Topiramate as a neuroprotective agent in a rat model of spinal cord injury
2018-01-05FiratNarinSahinHanaliogluHuseyinUstunKamerKilincBurcakBilginer
Firat Narin, Sahin Hanalioglu,, Huseyin Ustun, Kamer Kilinc, Burcak Bilginer
1 Department of Neurosurgery, Hacettepe University Faculty of Medicine, Ankara, Turkey
2 Department of Pathology, Ankara Training and Research Hospital, Ankara, Turkey
3 Department of Biochemistry, TOBB University of Economics and Technology Faculty of Medicine, Ankara, Turkey
Topiramate as a neuroprotective agent in a rat model of spinal cord injury
Firat Narin1, Sahin Hanalioglu1,*, Huseyin Ustun2, Kamer Kilinc3, Burcak Bilginer1
1 Department of Neurosurgery, Hacettepe University Faculty of Medicine, Ankara, Turkey
2 Department of Pathology, Ankara Training and Research Hospital, Ankara, Turkey
3 Department of Biochemistry, TOBB University of Economics and Technology Faculty of Medicine, Ankara, Turkey
Topiramate (TPM) is a widely used antiepileptic and antimigraine agent which has been shown to exert neuroprotective effects in various experimental traumatic brain injury and stroke models. However, its utility in spinal cord injury has not been studied extensively. Thus, we evaluated effects of TPM on secondary cellular injury mechanisms in an experimental rat model of traumatic spinal cord injury (SCI). After rat models of thoracic contusive SCI were established by free weight-drop method, TPM (40 mg/kg) was given at 12-hour intervals for four times orally. Post TPM treatment, malondialdehyde and protein carbonyl levels were signi ficantly reduced and reduced glutathione levels were increased, while immunoreactivity for endothelial nitric oxide synthase, inducible nitric oxide synthase, and apoptotic peptidase activating factor 1 was diminished in SCI rats. In addition, TPM treatment improved the functional recovery of SCI rats.This study suggests that administration of TPM exerts neuroprotective effects on SCI.
nerve regeneration; spinal cord injury; topiramate; neuroprotection; oxidative damage; nitric oxide; motor function; neural regeneration
Introduction
Spinal cord injury (SCI), complete or partial, is a significant public health problem because of its consequences due to loss of motor, sensory, autonomic, and re flex functions of the spinal cord. SCI is thought to occurviatwo mechanisms: a primary mechanical injury and a secondary injury in which one or multiple mechanisms, triggered by the primary injury, are involved(Azbill et al., 1997; Dumont et al., 2001). Currently, there are no treatment options, except prevention, to manage destruction and primary injury following SCI. Therefore, most experimental and clinical studies on SCIs aim to prevent secondary injury.Major mechanisms mediating secondary injury include, but are not limited to, ischemia, glutamate excitotoxicity, inflammation, free oxygen radicals, apoptosis, cytoskeletal degradation,and demyelination (Azbill et al., 1997; Lu et al., 2000; Dumont et al., 2001; Park et al., 2004). Notably, following spinal cord trauma, excessive amounts of glutamate are released, and neural and glial cells are lost via excitotoxic and other secondary mechanisms (Wrathall et al., 1996; Park et al., 2004).
Topiramate (TPM) is an antiepileptic drug that has a wide variety of applications in clinical practice (Dinoff et al., 2003;Shank and Maryanoff, 2008). TPM inhibits voltage-gated Na+ channels and selectively antagonizes glutamate receptor subtypes kainate and α-amino-3-hydroxy-5-methyl isoxazole propionate (AMPA) receptors (Dinoff et al., 2003; Gensel et al., 2013). Additionally, it weakly inhibits the carbonic anhydrase (CA) (Dodgson et al., 2000). Its bene ficial effects in neurological recovery have been demonstrated, albeit without signi ficant anatomical/histopathological changes, in two experimental models of traumatic brain injury (TBI) (Hoover et al., 2004; Kouzounias et al., 2011). In some experimental stroke studies, TPM decreased the infarct volume and showed antioxidant effects. Moreover, it is considered to be protective against neurological damage following severe ischemia/hypoxia in neonates (Shank et al., 2000). Although TPM is well-tolerated and bene ficial in reducing post-injury pain in patients with SCI (Dinoff et al., 2003), its use in SCI has not been extensively studied. Only one study in the literature reported neuroprotective and oligodendroglia-sparing effects of TPM in a model of unilateral cervical SCI (Gensel et al.,2013). In the present study, we aimed to investigate antioxidant, antiapoptotic, neuroprotective, and behavioral effects of TPM in an experimental rat model of thoracic contusive SCI.
Materials and Methods
Animals
Experimental protocols performed in this study were approved by Hacettepe University Animal Research Ethics Committee with the approval number 2009/15-2 and were in compliance with the European Union (EU) directive(2010/63/EU) on the protection of animals used for scientific purposes. Forty 6–8-week-old male Wistar rats weighing 150–200g were used. Animals were housed in Hacettepe University Experimental Animal Facility at room temperature under a 12-hour dark/light cycle and permitted food and waterad libitum.
The rats were randomly divided into four groups with 10 rats in each group. Rats in sham-operated group underwent only laminectomy. Rats in SCI only group underwent laminectomy and SCI induction but did not receive any treatment. Rats in the TPM group were administered 40 mg/kg TPM (TOPAMAX®,Janssen Pharmaceuticals, Raritan, NJ, USA) (2 mL, dissolved in saline) intraperitoneally 30 minutes after laminectomy and SCI induction and via oral gavage (2 mL, dissolved in saline)at 12, 24, 36, and 48 hours after the procedure. The TPM dosage regimen was selected based on previous studies with an experimental rat model of TBI (Hoover et al., 2004; Kouzounias et al., 2011). Rats in the SCI + vehicle group were administered equivalent volumes (2 mL) of 0.9% isotonic saline solution intraperitoneally 30 minutes after injury andviaoral gavage at 12, 24, 36, and 48 hours after injury.
Furthermore, five rats in each group were randomly selected and sacri ficed 24 hours after SCI (the remaining five rats in each group were followed for 4 weeks before sacri fice). Under general anesthesia by intraperitoneal injection of 60 mg/kg ketamine hydrochloride (Ketalar 5% solution, Eczacıbaşıİlaç Sanayi under Parke-Davis license; Istanbul, Turkey) and 8 mg/kg xylazine (Rompun 2% solution; Bayer, Istanbul, Turkey), rats underwent intracardiac perfusion of saline. Additionally, 1-cm-long spinal cord segments encompassing the injury site (approximately the T9level) were harvested under microscopic guidance. These segments were divided into two pieces (at the level of the trauma epicenter) for biochemical and immunohistochemical studies. The remaining rats were followed up for 4 weeks and were evaluated for their behavioral/functional impairment using motor function scores(MFS) and inclined plane scores (IPS) at the end of that period. Following functional evaluation, those rats (n= 5 rats/group) were similarly sacrificed and1-cm-long spinal cord segments encompassing the injury site (approximately the T9level) were harvested under microscopic guidance and embedded in paraffin to be used for histopathological analysis and lesion area measurements.
Surgical procedure
All animals were anesthetized by intraperitoneal injection of 60 mg/kg ketamine hydrochloride and 8 mg/kg xylazine. Following anesthesia, all rats were placed in the prone position and skin incision and paravertebral muscle dissection were performed at the T5–12and T7–11vertebral levels, respectively.The dura was exposed following microscope-assisted laminectomy at the T8–10vertebral levels.
A weight-drop model, modified from Allen’s method(Koozekanani et al., 1976), was used to induce traumatic SCI through a custom-made device. Force was applied by dropping a 5 g weight of spherical lead through a 10-cm guide tube, which was positioned perpendicular to the exposed spinal cord. Immediately after trauma, the muscles and skin incision were sutured using 3-0 silk sutures. The rats were returned to a temperature- and humidity-controlled chamber.Manual expression was performed thrice per day, until re flex emptying of the urinary bladder was observed.
Histopathological evaluation using hematoxylin and eosin
At the end of 4 weeks, five rats from each group were deeply anesthetized and 4% paraformaldehyde was perfused intracardially. Spinal cord segments around the injury site (T9 level) were removed under the microscope (Zeiss OPMI99,Oberkochen, Germany) and left in 10% formaldehyde for 1 week. For pathological evaluation, sections were cut (5 μm thick) from formalin-fixed and paraffin-embedded spinal cord segments. They were stained with hematoxylin and eosin. The slides were viewed under light microscopy to examine structural changes. Lesion size and total spinal cord cross-sectional area were measured at the lesion epicenter level. The proportion of the lesion size to the total area of spinal cord on each section was recorded as a percentage. Three sections with the largest lesion area were evaluated and percentages were averaged for each animal.
Biochemical analysis
For biochemical analyses, five rats from each group were sacrificed 24 hours after SCI. The harvested samples were stored at−20°C for further biochemical analysis,i.e., lipid peroxidation(MDA), and determination of protein carbonyl (PC) and reduced glutathione (GSH) levels.
To analyze lipid peroxidation, malondialdehyde (MDA)level was measured, as described previously (Miharaand Uchiyama, 1978). After weight measurement, frozen tissue samples were homogenized in 1:10 (w:v) potassium phosphate buffer(50 mM, pH 7.4) using a Dounce homogenizer. The homogenate (0.5 mL) was mixed with 3 mL 1% phosphoric acid and 1 mL 0.67% thiobarbituric acid (TBA) was added. Tubes were placed in boiling water for 45 minutes. After cooling the tubes,TBA-reactive substances (TBARS) were extracted into n-butanol and the absorbance was measured at 532 nm. The molar absorptivity of the TBA–MDA complex was taken as 1.56 ×105/M/cm; thus, tissue lipid peroxide levels (as TBARS) were calculated as nanomole per gram wet tissue (nmol/g).
To evaluate the oxidative damage, the tissue PC level was measured, as described previously (Levine et al., 1990) using assay kits (Cayman Chemical Company, Ann Arbor, MI,USA). Proteins were precipitated by adding 20% trichloroacetic acid and were redissolved in dinitrophenylhydrazine,and the absorbance was read at 370 nm. The PC level was expressed as nanomole per milligram tissue (nmol/mg).
Reduced glutathione (GSH) levels were calculated using assay kits (Cayman Chemical Company) by the Ellman method(Ellman, 1959). GSH is reacted with 5,5′-dithiobis (2-nitrobenzoic acid) resulting in the formation of a product that has absorbance at 410 nm. Results were expressed as nanomole per gram tissue (nmol/g).
Immunohistochemical evaluation
Five rats in each group were sacri ficed 24 hours after SCI, and spinal cord segments at the T9level and below were harvested. Sections were cut (5 μm thick) from formalin-fixed and paraffin-embedded spinal cord segments and were prepared for immunohistochemical staining. Moreover, a few slices were stained with hematoxylin and eosin and were evaluated to con firm SCI (Figure 1A). All sections were applied to poly-L-lysine-coated slides and were dewaxed in xylene. Sections were washed in decreasing series of ethanol and water.Cold hydrogen peroxide (3%) in distilled water was applied to block endogenous peroxidases for 30 minutes. Furthermore,the sections were washed with phosphate buffered saline(PBS) and incubated at 4°C with an endothelial nitric oxide synthase (eNOS) rabbit polyclonal antibody (Lab Vision Corp.,
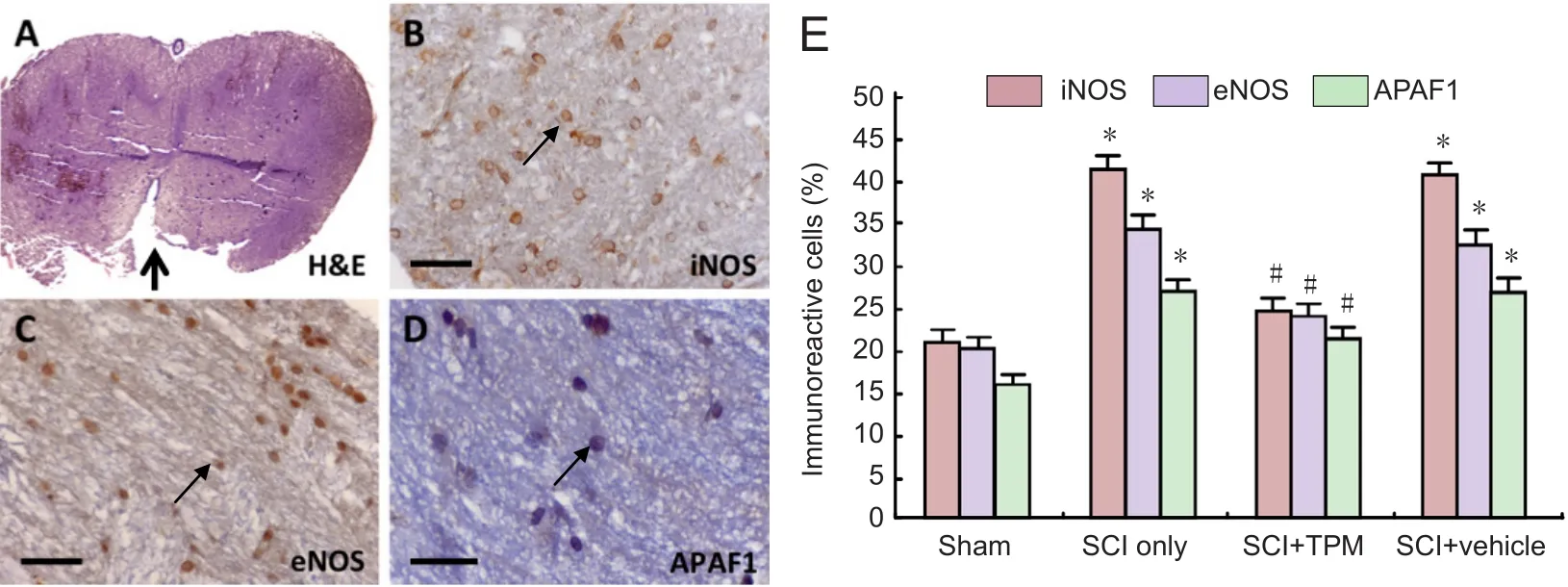
Figure 1 Representative microscopic images of spinal cord sections (A–D) and quanti fication of iNOS, eNOS, and APAF-1 immunoreactivity(E) in experimental groups at 24 hours following SCI.

Table 1 Motor function score (MFS) proposed by Farooque et al. (1999)
Fremont, CA, USA), inducible NOS (iNOS) rabbit polyclonal antibody (Lab Vision Corp., Fremont, CA, USA), or apoptotic peptidase activating factor 1 (APAF-1) epitope specific rabbit antibody (Lab Vision Corp., Fremont, CA, USA), at a dilution of 1/200 in PBS for NOS antibody and at 1/100 for APAF-1 antibody, for 16 hours at 4°C. Moreover, the sections were incubated with biotinylated goat anti-rabbit IgG secondary antibodies (Thermo Fisher Scienti fic, Waltham, MA, USA), washed with PBS thrice, and incubated with an avidin-biotin-peroxidase complex and were then visualized using a chromogenic reaction with diaminobenzidine (DAB). Slides were examined under the microscope (Leica, DM2000) by an experienced histopathologist who was blinded to the study groups (Figure 1B–D). For each animal, three sections around the lesion epicenter were evaluated. For each section, five random regions (including gray and white matter) were inspected with 40× magni fication.The percentage of positively immunolabeled cells over total cells in each region was determined. Then, average percentage for five regions in each section was calculated. This calculation was performed for three tissue sections per animal, and the overall average percentage was calculated for each animal.
Behavioral evaluation
We assessed behavioral changes in rats with the hind limb motor function score (MFS) and inclined plane score (IPS)at 4 weeks post-injury. MFS was measured, as described previously (Farooque et al., 1999). A description of MFS is given in Table 1. The rats were observed for 1 minute while moving on a horizontal plane of 0.7 × 0.9 m2. The hip, knee, and ankle joint movements were recorded.
We also used IPS (Rivlin and Tator, 1977) to evaluate behavioral changes in the rats. The highest angle at which a rat can support its weight for 5 seconds on an inclined plane(30 × 60 cm2) measured at 0°–90° was recorded. MFS and IPS were evaluated by a neurosurgeon blinded to the study groups and results were compared between groups.
Statistical analysis
Data were analyzed using SPSS 15.0 for Windows (SPSS, Chicago, IL, USA). Results were expressed as the mean ± SE. For statistical comparisons between groups, the Kruskal-Wallis test was used, and for dual comparison, the Mann-WhitneyUtest was used. APvalue of < 0.05 was considered statistically signi ficant.
Results
Oxidative stress
A comparison of biochemical findings among all experimental groups at 24 hours after SCI is illustrated in Figure 2.MDA levels were signi ficantly increased in the SCI only and SCI + vehicle groups than in the sham-operated group (allP< 0.01). MDA levels in the SCI + TPM group were signi ficantly reduced compared with those in the SCI + vehicle group (P= 0.005).
In the SCI + TPM group, MDA levels were significantly reduced compared with those in the SCI + vehicle group (P= 0.005). Nevertheless, there was still statistically signi ficant difference between sham-operated and SCI + TPM groups (P< 0.001).
In the SCI and SCI + vehicle groups, PC levels were signi ficantly increased compared with those in the sham-operated group (P< 0.001). PC levels were signi ficantly reduced in the SCI + TPM group than in the SCI + vehicle group (P= 0.04).Despite this reduction, there was still a statistically signi ficant difference between sham-operated and SCI + TPM groups (P= 0.01). Reduced GSH levels were signi ficantly diminished in the SCI only and SCI + vehicle groups than in the sham-operated group (P< 0.001). Reduced GSH levels in the SCI +TPM group were signi ficantly elevated compared with those in the SCI + vehicle group (P= 0.005). Despite this increase,there was still a statistically signi ficant difference between sham-operated and SCI + TPM groups (P< 0.001).
Histopathological and immunohistochemical changes
At 4 weeks post-injury, histopathological examination showed that necrosis, infiltration of inflammatory cells, demyelination, and vacuolation were observed in the SCI only group.These findings were less pronounced in the SCI + TPM group than in the SCI and SCI + vehicle groups. The ratio of the lesion area to the whole spinal cord section area was 36.2 ± 9.4%,22.4 ± 8.4%, and 35.4 ± 8.1% in the SCI only, SCI + TPM, and SCI + vehicle groups, respectively. The lesion area was smaller in the SCI + TPM group than that in the SCI only and SCI +vehicle groups, although the difference did not reach statistical signi ficance (P= 0.075 andP= 0.12, respectively; Figure 3). At 24 hours post-injury, iNOS, eNOS, and APAF-1 immunoreactivities were signi ficantly different among the groups (P= 0.001). iNOS, eNOS, and APAF-1 immunoreactivities were significantly lower in the SCI + TPM group than in the SCI only and SCI + vehicle groups (allP= 0.001); however, iNOS,eNOS, and APAF-1 immunoreactivities in the SCI+ TPM group were signi ficantly greater than those in the sham-operated group (allP= 0.001; Figure 1E).
Behavioral changes
The highest MFS and IPS values were detected in the sham-operated group (P= 0.01). MFS and IPS values in the SCI + TPM group were signi ficantly higher than those in the SCI only and SCI+ vehicle groups (bothP= 0.001); however,there was also a statistically signi ficant difference between sham-operated and SCI + TPM groups (P= 0.001; Figure 4).
Discussion
SCI is a major public health problem resulting in motor,sensory, and autonomic dysfunction. The pathophysiology of SCI is complex and includes primary and secondary injury mechanisms. The severity of the primary injury varies with the amplitude and duration of the trauma energy and it is almost impossible to intervene with this process. Secondary injury is induced by primary mechanical insult and occurs because of a cascade of various pathophysiologic processes within days following the initial trauma (Dumont et al., 2001). These processes include in flammation, ischemia, edema, increased glutamate levels, free oxygen radicals and cell membrane damage, lipid peroxidation, vascular mediators and nitric oxide (NO) production, and activation of proapoptotic mediators (Azbill et al., 1997; Lu et al., 2000; Dumont et al., 2001; Park et al., 2004).Necrosis and apoptosis cause neuronal loss, destruction of the axon-myelin structure, and finally loss of function (Schwartz and Osborne, 1993).
We showed that TPM, an AMPA receptor inhibitor and widely used antiepileptic medication, provides effective biochemical, histological, and functional protection against SCI when delivered within 30 minutes after injury. To the best of our knowledge, this is the second study con firming the neuroprotective effects of TPM in SCI. In a previous report, in a model of cervical spinal cord contusion injury, Gensel et al.(2012) showed that TPM, if delivered 15 minutes after SCI,increases tissue sparing and preserves oligodendrocytes and neurons at 48 hours post-injury. They also showed that TPM was superior to NBQX, a well-documented neuroprotective AMPA receptor antagonist, in the protection of neurons and oligodendrocytes. The present study supports and adds new dimensions to this former study (Gensel et al., 2012). Initially, we showed that TPM also provides protection against oxidative damage after SCI as re flected by reduced lipid peroxidation and protein carbonylation and increased reduced GSH levels. Oxidative stress is a hallmark of secondary injury after spinal trauma (Azbill et al., 1997; Dumont et al., 2001;Vaziri et al., 2004; Jia et al., 2012). Thus, alleviating oxidative stress is considered as an effective therapeutic strategy for SCI. Two agents methylprednisolone and 21-aminosteroid tirilazad possess signi ficant antioxidant activities and improve recovery of patients with SCI in clinical trials, whereas many others, including carotenoids and phenolic compounds, are also protective in experimental studies (Bilginer et al., 2009; Jia et al., 2012). Protection against oxidative damage by TPM, as shown in animal models of epilepsy, might be directly due to antioxidant properties and indirectly due to other mechanisms of action, taking into account the complex nature of pathophysiological processes involved in SCI (Cárdenas-Rodríguez et al., 2013; Naziroglu and Yürekli, 2013).
NO has a major role in secondary injury (Dawson et al.,1993; Estevez et al., 1998; Conti et al., 2007; Garry et al., 2015).Nitric oxide synthase (NOS) has three subtypes: neuronal NOS(nNOS), endothelial NOS (eNOS), and finally inducible NOS(iNOS) found in macrophages and glia. It has been demonstrated that NO can exert both protective and detrimental effects in SCI depending on several factors, such as level of produced NO, activity of different synthase isoforms, cellular source of production, and time of release (Satake et al., 2000;Dumont et al., 2001; Vaziri et al., 2004; Conti et al., 2007; Yang et al., 2007). High NO levels produced by upregulated nNOS and iNOS, several hours to days after the trauma, were found to be neurotoxic (Conti et al., 2007). Previous studies suggested that eNOS and iNOS have opposing roles in the pathophysiology of SCI. The expression of NOS subtypes increases during the initial days after spinal cord trauma (Vaziri et al.,2004; Conti et al., 2007; Yang et al., 2007). eNOS is known as a potent vasodilatator exhibiting neuroprotective effects after cerebral ischemia. Additionally, apoptosis is reduced by NO produced by eNOS (Estevez et al., 1998). Phosphorylation of nNOS might have an important role in regeneration because of increased blood flow at the lesion site (Osuka et al., 2007).Effects of iNOS on in flammation and apoptosis after SCI have been reported previously (Satake et al., 2000). Mediators, such as tumor necrosis factor α (TNF-α) and interleukin 1β (IL-1β),secreted during SCI increase the expression of iNOS and result in the upregulation of iNOS in macrophages and astrocytes(Dawson et al., 1993; Satake et al., 2000). High iNOS levels inhibit mitochondrial functions and stimulate apoptosis and excitotoxic neuronal death (Satake et al., 2000; Wu et al., 2007).In our study, we have found that following the trauma, eNOS and iNOS levels were increased at the lesion site, and they were diminished after administration of TPM. This finding shows that these two enzymes may together have roles in the pathophysiology of SCI. Further studies are needed to identify mechanisms by which TPM lowers NOS levels and whether this decrease indeed translates into a protective effect. We also found that the activity of APAF-1, an apoptotic marker (Shakeri et al., 2017), was lower in the SCI + TPM group than in SCI only and SCI + vehicle groups. This finding also suggested that TPM may directly or indirectly inhibit apoptosis.
For evaluation of the functional outcome after SCI, MFS and IPS were used as a measurement of neurological recovery. In our study, functional recovery was found in SCI rats following TPM treatment.
Being a broad spectrum anticonvulsant and having multi-mechanistic properties (Shank and Maryanoff, 2008),TPM might well be a promising therapy for treating SCI. In addition to antagonizing glutamate receptors, it enhances the effects of the inhibitory neurotransmitter (i.e., GABA), reduces the activity of voltage-gated sodium and calcium channels, and blocks the in flux of calcium into cells (Shank et al., 2000; Dinoff et al., 2003; Shank and Maryanoff, 2008).
Unlike other glutamate receptor antagonists that have failed clinical trials because of undesired side effects (Walters et al.,2005; Chen and Lipton, 2006), TPM is available clinically for migraine and epileptic seizure treatment and is well-tolerated by individuals with SCI (Shank et al., 2000; Dinoff et al., 2003;Shank and Maryanoff, 2008). Because it is clinically used to reduce pain related to SCI, systemic administration of TPM may prove effective for acute phase SCI treatment as well.
To conclude, in this study, biochemical, immunohistochemical, and neurobehavioral findings support the neuroprotective role of TPM in SCI. We found that TPM treatment diminished oxidative damage after SCI. Immunohistochemical parameters signi ficantly subsided, except for eNOS levels, after TPM treatment in the acute phase of SCI. Additionally, TPM treatment also improved function after SCI. This study provides evidence for neuroprotective effects of TPM in SCI; however, further experimental and clinical studies are needed to explore its mechanism of action, evaluate its short- and long-term effects,establish an optimal dosing scheme, and assess its combination with other therapeutics.
Author contributions:FN and BB designed this study. FN and SH performed experiments. SH, KK, HU and BB analyzed data. FN and SH wrote the paper. All authors approved the final version of the paper.
Con flicts of interest:All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership,or other equity interest; and expert testimony or patent-licensing arrangements), or non- financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter ormaterials discussed in this manuscript.
Research ethics:Experimental protocols performed in this study were approved by Hacettepe University Animal Research Ethics Committee with the approval number 2009/15-2 and were in compliance with the European Union (EU) directive (2010/63/EU) on the protection of animals used for scienti fic purposes.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under identical terms.
Azbill RD, Mu X, Bruce-Keller AJ, Mattson MP, Springer JE (1997) Impaired mitochondrial function, oxidative stress and altered antioxidant enzyme activities following traumatic spinal cord injury. Brain Res 765:283-290.
Bilginer B, Onal MB, Narin F, Ustun H, Kılınc K, Akalan N (2009) Antiapoptotic andneuroprotective effects of mycophenolatemofetil after acute spinal cord injury in young rats. Childs Nerv Syst 25:1555-1561.
Cárdenas-Rodríguez N, Coballase-Urrutia E, Huerta-Gertrudis B, García-Cruz ME, Pedraza-Chaverri J, Coria-Jiménez R, Bandala C, Ruíz-García M (2013) Antioxidant activity of topiramate: an antiepileptic agent.Neurol Sci 34:741-747.
Chen HS, Lipton SA (2006) The chemical biology of clinically tolerated NMDA receptor antagonists. J Neurochem 97:1611-1626.
Conti A, Miscusi M, Cardali S, Germano A, Suzuki H, Cuzzocrea S, Tomasello F (2007) Nitric oxide in the injured spinal cord: synthases crosstalk, oxidative stressand in flammation. Brain Res Rev 54:205-218.
Dawson VL, Dawson TM, Bartley DA, Uhl GR, Snyder SH (1993) Mechanisms of nitric oxide-mediated neurotoxicity in primary brain cultures.J Neurosci 13:2651-2661.
Dinoff BL, Richards JS, Ness TJ (2003) Use of topiramate for spinal cord injury related pain. J Spinal Cord Med 26:401-403.
Dodgson SJ, Shank RP, Maryanoff BE (2000) Topiramate as an inhibitor of carbonic anhydrase isoenzymes. Epilepsia 41 Suppl 1:S35-39.
Dumont RJ, Okonkwo DO, Verma S, Hurlbert RJ, Boulos PT, Ellegala DB,Dumont AS(2001) Acute spinal cord injury, part I: pathophysiologic mechanisms. Clin Neuropharmacol 24:254-264.
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70-77.
Estevez AG, Spear N, Thompson JA, Cornwell TL, Radi R, Barbeito L, Beckman JS(1998) Nitric oxide-dependent production of cGMP supports the survival of rat embryonic motor neurons cultured with brain-derived neurotrophic factor. J Neurosci 18:3708-3714.
Farooque M, Isaksson J, Jackson DM, Olsson Y (1999) Clomethiazole(ZENDRA, CMZ) improves hind limb motor function and reduces neuronal damage after severe spinal cord injury in rat. Acta Neuropathol 98:22-30.
Garry PS, Ezra M, Rowland MJ, Westbrook J, Pattinson KT (2015) The role of the nitric oxide pathway in brain injury and its treatment--from bench to bedside. Exp Neurol 263:235-243.
Gensel JC, Tovar CA, Bresnahan JC, Beattie MS (2012) Topiramate treatment is neuroprotective and reduces oligodendrocyte loss after cervical spinal cord injury. PLoS One 7:e33519.
Hoover RC, Motta M, Davis J, Saatman KE, Fujimoto ST, Thompson HJ,Stover JF, Dichter MA, Twyman R, White HS, McIntosh TK (2004) Differential effects of the anticonvulsant topiramate on neurobehavioural and histological outcomes following traumatic brain injury in rats. J Neurotrauma 21:501-512.
Jia Z, Zhu H, Li J, Wang X, Misra H, Li Y (2012) Oxidative stress in spinal cord injury and antioxidant-based intervention. Spinal Cord 50:264-274.
Koozekanani SH, Vise WM, Hashemi RM, McGhee RB (1976) Possible mechanisms for observed pathophysiological variability in experimental spinal cord injury by the method of Allen. J Neurosurg 44:429-434.
Kouzounias K, Kimiskidis VK, Siozos T, Violaris K, Kostomitsopoulos N,Karayannakos PE, Sotirakoglou K, Nanassis K (2011) Topiramate promotes neurological recovery in a new model of traumatic brain injury in rats. Neuroscience 183:171-177.
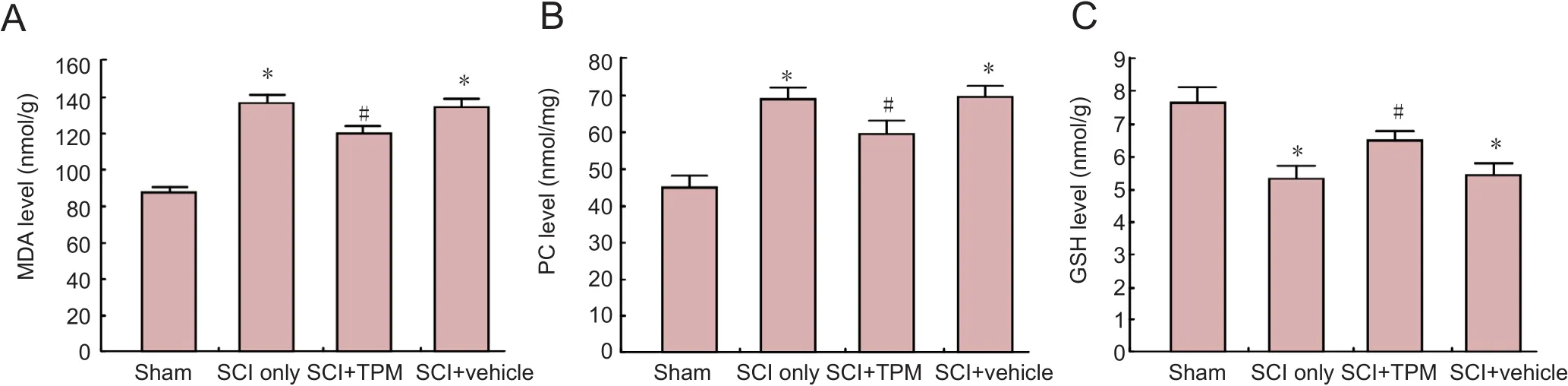
Figure 2 Biochemical assessment results of experimental groups at 24 hours after injury.

Figure 4 Behavioral assessment results of experimental groups at 4 weeks post-injury.
Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz AG, Ahn BW,Shaltiel S, Stadtman ER (1990) Determination of carbonyl content in oxidatively modi fied proteins. Methods Enzymol 186:464-478.
Lu J, Ashwell KW, Waite P (2000) Advances in secondary spinal cord injury: role of apoptosis. Spine (Phila Pa 1976) 25:1859-1866.
Mihara M, Uchiyama M (1978) Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem 86:271-278.
Nazıroğlu M, Yürekli VA (2013) Effects of antiepileptic drugs on antioxidant and oxidant molecular pathways: focus on trace elements. Cell Mol Neurobiol 33:589-599.
Osuka K, Watanabe Y, Usuda N, Atsuzawa K, Aoshima C, Yamauchi K,Takayasu M, Yoshida J (2007) Phosphorylation of neuronal nitric oxide synthase at Ser847 in the nucleus intermediolateralis after spinal cord injury in mice. Neuroscience 145:241-247.
Park E, Velumian AA, Fehlings MG (2004) The role of excitotoxicity in secondary mechanisms of spinal cord injury: a review with an emphasis on the implications for white matter degeneration. J Neurotrauma 21:754-774.
Rivlin AS, Tator CH (1977) Objective clinical assessment of motor function after experimental spinal cord injury in the rat. J Neurosurg 47:577-581.
Satake K, Matsuyama Y, Kamiya M, Kawakami H, Iwata H, Adachi K,Kiuchi K(2000) Nitric oxide via macrophage iNOS induces apoptosis following traumatic spinal cord injury. Brain Res Mol Brain Res 85(1-2):114-122.
Schwartz LM, Osborne BA (1993) Programmed cell death apoptosis and killer genes. Immunol Today 14:582-590.
Shakeri R, Kheirollahi A, Davoodi J (2017) Apaf-1: Regulation and function in cell death. Biochimie 135:111-125.
Shank RP, Gardocki JF, Streeter AJ, Maryanoff BE (2000) An overview of the preclinical aspects of topiramate: pharmacology, pharmacokinetics,and mechanism of action. Epilepsia 41:S3-9.
Shank RP, Maryanoff BE (2008) Molecular pharmacodynamics, clinical therapeutics, and pharmacokinetics of topiramate. CNS Neurosci Ther 14:120-142.
Vaziri ND, Lee YS, Lin CY, Lin VW, Sindhu RK (2004) NAD(P)H oxidase, superoxide dismutase, catalase, glutathione peroxidase and nitric oxide synthase expression in subacute spinal cord injury. Brain Res 995:76-83.
Walters MR, Kaste M, Lees KR, Diener HC, Hommel M, De Keyser J, Steiner H, Versavel M (2005) The AMPA antagonist ZK 200775 in patients with acute ischaemic stroke: a double blind, multicentre, placebo-controlled safety and tolerability study. Cerebrovasc Dis 20:304-309.
Wrathall JR, Teng YD, Choiniere D (1996) Amelioration of functional de ficits from spinal cord trauma with systemically administered NBQX,an antagonist of non-N-methyl-D-aspartate receptors. Exp Neurol 137:119-126.
Wu XH, Yang SH, Duan DY, Cheng HH, Bao YT, Zhang Y (2007) Anti-apoptotic effect of insulin in the control of cell death and neurologic de ficit after acute spinal cord injury in rats. J Neurotrauma 24:1502-1512.
Yang JY, Kim HS, Lee JK (2007) Changes in nitric oxide synthase expression in young and adult rats after spinal cord injury. Spinal Cord 45:731-738.
How to cite this article:Narin F, Hanalioglu S, Ustun H, Kilinc K, Bilginer B (2017) Topiramate as a neuroprotective agent in a rat model of spinal cord injury. Neural Regen Res 12(12):2071-2076.
*Correspondence to:
Sahin Hanalioglu, M.D., Ph.D.,sahinhanalioglu@gmail.com.
orcid:0000-0003-4988-4938(Sahin Hanalioglu)
10.4103/1673-5374.221164
2017-09-26
Preparation for publication of this article has been partly supported by Turkish Neurosurgical Society.
Copyedited by Li CH, Song LP, Zhao M
杂志排行
中国神经再生研究(英文版)的其它文章
- Roles of neural stem cells in the repair of peripheral nerve injury
- Quanti fication of intermuscular and intramuscular adipose tissue using magnetic resonance imaging after neurodegenerative disorders
- Notch pathway inhibitor DAPT enhances Atoh1 activity to generate new hair cells in situ in rat cochleae
- Long non-coding RNA NONMMUG014387 promotes Schwann cell proliferation after peripheral nerve injury
- Autologous transplantation with fewer fibers repairs large peripheral nerve defects
- Diffusion tensor imaging of spinal microstructure in healthy adults: improved resolution with the readout segmentation of long variable echo-trains
