Mitochondrial protective and anti-apoptotic effects of Rhodiola crenulata extract on hippocampal neurons in a rat model of Alzheimer’s disease
2018-01-05JunmeiWangZeqiangQuJinlangWuPeterChungYuanshanZeng
Jun-mei Wang, Ze-qiang Qu, Jin-lang Wu, Peter Chung, Yuan-shan Zeng
1 Department of Histology and Embryology, Zhongshan School of Medicine, Sun Yat-sen University, Guangzhou, Guangdong Province, China
2 Department of Electron Microscope, Zhongshan School of Medicine, Sun Yat-sen University, Guangzhou, Guangdong Province, China
3 Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
4 Institute of Spinal Cord Injury, Sun Yat-sen University, Guangzhou, Guangdong Province, China
5 Co-innovation Center of Neuroregeneration, Nantong University, Nantong, Jiangsu Province, China
6 Guangdong Province Key Laboratory of Brain Function and Disease, Zhongshan School of Medicine, Sun Yat-sen University, Guangzhou,Guangdong Province, China
Mitochondrial protective and anti-apoptotic effects of Rhodiola crenulata extract on hippocampal neurons in a rat model of Alzheimer’s disease
Jun-mei Wang1,#, Ze-qiang Qu1,#, Jin-lang Wu2, Peter Chung3, Yuan-shan Zeng1,4,5,6,*
1 Department of Histology and Embryology, Zhongshan School of Medicine, Sun Yat-sen University, Guangzhou, Guangdong Province, China
2 Department of Electron Microscope, Zhongshan School of Medicine, Sun Yat-sen University, Guangzhou, Guangdong Province, China
3 Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
4 Institute of Spinal Cord Injury, Sun Yat-sen University, Guangzhou, Guangdong Province, China
5 Co-innovation Center of Neuroregeneration, Nantong University, Nantong, Jiangsu Province, China
6 Guangdong Province Key Laboratory of Brain Function and Disease, Zhongshan School of Medicine, Sun Yat-sen University, Guangzhou,Guangdong Province, China
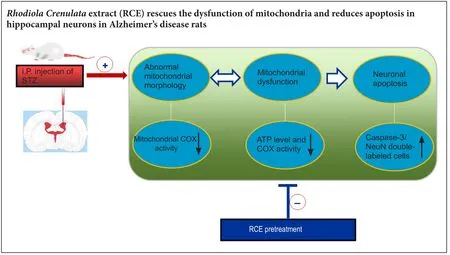
In our previous study, we found that the edible alcohol extract of the root of the medicinal plantRhodiola crenulata(RCE) improved spatial cognition in a rat model of Alzheimer’s disease. Another study from our laboratory showed that RCE enhanced neural cell proliferation in the dentate gyrus of the hippocampus and prevented damage to hippocampal neurons in a rat model of chronic stress-induced depression.However, the mechanisms underlying the neuroprotective effects of RCE are unclear. In the present study, we investigated the anti-apoptotic effect of RCE and its neuroprotective mechanism of action in a rat model of Alzheimer’s disease established by intracerebroventricular injection of streptozotocin. The rats were pre-administered RCE at doses of 1.5, 3.0 or 6.0 g/kg for 21 days before model establishment.ATP and cytochrome c oxidase levels were signi ficantly decreased in rats with Alzheimer’s disease. Furthermore, neuronal injury was obvious in the hippocampus, with the presence of a large number of apoptotic neurons. In comparison, in rats given RCE pretreatment, ATP and cytochrome c oxidase levels were markedly increased, the number of apoptotic neurons was reduced, and mitochondrial injury was mitigated. The 3.0 g/kg dose of RCE had the optimal effect. These findings suggest that pretreatment with RCE prevents mitochondrial dysfunction and protects hippocampal neurons from apoptosis in rats with Alzheimer’s disease.
nerve regeneration; Alzheimer’s disease; intracerebroventricular injection; streptozotocin; neuronal apoptosis; neuroprotection;cytochrome c oxidase; adenosine triphosphate; caspase-3; NeuN; neural regeneration
Introduction
Alzheimer’s disease (AD) is a degenerative disease of the central nervous system characterized by progressive cognitive dysfunction, including impairments in learning, memory and language. AD is strongly associated with perturbed energy metabolism, which is one of the leading causes of neural degeneration, the loss of cerebral neurons, and the formation of senile plaques and neuro fibrillary tangles (Cardoso et al., 2004). Reduced glucose utilization and abnormal oxidative metabolism are found in the brain of AD patients,and the decreased metabolism is positively correlated with the severity of dementia (Drzezga et al., 2005; Zhang et al.,2016). Excessive formation and secretion of amyloid-beta,which is a key factor in AD pathogenesis, has a mutually reinforcing relationship with glucose metabolism disorders(Sadowski et al., 2004; Hoyer et al., 2005; Kalpouzos et al.,2005). Insulin signal transduction impairment, glucose metabolism disorder and reduced energy generation also contribute to amyloid-beta deposition and tau hyperphosphorylation, leading to AD (Green field et al., 1999; Hoyer,2000). Before the presence of neuropathological damage and brain atrophy detected by radiological examination, AD patients exhibit abnormal glucose metabolism in the temporal cortex and hippocampus, suggesting that perturbed oxidative phosphorylation (Drzezga et al., 2005), mitochondrial dysfunction and energy metabolism decay are early events in AD. Bucht et al. (1983) found that plasma insulin levels in AD patients were abnormal after oral glucose tolerance test. Subsequent studies demonstrated that, in the fasting state, AD patients have a higher insulin level in the cerebrospinal fluid than normal subjects. Furthermore, AD patients exhibit lower tyrosine kinase activity and impaired insulin signaling, similar to the pathophysiology of non-insulin-dependent diabetes mellitus (De Keyser et al., 1994; Jafferali et al., 2000; Nicolls, 2004). These observations led to the novel proposition that insulin receptor desensitization might play an important role in the pathogenesis of sporadic AD(Gasparini et al., 2002; Watson and Craft, 2003; Carro and Torres-Aleman, 2004; Messier and Teutenberg, 2005; Revill et al., 2006).
Existing drugs for AD include cholinergic agents, nutritional preparations, anti-in flammatory agents, neurotrophic factors, antioxidant drugs and herbal preparations.Rhodiola crenulatais a perennial herb in the family Crassulaceae. It is abundantly and widely distributed in China. According to traditional Chinese folk medicine,Rhodiola crenulatacan improve endurance, resist altitude sickness (Chiu et al., 2013), and treat fatigue, depression, insomnia and impotence (Pooja et al., 2009). A recent study found thatRhodiola crenulatasupplement strikingly improves aerobic exercise performance after short-term high altitude training (Chen et al., 2014).Rhodiola crenulataexerts protective effects on chronic intermittent hypoxia-induced mitochondrial-dependent apoptosis in cardiac cells (Lai et al., 2015).Accumulating evidence indicates thatRhodiola crenulataprotects against cerebral ischemia-reperfusion injury in the rat brain (Song et al., 2006a, b). Pharmacological studies suggest thatRhodiola crenulatapromotes cognitive function and relieves brain fatigue (Darbinyan et al., 2000; Spasov et al., 2000), clears reactive oxygen species and reduces oxidative stress (Abidov et al., 2003; Wing et al., 2003; De Sanctis et al., 2004; Battistelli et al., 2005; Kanupriya et al., 2005; Yu et al., 2007; Pooja et al., 2009), enhances physical endurance(Spasov et al., 2000; De Bock et al., 2004), ameliorates metabolic dysfunction (Wang et al., 2012; Tian et al., 2013; Wang et al., 2013), bolsters immunity (Zhu et al., 2014) and exerts anti-tumor effects (Tu et al., 2008). In recent years,Rhodiola crenulatahas attracted increasing attention because of its cognitive protective effects, antioxidant effects, and ability to scavenge reactive oxygen species (Chen et al., 2012; Zhou et al., 2015).
In our previous study, an edible alcohol extract ofRhodiola crenulataroot (RCE) protected against spatial cognitive deficits in a rat model of AD induced by intracerebroventricular (ICV) injection of streptozotocin (STZ) (Qu et al.,2009). However, the mechanisms underlying the protective effects of RCE on learning and memory remain unclear. ICV injection of STZ causes a persistent disruption in glucose metabolism and energy production in the brain, leading to learning and memory impairment in rats (Hoyer, 2004;Hoyer and Lannert, 2008). We hypothesized that RCE might protect against mitochondrial dysfunction and improve glucose metabolism and energy production in neurons.Our preliminary studies (Chen et al., 2008a, 2009a; Qin et al., 2008) demonstrated that RCE promotes neural cell proliferation in the dentate gyrus of the hippocampus, and prevents damage to hippocampal neural cells in a rat model of depression induced by chronic stress. In our previous study, RCE pre-administration signi ficantly reduced oxidative stress in the hippocampus of rats administered STZ, accompanied by an improvement in spatial cognitive function(Qu et al., 2009). Furthermore, salidroside (the main active ingredient in RCE) promoted the neuronal differentiation of neural stem cells in vitro(Qu et al., 2012). We hypothesized that RCE would exert neuroprotective effects in AD rats by improving mitochondrial function and/or by reducing neuronal apoptosis. In the present study, we investigated the anti-apoptotic effect of RCE and examined its neuroprotective mechanism of action in AD rats, with the aim of providing a rational basis for the use of RCE in the treatment of central neurodegenerative diseases.
Materials and Methods
Preparation of RCE
RCE was provided by Holistol International Co., Ltd., Hong Kong Special Administrative Region, China. The medicinal plantRhodiola crenulatawas identified by Professor Ye Huagu of the Herbarium, South China Botanical Garden,The Chinese Academy of Sciences, China. The RCE used in this study is the edible alcohol extract ofRhodiola crenulataroot, which is a red-brown fine powder, with a rose fragrance. The extraction process is as follows: (1)Rhodiolacrenulataroots were cleaned and dried; (2) the roots were ground, yielding the powder; (3) the powder was extracted twice using 70% ethanol, 2 hours each, to yield the preliminary extract solution; (4) the solution was concentrated under vacuum; (5) the concentrated solution was precipitated twice using 90% ethanol, yielding a paste; (6) the paste was spray dried, yielding the final RCE powder. The yield was 3–5% (w/w), and the proportion of salidroside in the RCE preparation was 4% (w/w), as determined by high-performance liquid chromatography (HPLC). Certi ficate of analysis was provided by Sichuan Zhonghong Natural Medicine Co., Ltd., Chengdu, China.1.7 mM CaCl2and 2.2 mM dextrose) to prepare a 25 mg/mL working solution. Rats in the model and RCE groups were injected STZ at a dose of 1.5 mg/kg into the bilateral ventricles (3 μL/100 g body weight for each lateral ventricle). The rats in the normal control group received the same amount of arti ficial cerebrospinal fluid. Each injection lasted 10 minutes, and the needle was maintained in place for 10 minutes.The suture was disinfected with 0.01% benzalkonium bromide. To prevent infection, rats were given intramuscular penicillin (200,000 U, twice a day, for 3 days).
HPLC assay for ATP content in mitochondria
Sample preparation
Drug administration
Ninety adult, female, clean, Sprague-Dawley rats, 5 months old and weighing 220–250 g, were provided by the Experimental Animal Center of Sun Yat-sen University, China(license No. SCXK (Yue) 2009-0011). The rats were allowed free access to standard chow and tap water and housed in cages at 24 ± 2°C in a room with 50–60% relative humidity,under a 12-hour light/dark cycle, for at least 1 week before the experiment. The rats were randomly divided into five groups as follows: normal control group (n= 20), AD group(n= 20), high-dose RCE group (H-RC group;n= 15), moderate-dose RCE group (M-RC group;n= 20), and low-dose RCE group (L-RC group;n= 15 rats). Rats in the L-RC,M-RC and H-RC groups were respectively administered 1.5, 3.0 and 6.0 g/kg RCE. The RCE was diluted with 0.5%sodium carboxymethycellulose (Guangzhou Pharmaceutical Company, Guangzhou, Guangdong Province, China) water solution to produce working stocks of 1.2, 0.6 and 0.3 g/mL for the H-RC, M-RC and L-RC groups, respectively, and given orally every day at a single dose of 0.5 mL/100 g (body weight) by gavage for 21 days before STZ injection (Figure 1).Rats in the AD and normal control groups received an equal amount of 0.5% sodium carboxymethycellulose solution for 21 days.
AD induced by STZ
At days 1 and 3 after RCE or sodium carboxymethycellulose administration, a rat model of AD was established using ICV injection of STZ (Sigma, St. Louis, USA) (Figure 1). In brief, rats were anesthetized with 1% sodium pentobarbital(40 mg/kg; China Pharmaceutical Group Shanghai Chemical Reagent Company, Shanghai, China) by intraperitoneal injection, and shaved. Their heads were fixed with a stereotaxic apparatus (Jiangwan Instrument Factory, Shanghai,Jiangsu province, China). After the skin was disinfected with iodine and 75% alcohol, a 1.5-cm incision was made along the sagittal line, and the periosteum was cut to expose the cranial bone. Using the Stereotaxic Atlas of the Rat Brain for reference (Bao and Shu, 1991), the skull was drilled 0.8 mm posterior to the bregma and 2.2 mm lateral to the sagittal suture. Thereafter, a microsyringe was vertically inserted to a depth of 4.5 mm. STZ was dissolved in arti ficial cerebrospinal fluid (147 mM NaCl, 2.9 mM KCl, 1.6 mM MgCl2,
At 21 days after model establishment, 10 rats were decapitated under anesthesia. The brain was rapidly removed on ice, and the hippocampus was quickly dissected and kept at −20°C.The hippocampi were transferred to a glass homogenizer with 0.5 M perchloric acid, 10 μL/mg, and homogenized on ice. The homogenate was kept on ice for 30 minutes and centrifuged at 16,000 ×gat 4°C for 10 minutes. The pH was adjusted to 7.0 with 0.5 M KOH, placed on ice for 10 minutes, centrifuged and precipitated. The supernatant was stored at −80°C for HPLC detection.
Chromatography
ATP content in the hippocampus was quantitatively detected using reverse HPLC (Waters, Milford, MA, USA). Brie fly,a Hypersil BDS C-18 column (4.6 mm ID × 30 mm, 5 μm particle size) was washed with 150 mM NaH2PO4buffer solution (pH 6.45, filtered). Then, 20 mL standard solution was added to the column, and the residence time (approximately 13 minutes) was measured. At a wavelength of 254 nm, 20 μL of the sample solution was added and compared with the standard solution for chromatographic analysis.
Standard curve
Taking the peak (μV) as the vertical axis and ATP content as the abscissa, a standard curve was constructed according to the linear equationY= 64,535.14X− 7,026.75,r= 0.9999.
Detection of mitochondrial cytochrome c oxidase (COX)activity
Ten rats in each group were selected for assessment of COX activity. After the hippocampus was isolated and stored at–20°C, the tissue was cut into pieces and homogenized with saline (5% w/v) in a glass homogenizer. The homogenates were centrifuged at 700 ×gfor 16 minutes, and the supernatant was carefully removed. COX activity was determined with a microplate reader (BioRad Model 680, Shanghai, Jiangsu Province, China) using a COX quantitative detection kit (Shanghai Genius U.S. Genetic Medicine Technology Co., Ltd.).
Caspase-3/NeuN double immuno fluorescence detection for hippocampal neuronal apoptosis
Five rats in each group were used to obtain hippocampal tissue samples 21 days after model establishment. In brief,rats were intraperitoneally anesthetized with 1% sodium pentobarbital (40 mg/kg), and the heart was fully exposed.A cannula was inserted into the ascending aorta through the left ventricle, and perfused with 120 mL saline, with the right atrium cut for drainage. Pre-cooled 4% paraformaldehyde,approximately 300 mL, was perfused into the heart over a period of 30 minutes. The brain tissue was quickly taken out and fixed with 4% paraformaldehyde at 4°C for 24 hours,and sequentially immersed in 10%, 20% and 30% sucrose at 4°C. Subsequently, the specimens were embedded, and serial coronal slices of the hippocampus were obtained using a cryostat microtome (Thermo Shandon Limited, Altrincham Cheshire, UK). Slices were taken every 300 μm, with thicknesses of 40 and 15 μm. A total of 12 slices were taken from each sample, in two sets.
Five rats in each group were used for immunofluorescence staining, using four slices from each rat, for a total of 20 slices. In brief, the slices were rinsed with 0.01 M PBS and blocked with normal goat serum (1:10; Abcam, Cambridge, UK) for 20 minutes. The slices were then incubated with rabbit anti-rat caspase-3 polyclonal antibody (1:100;Beijing Zhongshan Golden Bridge, Beijing, China). Negative controls were incubated with 0.01 M PBS at 4°C for two nights. The slices were then rinsed with 0.01 M PBS,incubated with goat anti-rabbit IgG-CY3 antibody (1:400;Jackson ImmunoResearch Laboratories, Inc., Pennsylvania, USA) at 37°C for 1 hour, rinsed with 0.01 M PBS, and blocked with normal goat serum (1:10) for 20 minutes.The specimens were incubated with mouse anti-rat NeuN monoclonal antibody (1:100; Chemicon International Inc.,Billerica, MA, USA). Negative controls were incubated with 0.01 M PBS at 4°C for two nights. After rinsing with PBS,the specimens were incubated with goat anti-mouse IgGFITC antibody (1:100; Jackson Immunological Research) at 37°C for 1 hour, rinsed with PBS, mounted, and observed under a fluorescence microscope (Leica Microsystems Inc.,Wetzlar, Germany).
Five rats in each group were used for cell counting, using four slices from each rat, for a total of 20 slices. Caspase-3 and NeuN-positive cells in the hippocampal CA3 region were counted under 200× magni fication, and the percentage of caspase-3-positive cells to NeuN-positive cells was calculated.
Enzyme histochemistry and electron microscopic observation of mitochondrial COX
At 21 days after model induction, five rats each from the normal control, AD and M-RC groups were intraperitoneally anesthetized with 1% sodium pentobarbital (40 mg/kg).The rats were perfused as above, and the brains were fixed with 4% paraformaldehyde/0.5% glutaraldehyde/15% picric acid (0.1 M PB, pH 7.4). The brains were then immersed in 0.1 M phosphate buffer, and cut into 50-μm-thick slices on a vibratome (Zhejiang Xiangshan Science Instrument Factory,Ningbo, China).
The slices were rinsed with 0.1 M phosphate buffer, incubated with 4% sucrose solution (prepared with 0.1 M phosphate buffer) at 37°C for 30 minutes, and incubated with COX reaction mix [cytochrome c (Sigma) 1.5 mg,3,3′-diaminobenzidine 2.5 mg, sucrose 200 mg, dissolved in 5 mL 0.1 M phosphate buffer] at 37°C for 5 hours. After incubation, the specimens were rinsed with phosphate buffer,and the bilateral hippocampal CA3 regions were observed under a stereomicroscope (Leica) and then stored in 0.1 M phosphate buffer for further observation under an electron microscope.
Transmission electron microscopy
(1) Fixation: Hippocampal tissue was rinsed with 0.1 M phosphate buffer, fixed with 1% osmic acid for 1 hour, and rinsed again with phosphate buffer. (2) Dehydration: The tissue was dehydrated serially with 50% and 75% ethanol for 15 minutes,twice with 95% ethanol for 15 minutes each, three times with absolute alcohol for 10 minutes each, and three times with anhydrous acetone for 10 minutes each. (3) Permeabilization:The tissue was immersed in embedding liquid (1:1 ratio of Epon812 and acetone) at room temperature for 1 hour, and embedded in pure Epon 812 embedding solution overnight.(4) Embedding and polymerization: The slices were embedded in Epon 812, heated in a 36°C oven, and polymerized for 48 hours. (5) Finally, the slices were cut into ultra-thin slices using an AO ultramicrotome (Leica), stained with 2% uranyl acetate (10 minutes) and lead citrate (3 minutes), and photographed under the electron microscope (PHILIPS CM10,Royal Philips, Amsterdam, Holland).
Statistical analysis
Data are expressed as the mean ± SD, and analyzed using SPSS 11.5 software (SPSS, Chicago, IL, USA) by one-way analysis of variance. If homogeneity of variance was found,the mean value among groups was compared with the least significant difference test. If heterogeneity of variance was found, the comparison was done using Tamhane’s T2 test. A value ofP< 0.05 was considered statistically signi ficant.
Results
Effect of RCE on hippocampal ATP levels in AD rats
As shown in Figure 2, hippocampal ATP levels in the M-RC and normal control groups were higher than in the other three groups (P< 0.05). Hippocampal ATP levels were lower in the M-RC group than in the normal control group (P<0.05). There were no signi ficant differences in hippocampal ATP levels among the AD, L-RC and H-RC groups (P> 0.05).
Effect of RCE on hippocampal COX levels in AD rats
As shown in Figure 2, there was no signi ficant difference in hippocampal COX levels between the AD and H-RC groups(P> 0.05), which were signi ficantly lower than in the other three groups (P< 0.05). Among the normal control, L-RC and H-RC groups, hippocampal COX levels were significantly different for each pairwise comparison (P< 0.05).
Effect of RCE on hippocampal neuronal apoptosis in AD rats
Hippocampal neuronal apoptosis was detected with caspase-3 and NeuN double immunofluorescence labeling(Figure 3A). Caspase-3 and NeuN-positive signals were mainly distributed in the nucleus, and only a small amount was found in the cell body. In the normal control group,NeuN-labeled neurons were morphologically normal and higher in number, and only a few caspase-3-positive cells were observed. In the AD group, a small number of neurons were NeuN-positive, and a large number of caspase-3-positive cells were observed. In the RCE groups, caspase-3-positive cells were reduced to a degree. The M-RC group had the lowest number of caspase-3-positive cells, while the H-RC group had the highest number among the three RCE-administered groups.
The percentage of caspase-3/NeuN-double-labeled cells in the hippocampal CA3 region is shown in Figure 3B. This percentage reflects the degree of hippocampal neuronal apoptosis. As shown in Figure 3B, there was no signi ficant difference in the percentage of double-labeled cells between the AD and H-RC groups (P> 0.05), and these two groups were significantly different from the other 3 groups (P<0.05). Among the normal control, L-RC and M-RC groups,there were signi ficant differences in the percentage of double-labeled cells between each pairwise comparison (P<0.05). The percentage of double-labeled cells was lowest in the normal control group, in-between in the M-RC group,and highest in the L-RC group.
Effect of RCE on mitochondrial COX levels in hippocampal neurons in AD rats
By electron microscopy, the COX-positive high-electrondense particles, which represent COX enzymatic activity,were accumulated in the neuronal mitochondrial inner membrane and cristae (Figure 4). In the normal control group, mitochondria were small, round or rectangular, with a clearly-defined and uniform matrix, regularly distributed cristae, and a compact structure. A large number of COX-positive particles were visible in the plasma and neurites, as well as in the mitochondrial inner membrane and cristae. In the model group, mitochondrial swelling, a pale gray matrix, and ruptured or blurred cristae were found,and some mitochondrial outer membranes were incomplete or absent, with fewer COX-positive particles on the inner membrane and cristae. After treatment with 3.0 g/kg RCE,however, the decreased COX-positive particles on cristae and the pathological changes in mitochondrial morphology were all improved to a degree. The results are consistent with the ATP and COX levels measured in the homogenates.
Discussion
STZ is a nitrosourea derivative, and intraperitoneal injection can cause diabetes mellitus through the destruction of pancreatic β cells (Weiss, 1982; Bolzan and Bianchi, 2002; Kamat, 2015). In the central nervous system, STZ reduces insulin receptor phosphorylation and tyrosine kinase activity,increases tyrosine phosphatase activity and inhibits insulin signaling, thereby impairing glucose and energy metabolism(Hoyer, 1998; Lannert and Hoyer, 1998; Hoyer et al., 2000).The mechanism of STZ cytotoxicity remains unclear, but the alkylating effect of its metabolites can produce reactive oxygen groups, leading to oxidative stress and mitochondrial and nuclear DNA damage (Szkudelski, 2001; Bolzan and Bianchi, 2002; Gille et al., 2002). ICV injection of STZ results in persistent glucose metabolism and energy production disorder in rats, accompanied by reduced hippocampal choline acetyltransferase activity, oxidative stress, and learning and memory disorders (Hoyer and Lannert, 1999, 2008; Shoham et al., 2003; Hoyer, 2004; Sapcanin et al., 2008). Prior to the emergence of hyperglycemia, a series of AD-like pathological changes appears in rodents given ICV injection of STZ,such as brain atrophy and neurodegeneration, loss of central neurons, abnormal activation of glial cells, p53 and GSK-3β activation, hyperphosphorylation of tau protein, elevated amyloid-β levels, and abnormal mitochondrial morphology and function (Grünblatt et al., 2004, 2007; Chu and Qian,2005; Lester-Coll et al., 2006; Salkovic-Petrisic et al., 2006;Hoyer and Lannert, 2007; Du et al., 2015). Many research groups have used this as a model of sporadic AD (Sharma and Gupta, 2002; Veerendra Kumar and Gupta, 2003;Sonkusare et al., 2005; Shoham et al., 2007; Tahirovic et al.,2007).
Mitochondrial energy metabolism disorder plays a crucial role in neurodegenerative diseases (Blass, 1999; Bubber et al., 2005). Mitochondrial dysfunction promotes and participates in AD occurrence and development, and it is an important factor in the pathogenesis of AD (Lustbader et al.,2004; Hauptmann et al., 2006; Moreira et al., 2006). Mitochondria not only synthesize most of the cell’s ATP, but they also produce reactive oxygen species, such as superoxide anion, regulate cellular redox potential and signal transduction, and control apoptosis and gene expression (Newmeyer and Ferguson-Miller, 2003).
By electron microscopy, mitochondrial morphology is abnormal in the brain tissue of AD patients. In brain homogenates, the function and expression of several enzyme systems involved in mitochondrial energy generation are affected. Analysis of autopsy samples from AD patients shows that COX activity is decreased in the hippocampus,cerebellum, thalamus and other brain areas (Drzezga et al.,2005). Furthermore, COX activity is decreased, and there are changes in the activities of respiratory chain complexes I and III. Changes in mitochondrial morphology are also found in neurons in the hippocampus, cortex and hypothalamus (Baloyannis, 2006). Morphological abnormalities precede the formation of neuro fibrillary tangles, and mitochondrial degeneration may be one of the earliest pathological changes in AD (Maurer et al., 2000; Hirai et al., 2001).Ultrastructural studies show that mitochondria are small in normal neurons, round or columnar in shape, with a dense and uniform matrix, and regular cristae distribution. In comparison, lesioned mitochondria exhibit matrix changes and little or no residual cristae (Baloyannis, 2006).

Figure 1 Drug administration and procedure for model establishment.

Figure 2 Effect of RCE on the levels of ATP (µg/mL) and COX enzymatic activity (U/µg•min) in the hippocampal mitochondria of Alzheimer’s disease rats.

Figure 3 Caspase-3/NeuN immuno fluorescence double labeling of cells in the hippocampal CA3 region of Alzheimer’s disease rats treated with RCE.

Figure 4 Ultrastructural changes in mitochondria and COX enzyme staining in hippocampal neurons in Alzheimer’s disease rats treated with RCE (transmission electron microscopy).
In previous studies, ICV injection of STZ caused serious oxidative injury and signi ficant spatial learning and memory disorders in the rat hippocampus, and pre-administration of RCE signi ficantly reduced oxidative stress and improved the spatial cognitive defects (Qu et al., 2009). Oxidative stress in cells results in mitochondrial injury, leading to mitochondrial respiratory dysfunction. Mitochondria are the main site of oxidative phosphorylation, and are an important source and target of reactive oxygen species. Mitochondria are prone to free radical attack and oxidative damage to their mitochondrial DNA, particularly as they lack histones,have no proofreading function, and do not have an effective DNA repair mechanism. More than 95% of intracellular reactive oxygen species are generated from mitochondrial oxidative phosphorylation. Mitochondrial dysfunction further increases reactive oxygen species levels, and this vicious cycle results in excessive oxidative stress, eventually leading to neuronal death.
In the present study, we demonstrated that ICV injection of STZ significantly decreases ATP content and COX enzyme activity in the rat hippocampus. Enzyme histochemistry and electron microscopy showed that COX-positive electron-dense particles in the model group were reduced compared with the normal control group, and mitochondrial swelling was found, with no or only residual cristae in the model group. Furthermore, caspase-3-positive signals were observed in the majority of hippocampal neurons in the model group.
Our current findings suggest that ICV injection of STZ in rats induces damage to neuronal mitochondria, thereby leading to neuronal apoptosis. Pre-treatment with RCE increased COX enzyme content and activity, as well as ATP content in the hippocampus. Furthermore, RCE ameliorated neuronal mitochondrial morphology and function,and reduced the percentage of caspase-3-positive cells. This suggests that RCE pre-treatment protects against neuronal apoptosis by preserving neuronal mitochondrial structure and function.
The root and stem of Rhodiola have great therapeutic potential, containing 40 different chemical components.The main pharmacologically active ingredients are salidroside and p-tyrosol, rosavin, pyridine, rhodiosin and rhodionin (Wang and Wang, 1992; Yu et al., 1993; Nakamura et al., 2008). Phytochemical investigations revealed that salidroside, rosavins and p-tyrosol are the most abundant compounds and are thought to account for the therapeutic activities of the plant (Cui et al., 2003). Most studies have focused on the most bioactive ingredient, salidroside (Cui et al., 2003; Nakamura et al., 2008), because of its strong antioxidant activity (Yu et al., 2007).In vitrostudies have shown that salidroside stimulates erythropoiesis (Qian et al., 2011) promotes hippocampal cell proliferation (Chen et al., 2009b) and promotes the differentiation of bone marrow mesenchymal stem cells into hepatocytes (Ouyang et al.,2010). Furthermore, salidroside prevents apoptosis induced by hydrogen peroxide in human neuroblastoma SH-SY5Y cells (Zhang et al., 2007), reduces reactive oxygen species levels in neural stem cells in the rat hippocampus, ameliorates apoptosis and necrosis, and promotes the proliferation and differentiation of neural stem cells (Qu et al., 2012).Tyrosol is also relatively well studied, as it is an important bioactive ingredient in a variety of foods, such as white wine and olive oil (Di Benedetto et al., 2007; St-Laurent-Thibault et al., 2011). Tyrosol has numerous actions, including antioxidant activity (Di Benedetto et al., 2007; Loru et al., 2009),neural protective functions (Bu et al., 2007), anti-in flammatory effects, anti-tumor effects (Giovannini et al., 2002), and cardio-protective effects (Samuel et al., 2008). However, the actions of rosavins, which include rosavin, rosarin and rosin, remain unclear.
The RCE used in this study is an edible alcohol extract,and the concentration of the most important component,salidroside, was approximately 4% (w/w), as assayed by HPLC. Hence, in the present study, we speculate that the neuroprotective effects of RCE in AD rats are attributable to salidroside. Salidroside possesses a potent reactive oxygen species scavenging function and anti-apoptotic effects.The potential mechanisms underlying the protective effects of salidroside include: (1) modulation of apoptosis-related processes such as alteration of gene expression (e.g. downregulation of the pro-apoptotic gene Bax and/or up-regulation of the anti-apoptotic genes Bcl-2 and Bcl-X(L)) (Yu et al., 2008; Yang et al., 2013), restoration of the mitochondrial membrane potential (Zhang et al., 2010) and suppression of cytochrome c release and caspase cascade activation (Cai et al., 2008); (2) suppression of the excessive entry of Ca2+and the release of calcium stores and inhibition of the elevation in intracellular calcium levels (Cao et al., 2006; Chen et al.,2008b); and (3) inhibition of nitric oxide (NO) synthase activity and reduction of NO production by inhibition of the NF-κB-iNOS-NO signaling pathway (Chen et al., 2009b;Zhang et al., 2011). More studies on the effects of salidroside on the mitochondrial apoptotic pathway are needed to clarify the mechanisms underlying the neuroprotective functions of RCE.
We found that the protective effect of RCE exhibited a parabolic curve pattern, as previously described (Lazarova et al., 1986; Petkov et al., 1986). We found that 0.1 mL RCE signi ficantly improved learning and memory functions in rats,while the 0.02 and 1.0 mL doses had no significant effect.High doses of RCE failed to improve the functions, perhaps because of other components within the extract (Pooja et al.,2009). As mentioned above, RCE contains more than 40 different compounds, and it is possible that other compounds have effects that antagonize those of salidroside. Because of the complexity of herbal extracts, we cannot overcome the potentially adverse effects of other components, which may affect the efficacy of high doses of the extract.
In summary, ICV injection of STZ leads to abnormal changes in mitochondrial structure and function in the rat hippocampus, ultimately resulting in elevated levels of hippocampal neuronal apoptosis. Pretreatment with RCE protects against mitochondrial morphological and functional damage in hippocampal neurons, thereby reducing neuronal apoptosis in the STZ-induced rat model of AD. Our findings provide a basis for future studies on the use of RCE for the treatment of neurodegenerative diseases such as AD.
Author contributions:YSZ, ZQQ and JMW provided data, ensured the integrity of the data, and participated in study conception and design. ZQQ, YSZ and JMW analyzed the data. JMW and ZQQ wrote the paper, and were in charge of paper authorization. ZQQ, JLW and ZQQ performed statistical analysis, and provided technical or data support.ZQQ obtained the funding. YSZ served as principle investigators. All authors approved the final version of the paper.
Con flicts of interest:None declared.
Research ethics:The experimental procedure followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 8023, revised 1978). All efforts were made to minimize the number and suffering of the animals used in the experiments.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under identical terms.
Abidov M, Crendal F, Grachev S, Seifulla R, Ziegenfuss T (2003) Effect of extracts from Rhodiola rosea and Rhodiola crenulata (Crassulaceae) roots on ATP content in mitochondria of skeletal muscles.Bull Exp Biol Med 136:585-587.
Baloyannis SJ (2006) Mitochondrial alterations in Alzheimer’s disease.J Alzheimers Dis 9:119-126.
Bao XM, Shu SY (1991) Stereotactic Atlas of Rat Brain. Beijing: People’s Health Publishing House.
Battistelli M, De Sanctis R, De Bellis R, Cucchiarini L, Dachà M, Gobbi P (2005) Rhodiola rosea as antioxidant in red blood cells: ultrastructural and hemolytic behaviour. Eur J Histochem 49:243-254.
Blass JP (1999) Mitochondria, neurodegenerative diseases, and selective neuronal vulnerability. Ann N Y Acad Sci 893:434-439.
Bolzan AD, Bianchi MS (2002) Genotoxicity of streptozotocin. Mutat Res 512:121-134.
Bu Y, Rho S, Kim J, Kim MY, Lee DH, Kim SY, Choi H, Kim H (2007)Neuroprotective effect of tyrosol on transient focal cerebral ischemia in rats. Neurosci Lett 414:218-221.
Bubber P, Haroutunian V, Fisch G, Blass JP, Gibson GE (2005) Mitochondrial abnormalities in Alzheimer brain: mechanistic implications. Ann Neurol 57:695-703.
Bucht G, Adolfsson R, Lithner F, Winblad B (1983) Changes in blood glucose and insulin secretion in patients with senile dementia of Alzheimer type. Acta Med Scand 213:387-392.
Cai L, Wang H, Li Q, Qian Y, Yao W (2008) Salidroside inhibits H2O2-induced apoptosis in PC12 cells by preventing cytochrome c release and inactivating of caspase cascade. Acta Biochim Biophys Sin (Shanghai) 40:796-802.
Cao LL, Du GH, Wang MW (2006) The effect of salidroside on cell damage induced by glutamate and intracellular free calcium in PC12 cells. J Asian Nat Prod Res 8:159-165.
Cardoso SM, Santana I, Swerdlow RH, Oliveira CR (2004) Mitochondria dysfunction of Alzheimer’s disease cybrids enhances Abeta toxicity. J Neurochem 89:1417-1426.
Carro E, Torres-Aleman I (2004) The role of insulin and insulin-like growth factor I in the molecular and cellular mechanisms underlying the pathology of Alzheimer’s disease. Eur J Pharmacol 490:127-133.
Chen CY, Hou CW, Bernard JR, Chen CC, Hung TC, Cheng LL,Liao YH, Kuo CH (2014) Rhodiola crenulata- and Cordyceps sinensis-based supplement boosts aerobic exercise performance after short-term high altitude training. High Alt Med Biol 15:371-379.
Chen D, Fan J, Wang P, Zhu L, Jin Y, Peng Y, Du S (2012) Isolation,identification and antioxidative capacity of water-soluble phenylpropanoid compounds from Rhodiola crenulata. Food Chem 134:2126-2133.
Chen QG, Zeng YS, Tang JY, Qin YJ, Chen SJ, Zhong ZQ (2008a)Effects of Rhodiola rosea on body weight and intake of sucrose and water in depressive rats induced by chronic mild stress. Zhong Xi Yi Jie He Xue Bao 6:952-955.
Chen QG, Zeng YS, Qu ZQ, Tang JY, Qin YJ, Chung P, Wong R,Hagg U (2009a) The effects of Rhodiola rosea extract on 5-HT level,cell proliferation and quantity of neurons at cerebral hippocampus of depressive rats. Phytomedicine 16:830-838.
Chen X, Liu J, Gu X, Ding F (2008b) Salidroside attenuates glutamate-induced apoptotic cell death in primary cultured hippocampal neurons of rats. Brain Res 1238:189-198.
Chen X, Zhang Q, Cheng Q, Ding F (2009b) Protective effect of salidroside against H2O2-induced cell apoptosis in primary culture of rat hippocampal neurons. Mol Cell Biochem 332:85-93.
Chiu TF, Chen LL, Su DH, Lo HY, Chen CH, Wang SH, Chen WL(2013) Rhodiola crenulata extract for prevention of acute mountain sickness: a randomized, double-blind, placebo-controlled, crossover trial. BMC Complement Altern Med 13:298.
Chu WZ, Qian CY (2005) Expressions of Abeta1-40, Abeta1-42,tau202, tau396 and tau404 after intracerebroventricular injection of streptozotocin in rats. Di Yi Jun Yi Da Xue Xue Bao 25:168-170, 173.
Cui S, Hu X, Chen X, Hu Z (2003) Determination of p-tyrosol and salidroside in three samples of Rhodiola crenulata and one of Rhodiola kirilowii by capillary zone electrophoresis. Anal Bioanal Chem 377:370-374.
Darbinyan V, Kteyan A, Panossian A, Gabrielian E, Wikman G,Wagner H (2000) Rhodiola rosea in stress induced fatigue--a double blind cross-over study of a standardized extract SHR-5 with a repeated low-dose regimen on the mental performance of healthy physicians during night duty. Phytomedicine 7:365-371.
De Bock K, Eijnde BO, Ramaekers M, Hespel P (2004) Acute Rhodiola rosea intake can improve endurance exercise performance. Int J Sport Nutr Exerc Metab 14:298-307.
De Keyser J, Wilczak N, Goossens A (1994) Insulin-like growth factor-I receptor densities in human frontal cortex and white matter during aging, in Alzheimer’s disease, and in Huntington’s disease.Neurosci Lett 172:93-96.
De Sanctis R, De Bellis R, Scesa C, Mancini U, Cucchiarini L, Dacha M (2004) In vitro protective effect of Rhodiola rosea extract against hypochlorous acid-induced oxidative damage in human erythrocytes. BioFactors 20:147-159.
Di Benedetto R, Varì R, Scazzocchio B, Filesi C, Santangelo C,Giovannini C, Matarrese P, D’Archivio M, Masella R (2007) Tyrosol, the major extra virgin olive oil compound, restored intracellular antioxidant defences in spite of its weak antioxidative effectiveness.Nutrition, Metabolism and Cardiovascular Diseases 17:535-545.
Drzezga A, Riemenschneider M, Strassner B, Grimmer T, Peller M,Knoll A, Wagenpfeil S, Minoshima S, Schwaiger M, Kurz A (2005)Cerebral glucose metabolism in patients with AD and different APOE genotypes. Neurology 64:102-107.
Du LL, Chai DM, Zhao LN, Li XH, Zhang FC, Zhang HB, Liu LB, Wu K, Liu R, Wang JZ, Zhou XW (2015) AMPK activation ameliorates Alzheimer’s disease-like pathology and spatial memory impairment in a streptozotocin-induced Alzheimer’s disease model in rats. J Alzheimers Dis 43:775-784.
Gasparini L, Netzer WJ, Greengard P, Xu H (2002) Does insulin dysfunction play a role in Alzheimer’s disease? Trends Pharmacol Sci 23:288-293.
Gille L, Schott-Ohly P, Friesen N, Schulte im Walde S, Udilova N,Nowl H, Gleichmann H (2002) Generation of hydroxyl radicals mediated by streptozotocin in pancreatic islets of mice in vitro.Pharmacol Toxicol 90:317-326.
Giovannini L, Migliori M, Filippi C, Origlia N, Panichi V, Falchi M,Bertelli AA, Bertelli A (2002) Inhibitory activity of the white wine compounds, tyrosol and caffeic acid, on lipopolysaccharide-induced tumor necrosis factor-alpha release in human peripheral blood mononuclear cells. Int J Tissue React 24:53-56.
Greenfield JP, Tsai J, Gouras GK, Hai B, Thinakaran G, Checler F,Sisodia SS, Greengard P, Xu H (1999) Endoplasmic reticulum and trans-Golgi network generate distinct populations of Alzheimer beta-amyloid peptides. Proc Natl Acad Sci U S A 96:742-747.
Grünblatt E, Hoyer S, Riederer P (2004) Gene expression profile in streptozotocin rat model for sporadic Alzheimer’s disease. J Neural Transm 111:367-386.
Grünblatt E, Salkovic-Petrisic M, Osmanovic J, Riederer P, Hoyer S(2007) Brain insulin system dysfunction in streptozotocin intracerebroventricularly treated rats generates hyperphosphorylated tau protein. J Neurochem 101:757-770.
Hauptmann S, Keil U, Scherping I, Bonert A, Eckert A, Muller WE(2006) Mitochondrial dysfunction in sporadic and genetic Alzheimer’s disease. Exp Gerontol 41:668-673.
Hirai K, Aliev G, Nunomura A, Fujioka H, Russell RL, Atwood CS,Johnson AB, Kress Y, Vinters HV, Tabaton M, Shimohama S, Cash AD, Siedlak SL, Harris PL, Jones PK, Petersen RB, Perry G, Smith MA (2001) Mitochondrial abnormalities in Alzheimer’s disease. J Neurosci 21:3017-3023.
Hoyer A, Bardenheuer HJ, Martin E, Plaschke K (2005) Amyloid precursor protein (APP) and its derivatives change after cellular energy depletion. An in vitro-study. J Neural Transm (Vienna) 112:239-253.
Hoyer S (1998) Is sporadic Alzheimer disease the brain type of non-insulin dependent diabetes mellitus? A challenging hypothesis.J Neural Transm (Vienna) 105:415-422.
Hoyer S (2000) Brain glucose and energy metabolism abnormalities in sporadic Alzheimer disease. Causes and consequences: an update.Exp Gerontol 35:1363-1372.
Hoyer S (2004) Glucose metabolism and insulin receptor signal transduction in Alzheimer disease. Eur J Pharmacol 490:115-125.
Hoyer S, Lannert H (1999) Inhibition of the neuronal insulin receptor causes Alzheimer-like disturbances in oxidative/energy brain metabolism and in behavior in adult rats. Ann N Y Acad Sci 893:301-303.
Hoyer S, Lannert H (2007) Long-term abnormalities in brain glucose/energy metabolism after inhibition of the neuronal insulin receptor:implication of tau-protein. J Neural Transm Suppl:195-202.
Hoyer S, Lannert H (2008) Long-term effects of corticosterone on behavior, oxidative and energy metabolism of parietotemporal cerebral cortex and hippocampus of rats: comparison to intracerebroventricular streptozotocin. J Neural Transm (Vienna) 115:1241-1249.
Hoyer S, Lee SK, Loffler T, Schliebs R (2000) Inhibition of the neuronal insulin receptor. An in vivo model for sporadic Alzheimer disease? Ann N Y Acad Sci 920:256-258.
Jafferali S, Dumont Y, Sotty F, Robitaille Y, Quirion R, Kar S (2000)Insulin-like growth factor-I and its receptor in the frontal cortex,hippocampus, and cerebellum of normal human and alzheimer disease brains. Synapse 38:450-459.
Kalpouzos G, Eustache F, de la Sayette V, Viader F, Chételat G, Desgranges B (2005) Working memory and FDG-PET dissociate early and late onset Alzheimer disease patients. J Neurol 252:548-558.
Kamat PK (2015) Streptozotocin induced Alzheimer’s disease like changes and the underlying neural degeneration and regeneration mechanism. Neural Regen Res 10:1050-1052.
Kanupriya, Prasad D, Sai Ram M, Kumar R, Sawhney RC, Sharma SK, Ilavazhagan G, Kumar D, Banerjee PK (2005) Cytoprotective and antioxidant activity of Rhodiola imbricata against tert-butyl hydroperoxide induced oxidative injury in U-937 human macrophages. Mol Cell Biochem 275:1-6.
Lai MC, Lin JG, Pai PY, Lai MH, Lin YM, Yeh YL, Cheng SM, Liu YF,Huang CY, Lee SD (2015) Effects of rhodiola crenulata on mice hearts under severe sleep apnea. BMC Complement Altern Med 15:198.
Lannert H, Hoyer S (1998) Intracerebroventricular administration of streptozotocin causes long-term diminutions in learning and memory abilities and in cerebral energy metabolism in adult rats. Behav Neurosci 112:1199-1208.
Lazarova MB, Petkov VD, Markovska VL, Petkov VV, Mosharrof A(1986) Effects of meclofenoxate and Extr. Rhodiolae roseae L. on electroconvulsive shock-impaired learning and memory in rats.Methods Find Exp Clin Pharmacol 8:547-552.
Lester-Coll N, Rivera EJ, Soscia SJ, Doiron K, Wands JR, de la Monte SM (2006) Intracerebral streptozotocin model of type 3 diabetes:relevance to sporadic Alzheimer’s disease. J Alzheimers Dis 9:13-33.
Loru D, Incani A, Deiana M, Corona G, Atzeri A, Melis MP, Rosa A,Dessi MA (2009) Protective effect of hydroxytyrosol and tyrosol against oxidative stress in kidney cells. Toxicol Ind Health 25:301-310.
Lustbader JW, Cirilli M, Lin C, Xu HW, Takuma K, Wang N, Caspersen C, Chen X, Pollak S, Chaney M, Trinchese F, Liu S, Gunn-Moore F, Lue LF, Walker DG, Kuppusamy P, Zewier ZL, Arancio O, Stern D, Yan SS, et al. (2004) ABAD directly links Abeta to mitochondrial toxicity in Alzheimer’s disease. Science 304:448-452.
Maurer I, Zierz S, Möller HJ (2000) A selective defect of cytochrome c oxidase is present in brain of Alzheimer disease patients. Neurobiol Aging 21:455-462.
Messier C, Teutenberg K (2005) The role of insulin, insulin growth factor, and insulin-degrading enzyme in brain aging and Alzheimer’s disease. Neural Plast 12:311-328.
Moreira PI, Cardoso SM, Santos MS, Oliveira CR (2006) The key role of mitochondria in Alzheimer’s disease. J Alzheimers Dis 9:101-110.
Nakamura S, Li X, Matsuda H, Yoshikawa M (2008) Bioactive constituents from Chinese natural medicines. XXVIII. Chemical structures of acyclic alcohol glycosides from the roots of Rhodiola crenulata.Chem Pharm Bull (Tokyo) 56:536-540.
Newmeyer DD, Ferguson-Miller S (2003) Mitochondria: releasing power for life and unleashing the machineries of death. Cell 112:481-490.
Nicolls MR (2004) The clinical and biological relationship between Type II diabetes mellitus and Alzheimer’s disease. Curr Alzheimer Res 1:47-54.
Ouyang JF, Lou J, Yan C, Ren ZH, Qiao HX, Hong DS (2010) In-vitro promoted differentiation of mesenchymal stem cells towards hepatocytes induced by salidroside. J Pharm Pharmacol 62:530-538.
Petkov VD, Yonkov D, Mosharoff A, Kambourova T, Alova L, Petkov VV, Todorov I (1986) Effects of alcohol aqueous extract from Rhodiola rosea L. roots on learning and memory. Acta Physiol Pharmacol Bulg 12:3-16.
Pooja, Bawa AS, Khanum F (2009) Anti-inflammatory activity of Rhodiola rosea--”a second-generation adaptogen”. Phytother Res 23:1099-1102.
Qian EW, Ge DT, Kong SK (2011) Salidroside promotes erythropoiesis and protects erythroblasts against oxidative stress by up-regulating glutathione peroxidase and thioredoxin. J Ethnopharmacol 133:308-314.
Qin YJ, Zeng YS, Zhou CC, Li Y, Zhong ZQ (2008) Effects of Rhodiola rosea on level of 5-hydroxytryptamine, cell proliferation and differentiation, and number of neuron in cerebral hippocampus of rats with depression induced by chronic mild stress. Zhongguo Zhong Yao Za Zhi 33:2842-2846.
Qu ZQ, Zhou Y, Zeng YS, Li Y, Chung P (2009) Pretreatment with Rhodiola rosea extract reduces cognitive impairment induced by intracerebroventricular streptozotocin in rats: implication of anti-oxidative and neuroprotective effects. Biomed Environ Sci 22:318-326.
Qu ZQ, Zhou Y, Zeng YS, Lin YK, Li Y, Zhong ZQ, Chan WY (2012)Protective effects of a Rhodiola crenulata extract and salidroside on hippocampal neurogenesis against streptozotocin-induced neural injury in the rat. PLoS One 7:e29641.
Revill P, Moral MA, Prous JR (2006) Impaired insulin signaling and the pathogenesis of Alzheimer’s disease. Drugs of today (Barc)42:785-790.
Sadowski M, Pankiewicz J, Scholtzova H, Ji Y, Quartermain D, Jensen CH, Duff K, Nixon RA, Gruen RJ, Wisniewski T (2004) Amyloid-beta deposition is associated with decreased hippocampal glucose metabolism and spatial memory impairment in APP/PS1 mice.J Neuropathol Exp Neurol 63:418-428.
Salkovic-Petrisic M, Tribl F, Schmidt M, Hoyer S, Riederer P (2006)Alzheimer-like changes in protein kinase B and glycogen synthase kinase-3 in rat frontal cortex and hippocampus after damage to the insulin signalling pathway. J Neurochem 96:1005-1015.
Samuel SM, Thirunavukkarasu M, Penumathsa SV, Paul D, Maulik N(2008) Akt/FOXO3a/SIRT1-mediated cardioprotection by n-tyrosol against ischemic stress in rat in vivo model of myocardial infarction: switching gears toward survival and longevity. J Agric Food Chem 56:9692-9698.
Sapcanin A, So fic E, Tahirovic I, Salkovic-Petrisic M, Hoyer S, Riederer P (2008) Antioxidant capacity in rat brain after intracerebroventricular treatment with streptozotocin and alloxan--a preliminary study. Neurotox Res 13:97-104.
Sharma M, Gupta YK (2002) Chronic treatment with trans resveratrol prevents intracerebroventricular streptozotocin induced cognitive impairment and oxidative stress in rats. Life Sci 71:2489-2498.
Shoham S, Bejar C, Kovalev E, Weinstock M (2003) Intracerebroventricular injection of streptozotocin causes neurotoxicity to myelin that contributes to spatial memory deficits in rats. Exp Neurol 184:1043-1052.
Shoham S, Bejar C, Kovalev E, Schorer-Apelbaum D, Weinstock M(2007) Ladostigil prevents gliosis, oxidative-nitrative stress and memory deficits induced by intracerebroventricular injection of streptozotocin in rats. Neuropharmacology 52:836-843.
Song YY, Qi G, Li YP, Han JT (2006a) Efffect of salidroside on expression of tumor necrosis factor-α with global cerebral ischemia-reperfusion injury. Zhongcaoyao 37:907-908.
Song YY, QI G, Han JT, Li YP (2006b) Effect of salidroside on changes of interleukin beta of rats with global cerebral ischemia-reperfusion injury. Zhongguo Linchuang Kangfu 10:30-32.
Sonkusare S, Srinivasan K, Kaul C, Ramarao P (2005) Effect of donepezil and lercanidipine on memory impairment induced by intracerebroventricular streptozotocin in rats. Life Sci 77:1-14.
Spasov AA, Wikman GK, Mandrikov VB, Mironova IA, Neumoin VV (2000) A double-blind, placebo-controlled pilot study of the stimulating and adaptogenic effect of Rhodiola rosea SHR-5 extract on the fatigue of students caused by stress during an examination period with a repeated low-dose regimen. Phytomedicine 7:85-89.
St-Laurent-Thibault C, Arseneault M, Longpré F, Ramassamy C (2011)Tyrosol and hydroxytyrosol, two main components of olive oil, protect N2a cells against amyloid-beta-induced toxicity. Involvement of the NF-kappaB signaling. Curr Alzheimer Res 8:543-551.
Szkudelski T (2001) The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol Res 50:537-546.
Tahirovic I, So fic E, Sapcanin A, Gavrankapetanovic I, Bach-Rojecky L,Salkovic-Petrisic M, Lackovic Z, Hoyer S, Riederer P (2007) Brain antioxidant capacity in rat models of betacytotoxic-induced experimental sporadic Alzheimer’s disease and diabetes mellitus. J Neural Transm Suppl:235-240.
Tian JY, Chen L, Zhang XL, Li J, Han J, Yang XM, Zhang PC, Du PG,An LP, Ye F (2013) Investigation of a compound, compatibility of Rhodiola crenulata, Cordyceps militaris, and Rheum palmatum on metabolic syndrome treatment III--controlling blood glucose.Zhongguo Zhong Yao Za Zhi 38:1570-1576.
Tu Y, Roberts L, Shetty K, Schneider SS (2008) Rhodiola crenulata induces death and inhibits growth of breast cancer cell lines. J Med Food 11:413-423.
Veerendra Kumar MH, Gupta YK (2003) Effect of Centella asiatica on cognition and oxidative stress in an intracerebroventricular streptozotocin model of Alzheimer’s disease in rats. Clin Exp Pharmacol Physiol 30:336-342.
Wang J, Rong X, Li W, Yang Y, Yamahara J, Li Y (2012) Rhodiola crenulata root ameliorates derangements of glucose and lipid metabolism in a rat model of the metabolic syndrome and type 2 diabetes. J Ethnopharmacol 142:782-788.
Wang L, Zhang XL, Li MH, Tian JY, Zhang PC, Ye F (2013) Investigation of a compound, compatibility of Rhodiola crenulata, Cordyceps militaris, and Rheum palmatum, on metabolic syndrome treatment. V--Mechanisms on improving glucose metabolic disorders.Zhongguo Zhong Yao Za Zhi 38:1972-1976.
Wang S, Wang FP (1992) Studies on the chemical components of Rhodiola crenulata. Yao Xue Xue Bao 27:117-120.
Watson GS, Craft S (2003) The role of insulin resistance in the pathogenesis of Alzheimer’s disease: implications for treatment. CNS Drugs 17:27-45.
Weiss RB (1982) Streptozocin: a review of its pharmacology, efficacy,and toxicity. Cancer Treat Rep 66:427-438.
Wing SL, Askew EW, Luetkemeier MJ, Ryujin DT, Kamimori GH,Grissom CK (2003) Lack of effect of Rhodiola or oxygenated water supplementation on hypoxemia and oxidative stress. Wilderness Environ Med 14:9-16.
Yang X, Xu W, Zhao W, Zhao Y, Yang Y, Ling Y (2013) Synthesis and neuroprotective effects of the fluorine substituted salidroside analogues in the PC12 cell model exposed to hypoglycemia and serum limitation. Chem Pharm Bull (Tokyo) 61:1192-1196.
Yu P, Hu C, Meehan EJ, Chen L (2007) X-ray crystal structure and antioxidant activity of salidroside, a phenylethanoid glycoside. Chem Biodivers 4:508-513.
Yu S, Liu M, Gu X, Ding F (2008) Neuroprotective effects of salidroside in the PC12 cell model exposed to hypoglycemia and serum limitation. Cell Mol Neurobiol 28:1067-1078.
Yu WS, Chen XM, Li H, Yang L (1993) Polyphenols from Rhodiola crenulata. Planta Med 59:80-82.
Zhang L, Yu H, Zhao X, Lin X, Tan C, Cao G, Wang Z (2010) Neuroprotective effects of salidroside against beta-amyloid-induced oxidative stress in SH-SY5Y human neuroblastoma cells. Neurochem Int 57:547-555.
Zhang L, Yu H, Sun Y, Lin X, Chen B, Tan C, Cao G, Wang Z (2007)Protective effects of salidroside on hydrogen peroxide-induced apoptosis in SH-SY5Y human neuroblastoma cells. Eur J Pharmacol 564:18-25.
Zhang S, Chen X, Yang Y, Zhou X, Liu J, Ding F (2011) Neuroprotection against cobalt chloride-induced cell apoptosis of primary cultured cortical neurons by salidroside. Mol Cell Biochem 354:161-170.
Zhang S, Huang XY, Liu S, Li YJ, Zhao JC (2016) Effects of amyloid-beta 25-35 on expression of synapse-associated proteins in PC12 neurons Effects of amyloid-beta 25-35 on expression of synapse- associated proteins in PC12 neurons. Zhongguo Zuzhi Gongcheng Yanjiu 20:224-229.
Zhou JT, Li CY, Wang CH, Wang YF, Wang XD, Wang HT, Zhu Y,Jiang MM, Gao XM (2015) Phenolic compounds from the roots of Rhodiola crenulata and their antioxidant and inducing ifn-gamma Production activities. Molecules 20:13725-13739.
Zhu C, Guan F, Wang C, Jin LH (2014) The protective effects of Rhodiola crenulata extracts on Drosophila melanogaster gut immunity induced by bacteria and SDS toxicity. Phytother Res 28:1861-1866.
How to cite this article:Wang JM, Qu ZQ, Wu JL, Chung P, Zeng YS (2017) Mitochondrial protective and anti-apoptotic effects of Rhodiola crenulata extract on hippocampal neurons in a rat model of Alzheimer’s disease. Neural Regen Res 12(12):2025-2034.
Funding: This study was supported by grants of the Administrative Bureau of Chinese Traditional Medicine of Guangdong Province of China,No. 2007109; and the Medical Research Foundation of Guangdong Province of China, No. A20111154.
Graphical Abstract
*Correspondence to:Yuan-shan Zeng, M.D., Ph.D.,zengysh@mail.sysu.edu.cn.
#These authors contributed equally to this paper.
orcid:0000-0003-3804-5792(Yuan-shan Zeng)
10.4103/1673-5374.221160
2017-09-05
The authors thank Hong Kong Holistal International Ltd. for providing Rhodiola crenulata extracts.
Copyedited by Patel B, de Souza M, Yu J, Li CH, Qiu Y, Song LP, Zhao M
杂志排行
中国神经再生研究(英文版)的其它文章
- Roles of neural stem cells in the repair of peripheral nerve injury
- Advanced diffusion-weighted magnetic resonance imaging in the evaluation of white matter axons in patients with idiopathic normal pressure hydrocephalus
- The contribution of oligodendrocytes and oligodendrocyte progenitor cells to central nervous system repair in multiple sclerosis: perspectives for remyelination therapeutic strategies
- MicroRNAs in Parkinson’s disease and emerging therapeutic targets
- Surgical reconstruction of spinal cord circuit provides functional return in humans
- Environmental cues determine the fate of astrocytes after spinal cord injury
