 600 MHz)δ: 11.62 (br,2H,OH),10.84 (br,2H,OH),7.99 (d,J=7.8 Hz, 4H, ArH),7.57(br,4H,ArH),7.34~7.38 (m,8H,ArH), 7.28 (t, J=7.2 Hz, 4H, ArH),6.94(t,J=7.2 Hz,2H,ArH),6.76(d,J=7.8 Hz,2H,ArH),6.04(s,2H,CH),3.48(br, 4H, OCH2), 0.85(br,4H,CH2), 0.79(br,2H,CH2);MS(m/s,%):336.27(24),664.73(100),924.60 (42)。
1f, 产率32%, m.p. 234~236℃,IR (KBr,ν cm-1) : 3072 (w), 2939 (w), 2866 (w),1659 (vs),1609 (s),1564(s),1494(m),1451 (m),1347(w),1303 (w),1247(w),1092 (m),1303(w),1247(w),1092(m),1015(w),954(w),757(vs),1H NMR (CDCl3,600 MHz)δ:11.61(br,2H,OH),10.87(br,2H,OH),7.99(d,J=7.8 Hz,4H,ArH),7.59(br, 4H, ArH), 7.34~7.39(m,8H,ArH),7.28(t,J=13.8Hz, 4H, ArH),6.93(t,J=13.8Hz,2H,ArH),6.83(d,J=13.8Hz,2H,ArH),6.07(s,2H,CH),3.65(br,4H,OCH2),0.97(br,4H,CH2),0.70(br,4H,CH2);MS(m/s,%):335.73(33),678.73(67),938.33 (100)。
4 结果讨论
采用微波加热的方式,烷氧基桥联芳醛和4-羟基香豆素的缩合反应可以很快的完成,生成了烷氧基桥联的四香豆素衍生物。产物的结构通过IR, NMR等方法鉴定。在1H NMR中,原料中醛基氢峰已经基本消失,目标产物中羟基在11.50、11.30 ppm处,这是由于氢键的作用使得其化学位移向低场移动的结果。芳环氢集中在8.06~7.03 ppm处,而联接两个4-羟基香豆素环的桥上CH 次甲基的特征化学位移受苯环、双键和酯基的影响,在6.04 ppm左右,向低场移动较大。
[1] Ji S J,Zhou M F,Gu D G,et al. Efficient synthesis of bis(indolyl)methanes catalyzed by lewis acids in ionic liquids[J].Synlett, 2003, 35(10):2077-2079.
[2] Reddy A V, Ravinder K, Niranjan V L, et al. Zeolite catalyzed synthesis of bis (indolyl) methanes[J].Synthetic Communications, 2003, 33(21): 3687-3694.
[2] Bandgar B P, Shaikh K A. Organic reactions in aqueous media: InF3 catalysed synthesis of bis(indolyl)methanes in water under mild conditions[J].Journal of Chemical Research,2004, 1:34-36.
[4] Zolfigol M A, Salehi P, Shiri M. An efficient procedure for the preparation of mono, and di-bis-indolyl methanes catalyzed by molybdatophosphoric acid[J]. Phosphorus, Sulfur, and Silicon and the Related Elements[J]. Phosphorus, Sulfur and Silicon & the Related Elements, 2004, 179(11), 2273-2277.
[5] Koba Yashi S. In Lewis acid organic synthesis[M].Wiley-VCH: Weinheim, 2008: 883-907.
[6] Garbe T R, Kobayashi M, Shimizu N, et al. Indolyl carboxylic acids by condensation of indoles with α-Keto acids[J]. Journal of Natural Products, 2000, 63(5):596-598.
[7] Ramesh C, Banerje J, Pal R,et al. Silica supported sodium hydrogen sulfate and amberlyst-15: two efficient heterogeneous catalysts for facile synthesis of bis- and tris(1H-indol-3-yl)methanes from Indoles and carbonyl compounds[J]. Advanced Synthesis & Catalysis, 2003, 345(5): 557-559.
[8] Heravi M M, Baghernejad B, Hossein A, et al. A novel and facile synthesis of 2-(cyclohexylamino)-6,7-dihydro-3-aryl-1H-indole-4(5H)-ones via a one-pot multi-component reaction[J]. Tetrahedron Letters, 2008, 49:6101-6103.
[9] Ke B,Qin Y, He Q F,et al. Preparation of bisindolylalkanes from N-tert-butanesulfinyl aldimines[J]. Tetrahedron Letters, 2005, 46:1751-1753.
[10] 孙 晶,朱美军,杨息琴,等,4,4′-二甲酰基-α,ω-二苯氧基烷烃的微波干法催化合成[J].扬州大学学报 (自然科学版), 2004, 7(4):18-20.
