D-dimer may predict poor outcomes in patients with aneurysmal subarachnoid hemorrhage: a retrospective study
2018-01-05JunhuiLiuXiangkuiLiZhibiaoChenQiangCaiLongWangYinghuYeQianxueChen
Jun-hui Liu, Xiang-kui Li, Zhi-biao Chen, Qiang Cai, Long Wang, Ying-hu Ye, Qian-xue Chen,
1 Department of Neurosurgery, Renmin Hospital of Wuhan University, Wuhan, Hubei Province, China
2 Department of Neurosurgery, Affiliated Hospital of Shandong Medical College, Linyi, Shandong Province, China
D-dimer may predict poor outcomes in patients with aneurysmal subarachnoid hemorrhage: a retrospective study
Jun-hui Liu1, Xiang-kui Li2, Zhi-biao Chen1, Qiang Cai1, Long Wang1, Ying-hu Ye1, Qian-xue Chen1,*
1 Department of Neurosurgery, Renmin Hospital of Wuhan University, Wuhan, Hubei Province, China
2 Department of Neurosurgery, Affiliated Hospital of Shandong Medical College, Linyi, Shandong Province, China
Serum biomarkers may play a reliable role in predicting the outcomes of patients with aneurysmal subarachnoid hemorrhage. This study retrospectively analyzed the relationship between serum biomarkers on admission and outcomes in patients with aneurysmal subarachnoid hemorrhage. We recruited 146 patients with aneurysmal subarachnoid hemorrhage who were treated in Renmin Hospital of Wuhan University of China between 1 May 2014 and 30 March 2016. There were 57 males and 89 females included and average age of included patients was 57.03 years old. Serum samples were taken immediately on admission (within 48 hours after initial hemorrhage) and the levels of serum biomarkers were detected. Baseline information, complications, and outcomes at 6 months were recorded. Univariate and multivariate logistic regression analyses were used to explore the relationship between biomarkers and clinical outcomes. Receiver operating characteristic curves were obtained to investigate the possibility of the biomarkers predicting prognosis. Of the 146 patients, 102 patients achieved good outcomes and 44 patients had poor outcomes. Univariate and multivariate analyses showed that high World Federation of Neurosurgical Societies grade, high serum D-dimer levels, and high neurological complications were signi ficantly associated with poor outcomes. Receiver operating characteristic curves veri fied that D-dimer levels were associated with poor outcomes. D-dimer levels strongly correlated with neurological complications. In conclusion, we suggest that D-dimer levels are a good independent prognostic factor for poor outcomes in patients with aneurysmal subarachnoid hemorrhage.
nerve regeneration; aneurysmal subarachnoid hemorrhage; D-dimer; serum; biomarkers; complications; prognosis; logistic regression analysis; neural regeneration
Introduction
Aneurysmal subarachnoid hemorrhage (aSAH) is a devastating disease with an annual incidence of 2–25 per 100,000 persons (de Rooij et al., 2007; Liu et al., 2011; Takashima et al., 2017; Wang et al., 2017). Although the management of aSAH in the past two decades has rapidly developed, 24.8%of patients still die in the first year after initial hemorrhage and more than 40% of survivors have various degrees of disability (de Azúa López Zaida et al., 2015; Mukhtar et al.,2016). Several clinical studies had demonstrated that age,Hunt-hess grade, World Federation of Neurosurgical Societies (WFNS) scale grade, and Fisher grade on admission are associated with unfavorable outcomes in patients with aSAH(Rosengart et al., 2007; Jaja et al., 2015; Zhao et al., 2017). In spite of these clinical factors, it is still necessary for clinicians to find more functional outcome predictors because predicting outcomes solely based on clinical factors are sometimes inaccurate (Le Roux et al., 1996; Turner et al., 2015; Greenberg et al., 2017).
Biomarkers are easy to obtain and measure, and because of their potential role in the pathological process after aSAH,serum biomarkers might be more reliable in predicting the outcomes of patients with aSAH (Hong et al., 2014; Ding et al., 2016; Paschoal et al., 2016; Suzuki and Kawakita, 2016;Frontera et al., 2017). Clinical studies regarding the relationship between these biomarkers on admission and outcomes are limited and more studies are needed to verify the roles of serum biomarkers in predicting the prognosis of patients after aSAH. Numerous factors might lead to elevated levels of D-dimer after aSAH, such as enhanced fibrinolysis activity, disruption of vessels, tissue factor release, and dissolved clots (Larsen et al., 2012; Staykov and Schwab, 2013; Boluijt et al., 2015). Clinical studies have revealed that D-dimer levels are elevated in patients with aSAH (Parra, 2006). Few studies have investigated the relationship between D-dimer levels and poor outcomes, and their prognostic role in aSAH. Thus, this study aimed to explore the prognostic role of D-dimer levels in patients with aSAH in the hope the results might give some guidance to the management in aSAH.
Subjects and Methods
Subjects
A retrospective study was conducted in aSAH patients, who were treated in Renmin Hospital of Wuhan University of China between 1 May 2014 and 30 March 2016. Patients included were diagnosed with aSAH by head computed tomography (CT) and head CT angiography (CTA) (Ni et al., 2016) or digital subtraction angiography (DSA) (Bechan et al., 2015). The aSAH patients also received hemostatic agents, mannitol or furosemide, nimodipine, prophylactic anti-seizure drugs, antihypertensive drugs, and other symptomatic treatment if necessary immediately on admission. After patients were diagnosed with aSAH, they received either microsurgical clipping or coiling. After surgery, patients also received standard treatments, as well as head CT and CTA.An immediate CT scan was performed to con firm any pathological changes if patients had neurological deterioration.
Inclusion criteria: Patients presenting with all of the following criteria were considered for study inclusion: (1)aSAH by head CT and head CTA or DSA (Grasso et al.,2017); (2) time from initial hemorrhage to admission < 48 hours. Exclusion criteria: Patients with one or more of the following conditions were excluded from this study: (1)perimesencephalic nonaneurysmal SAH or SAH caused by head trauma, vascular anomaly, vascular malformation, or moyamoya disease; (2) bilateral mydriasis, dilated pupils, or other irreversible brainstem injury; (3) severe heart/renal/respiratory/liver dysfunction; (4) taking anticoagulant drugs or corticosteroids or infection diseases in the 1 month before hospitalization. The study followed the principles of theDeclaration of Helsinkiand was approved by the Medical Ethics Committee of the Renmin Hospital of Wuhan University of China.
Data collection
We recorded the details (name, sex, and age) of patients and asked patients or their relatives about the history of the disease process. Details of accompanying diseases were acquired,as well as history of smoking and drinking. The Glasgow Coma scale (GCS) (Green et al., 2017) and WFNS Scale (Sano et al., 2015) were used to assess neurological status. Patients were categorized by Fisher grade (Smith et al., 2005) according to the head CT presentations on admission. Aneurysm location and the size of the neck of aneurysm were also recorded. Patients received either surgical clipping or coiling as soon as possible. We recorded postoperative complications, including systematic complications and neurological complications. Neurological complications included delayed cerebral ischemia, hydrocephalus, and seizure. Delayed cerebral ischemia was defined as an unexplained neurological deterioration or a new infarct on head CT after surgery, but not surgery-related cerebral ischemia (Suarez, 2015; Foreman, 2016). Systematic complications were de fined as pneumonia, urinary tract infection, or sepsis (Zheng and Wong,2017). The Glasgow outcome scale (GOS) was used to assess the neurological function at 6 months after the initial hemorrhage (Turek et al., 2016). A favorable outcome was de fined as 3–5 on the GOS and 1–2 on the GOS was de fined as poor outcome (Giraldo et al., 2012; Elhadi et al., 2015).
Serum biomarker measurement
Serum samples were taken immediately on admission (within 48 hours after initial hemorrhage) and sent to clinical laboratory of Renmin Hospital of Wuhan University for further analysis. Standard quantitative assay techniques were used to detect the levels of serum biomarkers and all operations were consistent with manufacturers’ instructions (AU5800,Beckman, CA, USA). Normal values of the biomarkers were as follows: leukocytes: 4–10 × 109/L, blood platelets: 100–300× 109/L, K+: 3.5–5.5 mM, Na+: 135–145 mM, D-dimers:0–0.55 mg/L, C-reactive protein: 0–10 mg/L, lactic dehydrogenase: 135.0–215.0 U/L, and low density lipoprotein-cholesterol: < 3.1 mM (Rahmanian et al., 2015).
Statistical analysis
Descriptive analysis (mean, standard deviation, and median) was used for continuous variables and percentages for categorical variables. The analysis of the association between variables (baseline information and biomarkers on admission) and clinical outcome was evaluated using univariate logistic regression analysis and multivariate logistic regression was used for further analysis. If variables wereP< 0.05,receiver operating characteristic (ROC) curves were used to access the prediction ability of the variants. We conducted additional analyses of candidate biomarkers and compli-cations using logistic regression analysis and also explored the correlations between candidate biomarkers and baseline information. Correlations between variables were analyzed using Spearman’s rank correlation test. Statistical analyses were conducted using SPSS 21.0 software (IBM, Armork,NY, USA) and Medcalc (Medcalc, Ostend, Belgium).P< 0.05 was considered statistically signi ficant.
Results
Characteristics of aSAH patients
One hundred and forty-six patients with aSAH were included in this retrospective study, including 57 males and 89 females. One hundred and two of these patients achieved good outcomes at 6 months after initial hemorrhage, whereas 44 patients had poor outcomes. The mean ages of patients with good outcomes and poor outcomes were 56.68 ± 8.16 and 57.86 ± 9.92 years old, respectively. There were 50 patients with hypertension, 16 with diabetes, 27 patients who smoked. GCS scores were 3–8 in 31 patients, 9–11 in 17 patients, and 12–15 in 100 patients on admission. There were 101 patients with a WFNS grade of I–III and 45 patients with a WFNS grade of IV–V. Hunt and Hess scale grade was used to access the status of patients (Ghosh et al., 2012) with grades I–II in 80 patients and grades III–IV in 66 patients.The mean time to treatment commencement (initial hemorrhage to admission) was 12.78 hours. One hundred and eleven aSAH patients received neurosurgical clipping and 35 patients received endovascular coiling. Patients’ baseline details are shown in Table 1.

Table 1 Baseline information of 146 patients with aneurysmal subarachnoid hemorrhage
Postoperative complications
Both neurological and systematic complications were recorded in this study. The results showed that 18 patients suffered from delayed cerebral ischemia, 19 patients experienced seizures, and 15 patients developed chronic hydrocephalus. Thirty-four patients had systematic complications,including fever, infections, and abnormal renal function.Details are shown in Table 2.
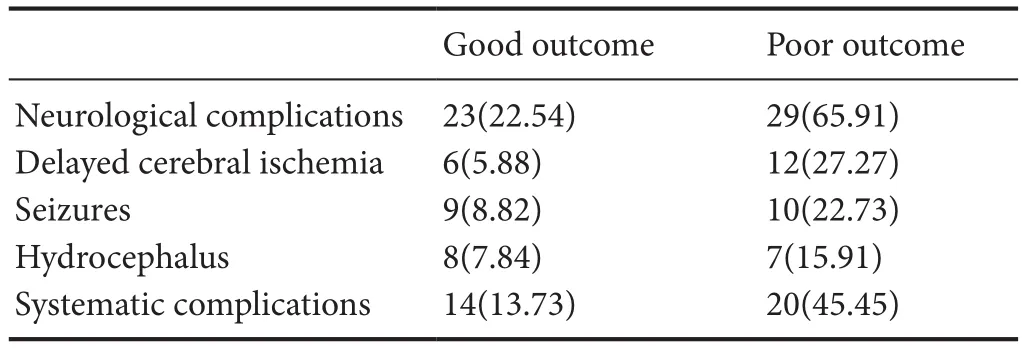
Table 2 Postoperative complications of survival patients with aneurysmal subarachnoid hemorrhage
Analyses of variables predicting poor outcomes
Results of the univariate and multivariate analyses are shown in Tables 3 and 4, respectively. WFNS grades IV–V, Fisher grades III–IV, D-dimer levels, C-reactive protein,systematic complications, and neurological complications were associated with poor outcomes (P< 0.05). We selected three main biomarkers (P< 0.05), which were associated with poor outcomes in univariate analyses to perform multivariate analysis. These results showed that WFNS grade and D-dimer levels were signi ficantly associated with poor outcomes (Table 4).
Prediction ability of biomarkers
We performed ROC to assess each biomarker’s prediction ability. The areas under the curve were 0.69, 0.86, 0.61,and 0.71 for C-reactive protein, D-dimers, leukocytes, and WFNS grade IV–V, respectively. The sensitivity of C-reactive protein, D-dimer, leukocytes, and WFNS IV–V in predicting poor outcomes was 70.45%, 82.09%, 50.00%, and 59.09%, respectively. The speci ficity of these four variables was 65.69%, 78.43%, 76.47% and 82.35%, respectively. Details are presented in Figure 1.

Table 3 Univariate analysis for variables of a poor outcome

Table 4 Multivariate analysis of variables indicating a poor outcome

Table 5 Results of Spearmen’s correlation between variables and D-dimer
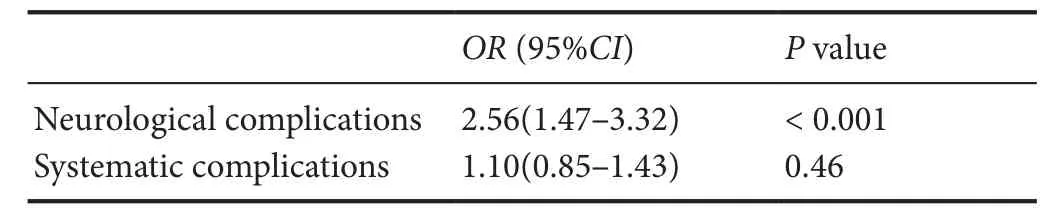
Table 6 Multivariate analysis for contribution of D-dimer to complications
Additional analysis of D-dimers
D-dimer levels were positively correlated with high WFNS grade, high Hunt-hess grade, and high Fisher grade, and were also strongly correlated with neurological complications (Table 5). Univariate and multivariate regression analyses were used to evaluate the contribution of D-dimer levels to complications. The results showed that D-dimer levels were significantly associated with neurological complications (OR= 2.56, 95%CI(1.47–3.32),P< 0.001), but not systematic complications (OR= 1.10, 95%CI(0.85–1.43),P= 0.46). Details are shown in Table 6.
Discussion

Figure 1 Diagnostic value of biomarkers for poor outcomes.
This was a retrospective study in which the patients were diagnosed with aSAH and underwent medical treatment within 48 hours. Preliminary findings showed that WFNS grades IV–V, Hunt and Hess scale grades III–V, Fisher grades III–IV, D-dimers, C-reactive protein, leukocytes, systematic complications, and neurological complications were associated with poor outcomes (Orakdogen et al., 2016; Aggarwal et al., 2017; Dinc et al., 2017). Several studies have demonstrated that higher WFNS, Fisher, and Hunt and Hess scale grades are associated with poorer outcomes in patients with aSAH (Sano et al., 2016; Zhao et al., 2016; Kilic et al., 2017;Ljubisavljevic et al., 2017). Moreover, a predictable model built by Zhao et al. (2017), which combined baseline information and postoperative pneumonia, had excellent discrimination. WFNS grade V and Fisher grades III–V were found to be independent factors for a poor outcome (OR= 3.728, 6.234, respectively). Moreover, they had excellent prognostic value in discriminating poor and good outcomes with an area under curve of 0.87 (van Donkelaar et al., 2017).Biomarkers, such as C-reactive protein, interleukin-6 (Ljubisavljevic et al., 2017), serum tumor necrosis factor-α (Sarrafzadeh et al., 2010; Sun et al., 2017), and erythrocyte distribution(Chugh et al., 2015), were all found to be associated with poor outcomes in aSAH patients. In flammatory factors on admission, including high-sensitivity-C-reactive protein and leukocyte levels, were shown to have independent associations with GOS scores at 3 months (Srinivasan et al., 2016). The level of C-reactive protein on admission failed to show a signi ficant association with poor outcomes, as well as neurological and systematic complications in this retrospective study (Romero et al., 2012; Badjatia et al., 2015). In this study, leukocyte levels were obviously higher in patients with poor outcomes,but it was not an independent risk factor for poor outcome.Leukocyte levels were also not a good prognostic factor for poor outcomes. This result was the same as a previous study(Rothoerl et al., 2006), where leukocytes had no significant relationship with clinical outcomes.
D-dimer is a fibrin degradation product and has been investigated in many diseases, such as deep vein thrombosis,acute aortic dissection, intracerebral hemorrhage, and also aSAH (Fujii et al., 1995; Suzuki et al., 1997; Ramchand et al., 2016; Fukuda et al., 2017). Patients with poor outcomes had higher D-dimer levels, which predicted poor outcomes independently. D-dimer levels were significantly higher in patients with SAH and were first found to be correlated with poor outcomes in 1995 by Fujii et al. Several studies have demonstrated that D-dimers are an independent predictor for delayed ischemic neurological de ficit and delayed cerebral ischemia in patients with aSAH. In our study, D-dimer levels showed significant correlation with neurological complications and were also significantly associated with neurological complications, which differs from previous results (Fukuda et al., 2017). It is believed that pathophysiological processes after SAH are complicated and that certain factors might play different roles at different times (de Lima Oliveira et al., 2015; van Lieshout et al., 2017). The dynamic changes of D-dimer levels, which were not investigated in our study, are very important. Ilveskero et al. (2005) found that D-dimer levels on admission and after surgery were elevated and correlated with long-term outcomes. In this retrospective study, we confirmed that D-dimer levels on admission had signi ficant association with poor outcomes.Furthermore, the area under the curve of D-dimers in ROC was 0.86, which indicated that it had good prognostic value in predicting poor outcomes. Similar findings were reported in a previous study and the results showed that D-dimer levels after admission could predict poor outcomes independently (Juvela and Siironen, 2006).
Elevation of D-dimer levels always indicates enhanced fibrinolysis activity in the human body and is a biomarker of a hypercoagulative state in patients with SAH (Filizzolo et al.,1978; Burchiel et al., 1984; Ilveskero et al., 2005). The possible mechanism of elevated plasma D-dimer levels might be as follows: The sudden disruption of vessels causes injury to the endothelium and subsequently releases tissue factor.Tissue factor release and dissolved subarachnoid clots may play important roles in fibronolysis, which led to D-dimer elevation (Fukuda et al., 2017). However, in previous studies(Mocco et al., 2006; Boluijt et al., 2015), no association was found between D-dimers on admission and delayed cerebral ischemia, which differs from the results in our study. Our results confirmed that elevated D-dimer levels are significantly associated with neurological complications (including delayed cerebral ischemia and seizures). Moreover, elevated D-dimer levels could be used as an independent prognostic factor for neurological complications.
In this study, we only investigated preoperative levels of D-dimer and this indicated that D-dimers have a signi ficant association with poor outcomes with good sensitivity and specificity in predicting poor outcomes in patients with aSAH. Blood samples were collected on admission, because many factors, such as surgery and deep venous thrombosis, might have effects on D-dimer levels after surgery(Delgado et al., 2006). Furthermore, preoperative D-dimer levels might provide more reliable evidence for fibrinolytic activation caused by the rupture of intracranial aneurysm,which might contribute to early brain damage and in-hospital complications. Elevated D-dimer levels on admission provide information about whether patients might achieve good outcomes or poor outcomes, as early as possible. In this study, we con firmed that D-dimer levels could be used as a prognostic factor for poor outcomes in aSAH patients.However, the real role of D-dimer levels as a prognostic factor for patients with aSAH is still unclear and needs further clinical studies, with strict inclusion and exclusion criteria,to explore its dynamic change before and after surgery.
Several limitations exist in this study. We did not explore the dynamic change of D-dimer levels in patients with aSAH and the number of patients was relatively small. D-dimer levels may differ between patients who received neurological clipping and underwent endovascular coiling, and further studies should focus on each of these groups.
In summary, plasma D-dimer levels are significantly elevated in aSAH patients with poor outcomes at 6 months after the initial hemorrhage. D-dimer levels had a signi ficant association with poor outcomes and neurological complications. D-dimers levels are good and independent prognostic factors for poor outcomes in aSAH patients.
Author contributions:JHL and XKL conceived and designed the study.ZBC, QC, LW and YHY performed experiments. JHL and LW collected clinical data. QXC and JHL wrote and edited the paper. All authors approved the final version of the paper.
Con flicts of interest:None declared.
Research ethics:The study followed the principles of the Declaration of Helsinki and was approved by the Medical Ethics Committee of the Renmin Hospital of Wuhan University of China.
Declaration of patient consent:The authors certify that they have obtained all appropriate patient consent forms. In the form patients have given his/her/their consent for their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under identical terms.
Aggarwal A, Dhandapani S, Praneeth K, Sodhi HB, Pal SS, Gaudihalli S, Khandelwal N, Mukherjee KK, Tewari MK, Gupta SK, Mathuriya SN (2017) Comparative evaluation of H&H and WFNS grading scales with modified H&H (sans systemic disease): A study on 1000 patients with subarachnoid hemorrhage. Neurosurg Revdoi:10.1007/s10143-017-0843-y.
Badjatia N, Monahan A, Carpenter A, Zimmerman J, Schmidt JM,Claassen J, Connolly ES, Mayer SA, Karmally W, Seres D (2015)In flammation, negative nitrogen balance, and outcome after aneurysmal subarachnoid hemorrhage. Neurology 84:680-687.
Bechan RS, van Rooij SB, Sprengers ME, Peluso JP, Sluzewski M, Majoie CB, van Rooij WJ (2015) CT angiography versus 3D rotational angiography in patients with subarachnoid hemorrhage. Neuroradiology 57:1239-1246.
Boluijt J, Meijers JC, Rinkel GJ, Vergouwen MD (2015) Hemostasis and fibrinolysis in delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage: a systematic review. J Cereb Blood Flow Metab 35:724-733.
Burchiel KJ, Hoffman JM, Bakay RA (1984) Quantitative determination of plasma fibrinolytic activity in patients with ruptured intracranial aneurysms who are receiving epsilon-aminocaproic acid: relationship of possible complications of therapy to the degree of fibrinolytic inhibition. Neurosurgery 14:57-63.
Chugh C, Nyirjesy SC, Nawalinski KP, Sandsmark DK, Frangos S,Maloney-Wilensky E, Stein SC, Levine JM, Kasner SE, Kumar MA(2015) Red blood cell distribution width is associated with poor clinical outcome after subarachnoid hemorrhage: a pilot study. Neurocrit Care 23:217-224.
de Azúa López Zaida R, Egea-Guerrero Juan J, Rivera-Rubiales G, Rodríguez-Rodríguez A, Vilches-Arenas Á, Murillo-Cabezas F (2015)Serum brain injury biomarkers as predictors of mortality after severe aneurysmal subarachnoid hemorrhage: preliminary results. Clin Chem Lab Med 53:e179-181.
de Lima Oliveira M, de Azevedo DS, de Azevedo MK, de Carvalho Nogueira R, Teixeira MJ, Bor-Seng-Shu E (2015) Encephalic hemodynamic phases in subarachnoid hemorrhage: how to improve the protective effect in patient prognoses. Neural Regen Res 10:748-752.
de Rooij NK, Linn FH, van der Plas JA, Algra A, Rinkel GJ (2007) Incidence of subarachnoid haemorrhage: a systematic review with emphasis on region, age, gender and time trends. J Neurol Neurosurg Psychiatry 78:1365-1372.
Delgado P, Alvarez-Sabín J, Abilleira S, Santamarina E, Purroy F,Arenillas JF, Molina CA, Fernández-Cadenas I, Rosell A, Montaner J (2006) Plasma d-dimer predicts poor outcome after acute intracerebral hemorrhage. Neurology 67:94-98.
Dinc N, Lescher S, Quick-Weller J, Berkefeld J, Platz J, Senft C, Seifert V, Konczalla J (2017) Outcome, prognostic factors, and follow-up results after subarachnoid hemorrhage from pericallosal artery aneurysms. World Neurosurg 99:566-571.
Ding YS, Sun B, Jiang JX, Zhang Q, Lu J, Gao GZ (2016) Increased serum concentrations of signal peptide-Cub-Egf domain-containing protein-1 in patients with aneurysmal subarachnoid hemorrhage.Clin Chim Acta 459:117-122.
Elhadi AM, Zabramski JM, Almefty KK, Mendes GA, Nakaji P, Mc-Dougall CG, Albuquerque FC, Preul MC, Spetzler RF (2015) Spontaneous subarachnoid hemorrhage of unknown origin: hospital course and long-term clinical and angiographic follow-up. J Neurosurg 122:663-670.
Filizzolo F, D’Angelo V, Collice M, Ferrara M, Donati MB, Porta M(1978) Fibrinolytic activity in blood and cerebrospinal fluid in subarachnoid hemorrhage from ruptured intracranial saccular aneurysm before and during EACA treatment. Eur Neurol 17:43-47.
Foreman B (2016) The pathophysiology of delayed cerebral ischemia. J Clin Neurophysiol 33:174-182.
Frontera JA, Provencio JJ, Sehba FA, McIntyre TM, Nowacki AS, Gordon E, Weimer JM, Aledort L (2017) The role of platelet activation and in flammation in early brain injury following subarachnoid hemorrhage. Neurocrit Care 26:48-57.
Fujii Y, Takeuchi S, Sasaki O, Minakawa T, Koike T, Tanaka R (1995)Hemostasis in spontaneous subarachnoid hemorrhage. Neurosurgery 37:226-234.
Fukuda H, Lo B, Yamamoto Y, Handa A, Yamamoto Y, Kurosaki Y,Yamagata S (2017) Plasma D-dimer may predict poor functional outcomes through systemic complications after aneurysmal subarachnoid hemorrhage. J Neurosurg 127: 284-290.
Ghosh S, Dey S, Maltenfort M, Vibbert M, Urtecho J, Rincon F, Jallo J(2012) Impact of Hunt-Hess grade on the glycemic status of aneurysmal subarachnoid hemorrhage patients. Neurol India 60:283-287.
Giraldo EA, Mandrekar JN, Rubin MN, Dupont SA, Zhang Y, Lanzino G, Wijdicks EF, Rabinstein AA (2012) Timing of clinical grade assessment and poor outcome in patients with aneurysmal subarachnoid hemorrhage. J Neurosurg 117:15-19.
Grasso G, Alafaci C, Macdonald RL (2017) Management of aneurysmal subarachnoid hemorrhage: State of the art and future perspectives.Surg Neurol Int 8:11.
Green SM, Haukoos JS, Schriger DL (2017) How to Measure the Glasgow Coma Scale. Ann Emerg Meddoi:10.1016/j.annemergmed.2016.12.016.
Greenberg JK, Guniganti R, Arias EJ, Desai K, Washington CW, Yan Y, Weng H, Xiong C, Fondahn E, Cross DT, Moran CJ, Rich KM,Chicoine MR, Dhar R, Dacey RG, Jr., Derdeyn CP, Zipfel GJ (2017)Predictors of 30-day readmission after aneurysmal subarachnoid hemorrhage: a case-control study. J Neurosurg 126:1847-1854.
Hong CM, Tosun C, Kurland DB, Gerzanich V, Schreibman D, Simard JM (2014) Biomarkers as outcome predictors in subarachnoid hemorrhage--a systematic review. Biomarkers 19:95-108.
Ilveskero S, Juvela S, Siironen J, Lassila R (2005) D-dimer predicts outcome after aneurysmal subarachnoid hemorrhage: no effect of thromboprophylaxis on coagulation activity. Neurosurgery 57:16-24; discussion 16-24.
Jaja BN, Lingsma H, Schweizer TA, Thorpe KE, Steyerberg EW, Macdonald RL, SAHIT collaboration (2015) Prognostic value of premorbid hypertension and neurological status in aneurysmal subarachnoid hemorrhage: pooled analyses of individual patient data in the SAHIT repository. J Neurosurg 122:644-652.
Juvela S, Siironen J (2006) D-dimer as an independent predictor for poor outcome after aneurysmal subarachnoid hemorrhage. Stroke 37:1451-1456.
Kilic M, Yilmaz I, Tanriverdi O, Akgun C, Musluman AM, Yilmaz A(2017) Factors that affect postoperative hydrocephalus development in aneurysmal subarachnoid hemorrhage: a clinical study. Turk Neurosurg 27:353-361.
Larsen CC, Sorensen B, Nielsen JD, Astrup J (2012) Reduced clot-stability during the first 6 hours after aneurysmal subarachnoid haemorrhage--a prospective case-control study. Thromb Res 129:e229-232.
Le Roux PD, Elliott JP, Newell DW, Grady MS, Winn HR (1996) Predicting outcome in poor-grade patients with subarachnoid hemorrhage: a retrospective review of 159 aggressively managed cases. J Neurosurg 85:39-49.
Liu BR, Xiao J, Qian J, Ma JM, Mao JH, Liu J (2011) Tru fill detachable coils for acute ruptured intracranial aneurysms in one case. Zhongguo Zuzhi Gongcheng Yanjiu 15:2253-2256.
Ljubisavljevic S, Milosevic V, Stojanov A, Ljubisavljevic M, Dunjic O,Zivkovic M (2017) Identi fication of clinical and paraclinical findings predictive for headache occurrence during spontaneous subarachnoid hemorrhage. Clin Neurol Neurosurg 158:40-45.
Mocco J, Ransom ER, Komotar RJ, Schmidt JM, Sciacca RR, Mayer SA, Connolly ES, Jr. (2006) Preoperative prediction of long-term outcome in poor-grade aneurysmal subarachnoid hemorrhage. Neurosurgery 59:529-538; discussion 529-538.
Mukhtar TK, Molyneux AJ, Hall N, Yeates DR, Goldacre R, Sneade M,Clarke A, Goldacre MJ (2016) The falling rates of hospital admission, case fatality, and population-based mortality for subarachnoid hemorrhage in England, 1999-2010. J Neurosurg 125:698-704.
Ni QQ, Tang CX, Zhao YE, Zhou CS, Chen GZ, Lu GM, Zhang LJ(2016) Single phase dual-energy CT angiography: one-stop-shop tool for evaluating aneurysmal subarachnoid hemorrhage. Sci Rep 6:26704.
Orakdogen M, Emon ST, Somay H, Engin T, Ates O, Berkman MZ(2016) Prognostic factors in patients who underwent aneurysmal clipping due to spontaneous subarachnoid hemorrhage. Turk Neurosurg 26:840-848.
Parra A (2006) Are D-dimer levels after aneurysmal subarachnoid hemorrhage predictive of outcome? Nat Clin Pract Neurol 2:592-593.
Paschoal EH, Yamaki VN, Teixeira RK, Paschoal Junior FM, Jong ALGS, Teixeira MJ, Yamada ES, Ribeiro-Dos-Santos A, Bor-Seng-Shu E (2016) Relationship between endothelial nitric oxide synthase(eNOS) and natural history of intracranial aneurysms: meta-analysis. Neurosurg Revdoi:10.1007/s10143-016-0761-4.
Rahmanian A, Mohebali N, Haghnegahdar A, Kamali Sarvestani E,Razmkon A, Kivelev J, Baghban F (2015) Serum levels of monocyte chemoattractant protein-1 correlate with poor clinical grades in cerebral aneurysms. Iran J Immunol 12:302-310.
Ramchand P, Nyirjesy S, Frangos S, Doer fler S, Nawalinski K, Quattrone F, Ju C, Patel H, Driscoll N, Maloney-Wilensky E, Stein SC,Levine JM, Kasner SE, Kumar MA (2016) Thromboelastography parameter predicts outcome after subarachnoid hemorrhage: an exploratory analysis. World Neurosurg 96:215-221.
Romero FR, Bertolini Ede F, Figueiredo EG, Teixeira MJ (2012) Serum C-reactive protein levels predict neurological outcome after aneurysmal subarachnoid hemorrhage. Arq Neuropsiquiatr 70:202-205.
Rosengart AJ, Schultheiss KE, Tolentino J, Macdonald RL (2007) Prognostic factors for outcome in patients with aneurysmal subarachnoid hemorrhage. Stroke 38:2315-2321.
Rothoerl RD, Axmann C, Pina AL, Woertgen C, Brawanski A (2006)Possible role of the C-reactive protein and white blood cell count in the pathogenesis of cerebral vasospasm following aneurysmal subarachnoid hemorrhage. J Neurosurg Anesthesiol 18:68-72.
Sano H, Inamasu J, Kato Y, Satoh A, Murayama Y, WFNS Cerebrovascular Diseases and Treatment Committee (2016) Modi fied world federation of neurosurgical societies subarachnoid hemorrhage grading system. Surg Neurol Int 7:S502-503.
Sano H, Satoh A, Murayama Y, Kato Y, Origasa H, Inamasu J, Nouri M, Cherian I, Saito N; members of the 38 registered institutions and WFNS Cerebrovascular Disease & Treatment Committee (2015)Modi fied World Federation of Neurosurgical Societies subarachnoid hemorrhage grading system. World Neurosurg 83:801-807.
Sarrafzadeh A, Schlenk F, Gericke C, Vajkoczy P (2010) Relevance of cerebral interleukin-6 after aneurysmal subarachnoid hemorrhage.Neurocrit Care 13:339-346.
Smith ML, Abrahams JM, Chandela S, Smith MJ, Hurst RW, Le Roux PD (2005) Subarachnoid hemorrhage on computed tomography scanning and the development of cerebral vasospasm: the Fisher grade revisited. Surg Neurol 63:229-234; discussion 234-235.
Srinivasan A, Aggarwal A, Gaudihalli S, Mohanty M, Dhandapani M,Singh H, Mukherjee KK, Dhandapani S (2016) Impact of early leukocytosis and elevated high-sensitivity C-reactive protein on delayed cerebral ischemia and neurologic outcome after subarachnoid hemorrhage. World Neurosurg 90:91-95.
Staykov D, Schwab S (2013) Clearing bloody cerebrospinal fluid: clot lysis, neuroendoscopy and lumbar drainage. Curr Opin Crit Care 19:92-100.
Suarez JI (2015) Diagnosis and Management of Subarachnoid Hemorrhage. Continuum (Minneap Minn) 21:1263-1287.
Sun XG, Ma Q, Jing G, Wang L, Hao XD, Wang GQ (2017) Early elevated levels of soluble triggering receptor expressed on myeloid cells-1 in subarachnoid hemorrhage patients. Neurol Sci 38:873-877.
Suzuki H, Kawakita F (2016) Tenascin-C in aneurysmal subarachnoid hemorrhage: deleterious or protective? Neural Regen Res 11:230-231.
Suzuki M, Kudo A, Otawara Y, Doi M, Kuroda K, Ogawa A (1997)Fibrinolytic activity in the CSF and blood following subarachnoid haemorrhage. Acta Neurochir (Wien) 139:1152-1154.
Takashima N, Arima H, Kita Y, Fujii T, Miyamatsu N, Komori M,Sugimoto Y, Nagata S, Miura K, Nozaki K (2017) Incidence, management and short-term outcome of stroke in a general population of 1.4 million Japanese-Shiga Stroke Registry. Circ Jdoi:10.1253/circj.CJ-17-0177.
Turek G, Lewszuk A, Kochanowicz J, Lyson T, Zielinska-Turek J,Gorbacz K, Mariak Z (2016) Early outcomes and perioperative complications of endovascular embolization in patients with aneurysmal SAH. Neurol Neurochir Pol 50:342-348.
Turner CL, Budohoski K, Smith C, Hutchinson PJ, Kirkpatrick PJ,Murray GD (2015) Elevated Baseline C-Reactive Protein as a Predictor of Outcome After Aneurysmal Subarachnoid Hemorrhage: Data From the Simvastatin in Aneurysmal Subarachnoid Hemorrhage(STASH) Trial. Neurosurgery 77:786-792; discussion 792-783.
van Donkelaar CE, Bakker NA, Veeger NJ, Uyttenboogaart M, Metzemaekers JD, Eshghi O, Mazuri A, Foumani M, Luijckx GJ, Groen RJ,van Dijk JM (2017) Prediction of outcome after subarachnoid hemorrhage: timing of clinical assessment. J Neurosurg 126:52-59.
van Lieshout JH, Dibue-Adjei M, Cornelius JF, Slotty PJ, Schneider T,Restin, T, Boogaarts HD, Steiger HJ, Petridis, AK, Kamp MA (2017)An introduction to the pathophysiology of aneurysmal subarachnoid hemorrhage. Neurosurg Revdoi:10.1007/s10143-017-0827-y.
Wang W, Jiang B, Sun H, Ru X, Sun D, Wang L, Wang L, Jiang Y, Li Y, Wang Y, Chen Z, Wu S, Zhang Y, Wang D, Wang Y, Feigin VL,NESS-China Investigators (2017) Prevalence, incidence, and mortality of stroke in China: results from a nationwide population-based survey of 480 687 adults. Circulation 135:759-771.
Zhao B, Yang H, Zheng K, Li Z, Xiong Y, Tan X, Zhong M, AMPAS study group (2016) Predictors of good functional outcomes and mortality in patients with severe rebleeding after aneurysmal subarachnoid hemorrhage. Clin Neurol Neurosurg 144:28-32.
Zhao B, Yang H, Zheng K, Li Z, Xiong Y, Tan X, Zhong M, AMPAS Study Group (2017) Preoperative and postoperative predictors of long-term outcome after endovascular treatment of poor-grade aneurysmal subarachnoid hemorrhage. J Neurosurg 126:1764-1771.
Zheng VZ, Wong GKC (2017) Neuroinflammation responses after subarachnoid hemorrhage: A review. J Clin Neurosci 42:7-11.
How to cite this article:Liu JH, Li XK, Chen ZB, Cai Q, Wang L, Ye YH, Chen QX (2017) D-dimer may predict poor outcomes in patients with aneurysmal subarachnoid hemorrhage: a retrospective study. Neural Regen Res 12(12):2014-2020.
Graphical Abstract
*Correspondence to:Qian-xue Chen, Ph.D.,chenqx666@sohu.com.
orcid:0000-0003-1398-2325(Qian-xue Chen)
10.4103/1673-5374.221158
2017-10-27
Copyedited by Yu J, Li CH, Qiu Y, Song LP, Zhao M
杂志排行
中国神经再生研究(英文版)的其它文章
- Roles of neural stem cells in the repair of peripheral nerve injury
- Advanced diffusion-weighted magnetic resonance imaging in the evaluation of white matter axons in patients with idiopathic normal pressure hydrocephalus
- The contribution of oligodendrocytes and oligodendrocyte progenitor cells to central nervous system repair in multiple sclerosis: perspectives for remyelination therapeutic strategies
- MicroRNAs in Parkinson’s disease and emerging therapeutic targets
- Surgical reconstruction of spinal cord circuit provides functional return in humans
- Environmental cues determine the fate of astrocytes after spinal cord injury
