Nitrogen uptake and transfer in broad bean and garlic strip intercropping systems
2018-01-04TANGQiuxiangHaileTewoldeLIUHongbinRENTianzhiJIANGPinganZHAILimeiLEIBaokunLINTaoLIUEnke
TANG Qiu-xiang, Haile Tewolde, LIU Hong-bin, REN Tian-zhi, JIANG Ping-an ZHAI Li-mei, LEI Bao-kun, LIN Tao, LIU En-ke
1 College of Agronomy, Xinjiang Agricultural University, Urumqi 830052, P.R.China
2 Institute of Agricultural Resources and Regional Planning, Chinese Academy of Agricultural Sciences/Key Laboratory of Nonpoint Source Pollution Control, Ministry of Agriculture, Beijing 100081, P.R.China
3 Crop Science Research Laboratory, USDA-ARS, MS 39762, USA
4 Agro-environmental Protection Institute, Ministry of Agriculture, Tianjing 300191, P.R.China
5 Institute of Agriculture Environmental Resources Research, Yunnan Academy of Agricultural Sciences, Kunming 650205,P.R.China
6 Institute of Industrial Crops, Xinjiang Academy of Agricultural Sciences, Urumqi 830091, P.R.China
7 Institute of Environment and Sustainable Development in Agriculture, Chinese Academy of Agricultural Sciences, Beijing 100081,P.R.China
RESEARCH ARTICLE
Nitrogen uptake and transfer in broad bean and garlic strip intercropping systems
TANG Qiu-xiang1,2, Haile Tewolde3, LIU Hong-bin2, REN Tian-zhi4, JIANG Ping-an1, ZHAI Li-mei2, LEI Bao-kun5, LIN Tao6, LIU En-ke7
1 College of Agronomy, Xinjiang Agricultural University, Urumqi 830052, P.R.China
2 Institute of Agricultural Resources and Regional Planning, Chinese Academy of Agricultural Sciences/Key Laboratory of Nonpoint Source Pollution Control, Ministry of Agriculture, Beijing 100081, P.R.China
3 Crop Science Research Laboratory, USDA-ARS, MS 39762, USA
4 Agro-environmental Protection Institute, Ministry of Agriculture, Tianjing 300191, P.R.China
5 Institute of Agriculture Environmental Resources Research, Yunnan Academy of Agricultural Sciences, Kunming 650205,P.R.China
6 Institute of Industrial Crops, Xinjiang Academy of Agricultural Sciences, Urumqi 830091, P.R.China
7 Institute of Environment and Sustainable Development in Agriculture, Chinese Academy of Agricultural Sciences, Beijing 100081,P.R.China
Utilization and transfer of nitrogen (N) in a strip intercropping system of garlic (Allium sativum L.) and broad bean (Vicia faba L.) have been investigated rarely. The objectives of this study were to quantify N uptake and utilization by intercropped broad bean and garlic and determine the magnitude of N transfer from broad bean to garlic. Field and pot trials were carried out in the Erhai Lake Basin in China using15N tracer applied to the soil or injected into broad bean plants. Strip intercropping of garlic and broad bean increased N absorption (47.2%) compared with sole crop broad bean (31.9%) or sole crop garlic(40.7%) and reduced soil residual N. Nearly 15% of15N injected into petioles of broad bean intercropped with garlic was recovered in garlic at harvest, suggesting that N could be transferred from broad bean to strip intercropped garlic. The findings provide a basis for evaluating legumes’ role in optimizing N fertilization when intercropped with non-legumes.
legumes, sole crop,15N abundance, nitrogen isotope, rhizoshpere
1. Introduction
Intercropping, which is the practice of growing two or more crops simultaneously on the same field, is the essence of Chinese traditional agriculture. Legumes are involved in 70% of more than 100 intercropping systems in China (Xiao et al. 2005). Broad bean (Vicia faba L.), which has a good ability to fix N2and adaptability to a wide range of soils, is planted on up to 2 million ha worldwide (Unkovich and Pate 2000) and has attracted attention of researchers in the last 20 years (Rochester et al. 1998; Unkovich and Pate 2000;Mayer et al. 2003).
Nitrogen (N) transfer from legumes to non-legumes has been reported extensively. For instance, Li et al. (2003)found that the N absorption of intercropped maize (Zea mays L.) and broad bean was higher than that of a sole crop maize or broad bean.15N tracer approach can be used to measure the N- fixing ability of legumes in an intercropping system directly and N absorption from different sources such as soil, fertilizer, and air (Zhu and Yang 1992). Using the15N tracer approach, the study of Danso et al. (1993)revealed that intercropping, compared with sole crop,improved N absorption by 26.3% and biomass by 21%.Fujita et al. (1990) found N transfer from pea (Pisum sativum L.) to sorghum (Sorghum bicolor L.) accounted for 32–58%of the total N absorption of sorghum in a pea-sorghum intercropping system. In other crops, the transfer of N from legumes to non-legumes was found to range from 2 to 17% in pea and oat (Avena sativa L.) (Papastylianou and Danso 1991), alfalfa (Medicago sativa L.) and bromegrass(Bromus riparius Rhem.), alfalfa and timothy-grass (Phleum pratense L.) (Ta et al. 1989; Tomm et al. 1994), maize and cowpea (Vigna unguiculata L.) (Eaglesham et al. 1981), and subterranean clover (Trifolium subterraneum L.) and annual ryegrass (Lolium rigidum Gaud.) (Ledgard et al. 1985). In the clover-ryegrass mixed stand, N transfer contributed strongly to the N budget of the companion ryegrass,especially in the stands where leaf fall contributed to the transfer. The uptake of clover-derived N by a companion grass may have implications for ley composition and feeding value as well as for the effects of mixed green manure and catch crops in agriculture (Dahlin and Stenberg 2010).Sierra and Desfontaines (2009) studied N transfer using15N leaf feeding method and concluded that N transfer from root exudates of jackbean (Canavalia ensiformis L.) would be a useful but minor process compared with N release from root turnover in soil. Other studies indicated that non-legums can bene fit from the N transfer from legumes in an intercropping system (Giller et al. 1991; Zhu and Yang 1992; Frey and Schüepp 1993; Høgh-Jensen and Schjoerring 2000), but the amount of N transfer varies greatly depending on crop species.
Garlic (Allium sativum L.) is another crop grown in the same regions where broad bean is grown in China, the two crops are rarely intercropped although both crops may bene fit from intercropping. Our earlier work revealed that intercropping of garlic and broad bean is suitable for planting in China because the practice improved yield and land use ef ficiency with land equivalent ratio of 1.15 (Tang et al. 2012,2013). The basis for these bene fits may be attributed to complementary N utilization and transfer between these two crops in an intercropping system. However, this has not been thoroughly investigated. The objective of this study was to use the15N tracer method to quantify N uptake and utilization by intercropped broad bean and garlic and determine the magnitude of N transfer from broad bean to garlic. The findings are expected to provide theoretical basis for evaluating role of legumes in N accumulation and optimizing N fertilization in an intercropping system of these two crops but also of other legumes and non-legumes.
2. Materials and methods
2.1. Experimental site
The study was carried out from September 2009 to May 2010 in a black loam soil in the Erhai Lake basin, China(100°13´E, 25°35´N). The location has a monsoon climate receiving an average of 800 to 1 100 mm annual rainfall,concentrated mostly in April to September. Other climatic characteristics of the location include 2 440 h annual sunshine duration, 240 frost-free days, about 15°C annual average temperature, and 69% annual average relative humidity. Garlic is the main crop in this region.
The study consisted of three complementary tests, two in the field in 1-m2mini-plots and one in the greenhouse in pots. The same soil was used in all three tests. Prior to initiating the study, the soil had 82.2 g kg–1organic matter,4.32 g kg–1total N, 2.49 mg kg–1ammonium N, 2.5 mg kg–1nitrate N, 30.5 mg kg–1Olsen-P, and pH 6.46.
2.2. Experimental design
Mini-plotsThe first of the two mini-plot tests, which is referred hereafter as Test 1, had three treatments, which included broad bean sole crop (Fig. 1), garlic sole crop(Fig. 2), and garlic-broad bean strip intercrop (Fig. 3).The design was a randomized complete block with four replications. Garlic cultivar Hongqixing and broad bean cultivar Yundou 06 were selected as the plant materials for the test. The sole crop broad bean mini-plots were planted with primed seeds at a spacing of 0.15 m between rows and 0.15 m between plants within a row. The sole crop garlic mini-plots were planted with cloves at a spacing of 0.08 m between rows and 0.06 m between cloves within a row.The planting of the intercropped mini-plots had a pattern of four rows of garlic (4G) after two rows of bean (2B) for a total of four rows of broad bean and eight rows of garlic(2B-4G-2B-4G). All mini-plots in the test had a dimension of 1 m×1 m each of which was established in the center of a larger plot measuring 5 m×4.8 m. The larger plots within a replication were spaced 0.3 m apart and, therefore, the mini-plots within a replication were spaced 4.3 m apart.

Fig. 1 Sole crop broad bean mini-plots.

Fig. 2 Sole crop garlic mini-plots.

Fig. 3 Broad bean and garlic intercropped mini-plots.
The larger plots were planted with the same crop or crop combination as the respective mini-plot. The field was prepared by moldboard plowing to a depth of 0.25 to 0.30 m before installing the mini-plots and planted with the respective crop or crops in October after installing plastic liners around each mini-plot. The liners were installed by digging a narrow trench around the mini-plots to 0.4 m depth, installing the plastic liner, and filling back the trench with the same soil. The liner was also long enough to form a shallow (≈0.2 m) berm for containment.
N was applied to the mini-plots as isotopic urea (10.15%15N) to trace N uptake and utilization. Garlic received 375 kg N ha–1if sole cropped or 300 kg N ha–1if strip intercropped with bean. Broad bean received 90 kg N ha–1whether in sole crop or intercropped. When intercropped, garlic occupied two-thirds of the total plot area and broad bean occupied one-third, so the total amount of N fertilizer applied to the intercropped plots was 230 kg N ha–1.
The N fertilizer was split-applied at three stages. All miniplots received 60 kg N ha–1just before planting. One half of the N for garlic was applied in late December when plants had about 3 to 4 leaves, the other half was applied in March of the following year when garlic was at the bulbil flower bud differentiation stage. The N for broad bean (30 kg ha–1) was applied in late December at the same time garlic had 3 to 4 leaves. The application of different N rates for garlic and broad bean when strip intercropped was accomplished by placing a calculated amounts of N fertilizer in shallow bands between the rows of garlic or broad bean plants.
The second mini-plot test, hereafter referred to as Test 2,involved two15N injection treatments imposed on garlic and broad bean intercropping to study the transfer of N from bean to garlic. The two N treatments included no15N injection (control) and15N injection in a randomized complete block design with four replications. The mini-plots in Test 2 had the same dimensions and arrangements and were planted with the same cultivars and strip intercrop planting patterns as described for Test 1. Each broad bean plant in the15N injection treatment received 1.125 mg N plant–1as (15NH4)2SO4to trace N transfer from bean to garlic. The injection of15N to bean plants was performed using a 25-μL micro-injector (Shanghai Medical Laser Instrument Factory,China) filled with 25 μL of 150 mmol L–1(15NH4)2SO4solution(99.17%15N abundance). Each broad bean plant in each15N injection mini-plot received this injection daily for 10 consecutive days starting 35 days after seed sowing. A total of 250 μL of 150 mmol L–1(15NH4)2SO4solution (equivalent to 1.125 mg15N) was injected to the base of petioles of leaves in the mid-section of the main stem. None of the two15N injection treatments received soil-applied N.
All mini-plots in both Test 1 and Test 2 received 78.6 kg P ha–1and 125 kg K ha–1. The entire amount of P as CaP2H4O8and K as K2SO4was applied to the soil before planting the crops. Four irrigations were applied for both Test 1 and Test 2 throughout the experiments.
Pot experimentThe pot experiment (Test 3) was intended to study whether adding unlabeled mineral N to the soil affects15N level in the plants to help elucidate the mechanism of N transfer from bean to garlic. Two soilapplied N treatments which included no N and 100 mg N kg–1(1.0 g N pot–1) in the form of unlabeled urea were applied to pots planted with mixed cropping of garlic and broad bean. All broad bean plants in one half of both soilapplied N treatments were injected with15N in the form of(15NH4)2SO4solution by the same procedure described for Test 2. The other half received no15N injection as the control.The design of the experiment was completely randomized with four treatment combinations being replicated four times(four pots per replication). All pots received 100 mg P kg–1soil (1.0 g P pot–1) and 126 mg K kg–1soil (1.26 g K pot–1).This experiment was performed in a greenhouse at the Dali Technology Innovation Center, Yunnan Province, China.The pots were 25 cm in diameter and 60 cm in height. Each pot was filled with 10 kg black loam soil collected from the same field where Test 1 and Test 2 were conducted after it was air-dried, sieved through 2-mm mesh, and mixed with the fertilizers for each treatment. Each pot received 800 mL of water before planting 20 garlic cloves and 10 broad bean seeds in each pot. Seedlings were thinned to 12 garlic and 7 broad bean plants. Adequate water was supplied throughout the crop growing period.
2.3. Sample collection and N measurement
Aboveground plant parts and roots of both garlic and broad bean in all three tests were collected. All plants in the 1-m2mini-plots from Test 1 and Test 2 were collected in late April.All plants from each pot were also collected from Test 3 in late April. If intercropped, the garlic plant samples were collected separately from the bean samples, which were further separated to stalks and seeds. Roots were harvested using the method of Böhme et al. (2005). In brief, the roots were dag up using a shovel to a depth of about 0.3 to 0.4 m,placed on a sieve, and shaken to separate loose soil from the roots. The roots were then washed with tap water ensuring to recover small roots that escaped the sieve. All aboveground plant parts and roots were fixed by exposing at 105°C for 30 min and dried at 70°C for 72 h before weighing.Soil samples from Test 2 and Test 3 were also collected from two soil zones: rhizosphere and non-rhizosphere. The non-rhizosphere samples in Test 2 were collected with a soil probe in the space between the garlic and bean rows to a depth of 60 cm in 20 cm increments (0–20, 20–40 and 40–60 cm). In Test 3, the non-rhizoshere samples were collected from the bulk soil after removing roots from the pots. The rhizoshpere samples from both Test 2 and Test 3 were collected by gently loosening the soil attached to root surfaces with ≈1 mm thickness. All soil samples were dried in the oven at 105°C for 30 min, followed by an additional 24 h at 75°C and saved for chemical analysis.
Total N in plant materials was measured by adding 5 mL of 18.4 mol L–1H2SO4, 1.5 g K2SO4, and 0.15 g CuSO4to dry and ground samples of 0.2 g garlic bulb, 0.2 g broad bean seed, 0.4 g garlic shoot, 0.4 g broad bean shoot, and 2 g soil in digestion tubes (Xiao et al. 2005). Following a thorough mixing, the solution was allowed to stand overnight, boiled to clear solution, and cooled before distillation. Boric acid was added to the distillate, titrated with sulfuric acid until the solution turned from green to pink, and the contents of total N in these solutions were calculated. Following the titration, the solution was supplemented with a few drops of sulfuric acid standard solution, and concentrated to 3 mL by heating on an electric hot plate to determine the content of15N using Finnigan MAT 251 mass spectrometer at Hebei Academy of Agricultural Sciences, Shijiazhuang, China.
2.4. Calculation
In Test 1 and Test 2, atom15N% excess was calculated as below:

Where,15Neis atom15N% excess in plant or soil sample,15Ntis atom15N% in plant or soil treated with15N fertilizer,15Ncis atom15N% in control plant or soil (not treated with15N fertilizer.
Percent of applied N that transferred from broad bean to garlic (NT, %) was calculated by the formula suggested by Johansen and Jensen (1996) as follows:

Where, Ngis total N uptake in garlic,15Negis garlic of15N excess, Nbis broad bean N uptake,15Nebis bean15N excess.
In Test 3, NT (%) was calculated as follows:

Where, Niis total injected N.
The competition intensity of garlic or broad bean when intercropped was calculated as suggested by Willey and Rao (1980):

Where, CRAand CRBis the competitive ratio of broad bean or garlic, respectively; YABis yield per unit area of crop A intercropped with crop B (per unit area was occupied by both crops); YBAis yield per unit area of crop B intercropped with crop A (per unit area was occupied by both crops);YAand YBare yield per unit area of crop A and crop B,respectively, grown as sole crops; PAis the proportion of intercropped area initially allocated to crop A; PBis the proportion of intercropped area initially allocated to crop B. Crop A is more competitive than crop B if CRA >1 and vice versa.
2.5. Statistical analysis
The data were statistically analyzed at randomized complete block design (Tests 1 and 2) or completely randomized design (Test 3) using SPSS statistics (SPSS Inc., ver. 19.0).Treatment means were compared using the LSD at 95%level of con fidence.
3. Results
3.1. N uptake
Fate of applied urea NIn Test 1, an average of 40.0% of the applied urea N was absorbed by the crops, 48.4% remained in the soil, and 11.6% was unaccounted for and considered lost in the cropping systems (Table 1). Fertilizer N balance differed among the three cropping systems. The largest N loss was observed in the broad bean sole crop, followed by garlic sole crop. Intercropping of garlic and broad bean lost the least amount of N (only 8.9% of the total applied)and had the largest N utilization (47.2%). The sole crop of broad bean retained the largest amount of N in the soil (up to 54.1%), probably indicating that broad bean as a legume does not rely on applied N. Overall, intercropping of garlic and broad bean improved the uptake of fertilizer N.
Vertical distribution of soil residual NWhen averaged across the three cropping systems, the retention of applied N was the highest in the upper 0–20 cm soil depth and decreased with increasing depth (Fig. 4). Despite receiving the least amount of N, the fraction of the total applied N that remained in the soil was the highest for broad bean sole crop than the other two systems at all three depths. This fraction in the 0–20 cm depth was higher for garlic sole crop than the garlic-bean intercrop, suggesting the intercropping of these two crops leads to better N utilization. The fraction of residual applied N at the other two depths was essentially the same for garlic and garlic-broad bean intercrop.
N uptake and partitioning to plant partsBoth garlic and broad bean absorbed more N when intercropped than when grown as sole crops. Garlic accumulated 214 kg N ha–1when sole crops, garlic absorbed more 27 kg N ha–1if intercropped with broad bean than as a sole crop(Fig. 5). Broad bean also absorbed more 15 kg N ha–1when intercropped with garlic than as a sole crop (Fig. 6). In theseed alone, broad bean accumulated 11 kg N ha–1when intercropped with garlic than as a sole crop. The relative partitioning of N to plant parts within a crop was not affected by intercropping.

Table 1 Fate of applied N in garlic and broad bean cropping system
3.2. N transfer from broad bean to garlic
Distribution of 15N abundanceBased on results from Test 2,the15N abundance (15N% of total N) in plant parts differed between the two crops (Table 2). On average, injecting broad bean pl
ants with15N in the field (Test 2) increased15N abundance in all plant parts of both garlic and broad bean plants (Table 2).
In broad bean, pods had the lowest15N abundance among the three plant parts regardless of the15N injection. There was no such difference between the two garlic plant parts.Injecting broad bean with15N did not positively or negatively affect the growth of either garlic or broad bean plant parts.
Based on results from the pot experiment (Test 3),applying N to the soil increased15N abundance in broad bean roots under both15N injection and no15N injection treatments (Table 3). There was no such increase in bean pods or stalks. In garlic, soil N application increased15N abundance in straws and root+bulb if the intercropped bean plants were not injected with15N. When the intercropped bean plants were injected with15N, soil N application did not affect15N in either garlic plant parts.
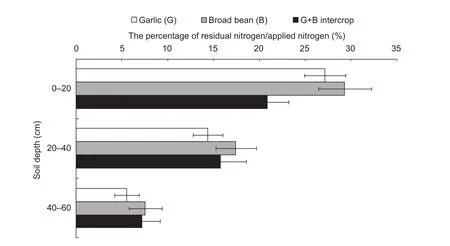
Fig. 4 The percentage of residual nitrogen/applied nitrogen in different soil depths after crop harvest. Error bars indicate SD.

Fig. 5 Total N uptake by garlic straw, root, and bulb under sole crop (garlic) vs. under garlic-broad bean intercrop. G, garlic; G+B intercrop, garlic and broad bean intercrop. Error bars indicate SD.

Fig. 6 Uptake of total applied N by broad bean root, stalk, and seed under bean sole crop (broad bean) vs. under garlic-broad bean intercrop. G, garlic; G+B intercrop, garlic and broad bean intercrop. Error bars indicate SD.
Injecting15N into bean plants intercropped with garlic increased15N abundance in all plant parts of broad bean and garlic (Table 3). While such increase is understandable with bean plants because of the direct15N injection, the increase in15N abundance in garlic plant parts suggests that15N transferred from broad bean to intercropped garlic plants.
N transfer between intercropped garlic and broad beanMuch of the total15N injected into broad bean plants (95%in Test 2 and 89% in Test 3) was recovered in the different plant parts of both bean and garlic. This suggests that the15N injected into broad bean petioles translocated to other plant parts of the bean plants (Table 4). Some fraction of this15N injected into bean plants also transferred to the intercropped garlic plants. In Test 2 in the field, 80% of the15N injected into broad bean plants remained in the bean plants, but up to 14.85% of it transferred to the intercropped garlic plants during the same growing season (Table 4). In Test 3 in pots, the transfer of15N from bean to garlic was 11.73% when N was applied to the soil and only 8.89%when no N was applied to the soil (Table 4). These results suggest the transfer of N from broad bean to garlic when intercropped would be in the range of 9 to 15% whether it is derived from biological N2fixation, soil-applied fertilizer,or foliarly applied or injected fertilizer.
15N residuals in the soilIn the field in Test 2, injecting15N into broad bean plants only slightly increased the level of15N in the bulk soil after harvesting the crops. The15N level in the non-rhizosphere (bulk) soil at the 0–20 cm depth after harvest was 1.33 mg kg–1(when the beans were not injected) vs. 1.34 mg kg–1(when the beans were injected with15N) (Table 5). Similar insigni ficant increases were measured in the other two depths. Injection, however,signi ficantly increased the15N level on the root surfaces of both crops. Understandably, this increase was signi ficantly greater on broad bean (1.66 mg kg–1or 41%) than on garlic (1.59 mg kg–1or 23%) rhizoshpere. When broad bean plants were not injected with15N, the bulk soil in the 0–20 cm depth and the rhizoshpere soil of both crops had similar15N levels. When15N was injected, however, the rhizoshpere soil of both crops had signi ficantly more15N than the bulk soil in the 0–20 cm depth. This probably suggests that15N released from injected bean plants is concentrated around the roots and decreases farther awayfrom roots, particularly deeper in the soil pro file. The bulk soil15N concentration clearly decline with depth regardless of the injection treatment.

Table 2 Biomass and 15N abundance in plant parts of intercropped garlic and broad bean in Test 2 with and without injection of 15N into broad bean plants
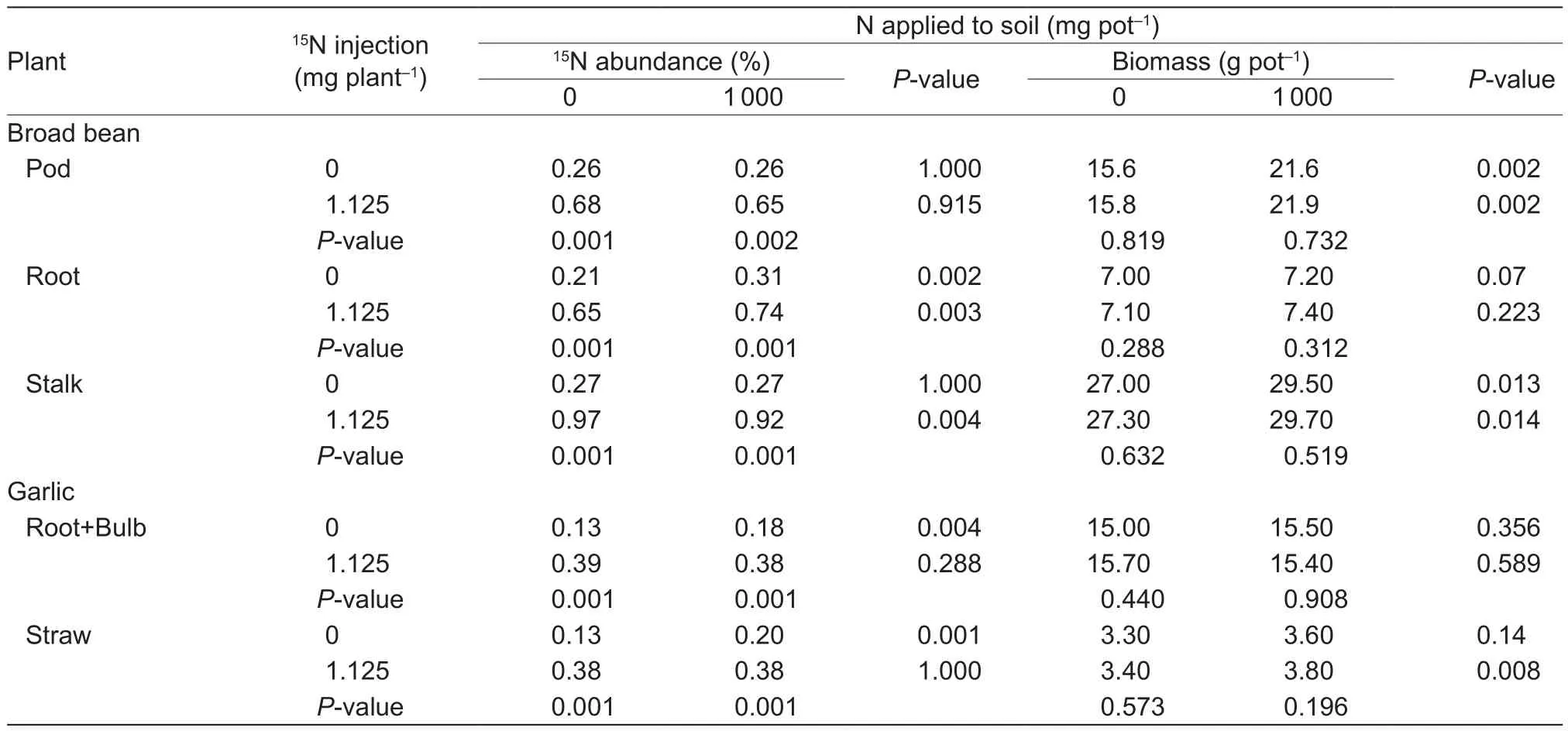
Table 3 Percent abundance of 15N in plant parts of garlic and broad bean that were mix-planted in pots in Test 31)
In Test 3, we tested whether adding fertilizer N to the soil affects15N level in the rhizoshpere and non-rhizoshpere soil.Applying N in the form of urea signi ficantly decreased15N concentration in the rhizoshpere soil of both crops but not in the bulk non-rhizoshpere soil, which may be an indication of preferential uptake for15N by both crops. Injection of15N to broad bean plants without soil-applied urea N did not result in elevated15N in the garlic or bean rhizoshpere relative to the bulk non-rhizoshpere soil as was found in Test 2 in the field. However, the lack of elevation may be attributed to the effect of root crowding in a limited soil space in the pots,probably leading to a more even distribution of the15N in rhizoshpere and non-rhizoshpere soil.
4. Discussion
4.1. Effects of intercropping on applied N utilization
Our results revealed that broad bean absorbed much less applied N than garlic, a large proportion of the applied N was found in the soil of broad bean. The proportion of applied N that remained in the soil after harvest was much less in garlic than that in broad bean. This suggests that broad bean was less dependent on applied N than garlic. This finding was consistent with the study of Li et al. (1999) on the intercropping of maize and broad bean.
We also found that intercropping garlic and broad bean promoted N utilization by both crops. Similar findings were reported in intercropped legumes and non-legumes (Anil et al. 1998; Carr et al. 1998; Chalk 1998; Carruthers et al.2000). Legumes obtain N through symbiotic N2fixation while non-legumes absorb a larger proportion of soil inorganic N because they are more competitive (Herridge et al. 1995).
The competitive ratio (CR) of legumes is different from non-legumes in intercropping systems (Fan et al. 2006). In our research, N fertilizer requirement of garlic was far higher than broad bean and we expected garlic competitiveness to be higher than that of broad bean. However, calculation of the CR of both garlic and broad bean revealed that,when intercropped, garlic had a CR of 0.65 compared to 1.55 for broad bean (Fig. 7). This suggests garlic was less competitive than broad bean when the two crops were intercropped. There are three reasons for this in our opinion.First, the amount of applied N to garlic in the intercropping treatment was less than the sole crop treatment. So the yield of the intercropped garlic had been reduced by shortage of N fertility. Increasing N application rate in an intercropping of legumes and non-legumes is known to change the CR(Xie et al. 2015). Second, broad bean plants were taller thangarlic in the intercropping and therefore had advantages over garlic in terms of competition for light which leads to higher broad bean yield. Third, the proportion of broad bean was less than garlic in the intercropping which has been known to affect CR (Wang et al. 2009).

Table 4 Amount of 15N injected to broad bean plants that was recovered in garlic plants in an intercropped garlic-broad bean system in mini-plots (Test 2) and in pots (Test 3)

Table 5 15N concentration in the rhizoshpere and non-rhizoshpere soil following the harvest of intercropped garlic and broad bean plants in mini-plots (Test 2) and in pots (Test 3)
Retention of applied15N in the soil was lower for garlic than for broad bean, likely because garlic depends on applied fertilizer N than broad bean. The retention of15N in the soil was the lowest when garlic and broad bean were intercropped suggesting that the utilization of applied N is improved when legumes and non-legumes are intercropped.The N uptake of intercropped broad bean was higher than sole crop broad bean (Fig. 6), but this may simply be because roots of the intercropped broad bean plants had access to the higher amount of N applied to garlic. Previous studies have proven that improved agronomic measures can increase the N- fixing ability of legumes and available N for the entire ecosystem in farmland (Peoples et al. 1995a, b;Jensen 1996; Van Kessel and Hartley 2000).
4.2. Possible mechanisms and necessary conditions for N transfer in an intercropping system
The possible mechanism and necessary conditions for N transfer between intercropped plants are still controversial.Many studies show that N transfers from legumes to gramineous plants (Ledgard et al. 1985; Ta et al. 1989; Fujita et al. 1990; Papastylianou and Danso 1991; Ofosu-Budu et al. 1993; Tomm et al. 1994; Johansen and Jensen 1996),while other studies show that little or no N transfers from legumes to non-legumes (Hamel et al. 1991; Papastylianou and Danso 1991; Danso et al. 1993; Trannin et al. 2000).In the present study,15N was injected to the petioles of broad bean plants intercropped with garlic in pot and field experiments. The15N injected to bean plants was recovered in the intercropped garlic plants at harvest, showing N could be transferred from broad bean to garlic during the course of the growing period.
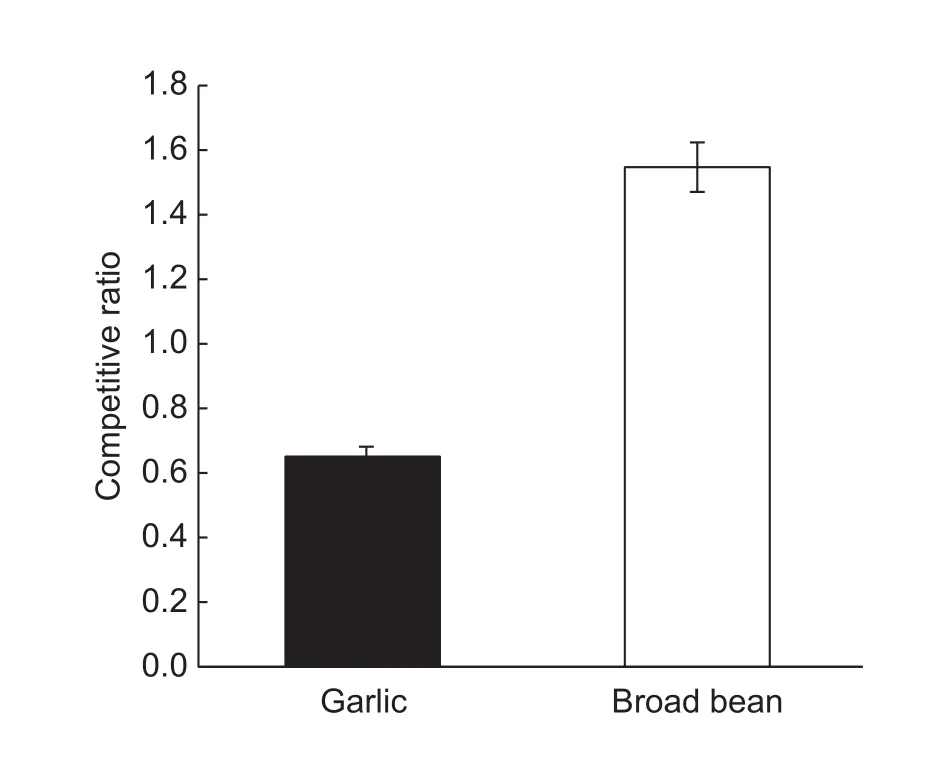
Fig. 7 The competitive ratio (CR) of garlic and broad bean in a strip intercropping system. Error bars indicate SD.
There may be two pathways for the N transfer. The first is a direct transfer through vesicular-arbuscular mycorrhiza(VAM) (Hamel et al. 1991; Johansen and Jensen 1996).Johansen and Jensen (1996) found that VAM inoculation of pea improved N transfer to barley. Another study by Ai et al. (2000) revealed that inoculation of VAM promoted N transfer from peanut to maize. However, Trannin et al.(2000) believed that VAM contributed less to N transfer.Most studies indicate that, in general, only a small amount of N transfers through VAM. The other pathway for N transfer is indirect. There are two indirect ways: (1)Roots of legume crops can secrete N-containing organic compounds (such as amino acids) and inorganic N (such as NH4+and NO3–) into the soil (Johansen and Jensen 1996).These exudates can then be absorbed by non-legumes growing in the same soil. (2) Decomposition of nodules and small roots excised from legume crops can release large amounts of mineral N to the rhizosphere through mineralization (Ta et al. 1989; Papastylianou and Danso 1991; Tomm et al. 1994; Johansen and Jensen 1996). This N becomes available for uptake by roots of non-legumes in the same soil. A smaller amount of N is transferred in the form of exudates than decomposition, but it can be measured by a short-term experiment. For example, Tomm et al. (1994) measured15N accumulation in intercropped Medicago sativa L. and Bromus japonicus Thunb during a 64 h period by labeling leaves, and speculated that the N transferring in a short-term period might be derived from root exudates, because the decomposition of roots required more time. The decomposition of root nodules, root rot,and net mineralization may be the main mechanisms for N transfer in long-term experiments. Trannin et al. (2000)proved that a large amount of N was transferred from legumes to gramineous plants if all shoots of legumes were cut. In general, the indirect transfer of N mediated by root decomposition seems to be the primary pathway for N transfer between intercropped legumes and nonlegumes. We speculated that the indirect transfer is the primary pathway of N transfer from broad bean to garlic in our research, but the direct pathways could have played a role also.
N use ef ficiency was improved in intercropping of garlic and broad bean in our research. Plants in general compete for resources when sharing a space. Such competition can take place between different individual plants of the same species (intraspeci fic competition) or different species(interspeci fic competition). There were considerable differences in resources usage observed both in different plants and different individuals of the same plant, which may be results of heterogeneity of the size and distribution of the root structure within a single species. It would cause asymmetrical plant competition for resources (Shipley and Keddy et al. 1994; Keddy et al. 2000). That is to say,the phenotypic structural heterogeneity of the roots is a product of the genetic variability within a species. The N utilization ef ficiency was improved in broad bean and garlic intercropping, and also promoted the N transfer. The amount of N transfer was greater in the pot and mini-plot with N application than that in the pot without N application. The reason may be that crop growth was stronger in the pot and mini-plot where N was applied, it was more bene ficial to bean N fixation and N transfer. Increasing the N application rate may have the opposite result, which needs further research.
5. Conclusion
Our study revealed that strip intercropping of garlic and broad bean, relative to sole cropping, substantially increased applied N absorption and utilization. The study also found that N could transfer from broad bean to garlic if intercropped.This transfer, which may also represent the amount of symbiotically fixed N2, may not exceed approximately 15%of that given to broad bean. The findings provide a basis for evaluating the role of legumes in optimizing N fertilization when intercropped with non-legumes.
Acknowledgements
The study was supported by the Special Fund for Agroscienti fic Research in the Public Interest, China (201003014)and the National Natural Science Foundation of China(31460143).
Ai W D, Li X L, Zuo Y M. 2000. Nitrogen transfers between maize and peanut by a common mycorrhizal fungi. Acta Agronomica Sinica,26, 473–481. (in Chinese)
Anil L, Park J, Phipps R H, Miller F A. 1998. Temperate intercropping of cereals for forage: A review of the potential for growth and utilization with particular reference to the UK.Grass and Forage Science,53, 301–317.
Böhme L, Langer U, Bhme F. 2005. Microbial biomass,enzyme activities and microbial community structure in two European long-term field experiments. Agriculture,Ecosystems & Environment,109, 141–152.
Carr P M, Martin G B, Caton J S, Poland W W. 1998. Forage and nitrogen yield of barley-pea and oat-pea intercrops.Agronomy Journal,90, 79–84.
Carruthers K, Prithiviraj B, Fe Q, Cloutier D, Martin R C, Smith D L. 2000. Intercropping corn with soybean, lupin and forages: Yield component responses. European Journal of Agronomy,12, 103–115.
Chalk P M. 1998. Dynamics of biologically fixed N in legumecereal rotations: A review. Australian Journal of Agricultural Research,49, 303–316.
Dahlin A S, Stenberg M. 2010. Transfer of N from red clover to perennial ryegrass in mixed stands under different cutting strategies. European Journal of Agronomy,33, 149–156.
Danso S K A, Pálmason F, Hardarson G. 1993. Is nitrogen transferred between field crops examining the question through a sweet-blue lupin (Lupinus angustifolius l.)-oats(Avena sativa) intercrop. Soil Biology and Biochemistry,25, 1135–1137.
Eaglesham A R J, Ayanaba A, Rao V R, Eskew D L. 1981.Improving the nitrogen nutrition of maize by intercropping with cowpea. Soil Biology and Biochemistry,13, 169–171.
Fan F L, Zhang F S, Song Y N, Sun J H, Bao X G, Guo T W, Li L. 2006. Nitrogen fixation of faba bean (Vicia faba L.) interacting with a non-legume in two contrasting intercropping systems. Plant and Soil,283, 275–286.
Frey B, Schüepp H. 1993. A role of vesicular-arbuscular (VA)mycorrhizal fungi in facilitating interplant nitrogen transfer.Soil Biology and Biochemistry,25, 651–658.
Fujita K, Ogata S, Matsumoto K, Masuda T, Godfred K, Budu O,Kuwata K. 1990. Nitrogen transfer and dry matter production in soybean and sorghum mixed cropping system at different population densities. Soil Science and Plant Nutrition,36,233–241.
Giller K E, Ormesher J, Awah F M. 1991. Nitrogen transfer from Phaseolus bean to intercropped maize measured using15N-enrichment and15N-isotope dilution methods. Soil Biology and Biochemistry,23, 339–346.
Hamel C, Furlan V, Smith D L. 1991. N2- fixation and transfer in a field-grown mycorrhizal corn and soybean intercrop.Plant and Soil,133, 177–185.
Herridge D F, Marcellos H, Felton W L, Turner G L, Peoples M B. 1995. Chickpea increases soil-N fertility in cereal systems through nitrate sparing and N2fixation. Soil Biology and Biochemistry,27, 545–551.
Høgh-Jensen H, Schjoerring J K. 2000. Below-ground nitrogen transfer between different grassland species: Direct quanti fication by15N leaf feeding compared with indirect dilution of soil15N. Plant and Soil,227, 171–183.
Jensen E S. 1996. Grain yield, symbiotic N2fixation and interspeci fic competition for inorganic N in pea-barley intercrops. Plant and Soil,182, 25–38.
Johansen A, Jensen E S. 1996. Transfer of N and P from intact or decomposing roots of pea to barley interconnected by an arbuscular mycorrhizal fungus. Soil Biology and Biochemistry,28, 73–81.
Keddy P, Gaudet C, Fraser L H. 2000. Effects of low and high nutrients on the competitive hierarchy of 26 shoreline plants.Journal of Ecology,88, 413–423.
Van Kessel C, Hartley C. 2000. Agricultural management of grain legumes: Has it led to an increase in nitrogen fixation?Field Crops Research,65, 165–181.
Ledgard S F, Freney J R, Simpson J R. 1985. Assessing nitrogen transfer from legumes to associated grasses. Soil Biology and Biochemistry,17, 575–577.
Li L, Yang S C, Li X L, Zhang F S, Christie P. 1999. Interspeci fic complementary and competitive interactions between intercropped maize and faba bean. Plant and Soil,212,105–114.
Li L, Zhang F S, Li X L, Christie P, Sun J H, Yang S C, Tang C X. 2003. Interspeci fic facilitation of nutrient uptake by intercropped maize and faba bean. Nutrient Cycling in Agroecosystems,65, 61–71.
Mayer J, Buegger F, Jensen E S, Schloter M, Heß J. 2003.Residual nitrogen contribution from grain legumes to succeeding wheat and rape and related microbial process.Plant and Soil,255, 541–554.
Ofosu-Budu K G, Fujita K, Gamo T, Akao S, Gamo T, Akao S.1993. Dinitrogen fixation and nitrogen release from roots of soybean cultivar bragg and its mutants Nts1116 and Nts1007. Soil Science and Plant Nutrition,39, 497–506.
Papastylianou I, Danso S K A. 1991. Nitrogen fixation and transfer in vetch and vetch-oats mixtures. Soil Biology and Biochemistry,23, 447–452.
Peoples M B, Herridge D F, Ladha J K. 1995a. Biological nitrogen fixation: An ef ficient source of nitrogen for sustainable agricultural production. Plant and Soil,174,3–28.
Peoples M B, Ladha J K, Herridge D F. 1995b. Enhancing legume N2fixation through plant and soil management.Plant and Soil,174, 83–101.
Rochester I J, Peoples M B, Constable G A, Gault R R. 1998.Faba beans and other legumes add nitrogen to irrigated cotton cropping systems. Australian Journal of Experimental Agriculture,38, 253–260.
Sierra J, Desfontaines L. 2009. Role of root exudates and root turnover in the below-ground N transfer from Canavalia ensiformis (jackbean) to the associated Musa acuminata(banana). Crop Pasture Science,60, 289–294.
Shipley B, Keddy P A. 1994. Evaluating the evidence for competitive hierarchies in plant communities. Oikos,69,340–345.
Ta T C, Faris M A, Macdowall F D H. 1989. Evaluation of15N methods to measure nitrogen transfer from alfalfa to companion timothy. Plant and Soil,114, 243–247.
Tang Q X, Ren T Z, Lei B K, Zhai L M, Hu W L, Luo X H, Zhang J Z, Liu H B. 2013. Economic and environmental bene fits of Vicia fava and garlic intercropping mode. Journal of Agriculture and Environmental Sciences,32, 816–826. (in Chinese)
Tang Q X, Ren T Z, Wilko S, Liu H B, Lei B K, Lin T, Zhang G L. 2012. Study on environmental risk and economic bene fits of rotation systems in farmland of erhai lake basin. Journal of Integrative Agriculture,11, 1038–1047.
Tomm G O, Kessel V C, Slinkard A E. 1994. Bi-directional transfer of nitrogen between alfalfa and bromegrass: Short and long term evidence. Plant and Soil,164, 77–86.
Trannin W S, Urquiaga S, Guerra G, Ibijbijen J, Cadisch G.2000. Interspecies competition and N transfer in a tropical grass-legume mixture. Biology and Fertility of Soils,32,441–448.
Unkovich M J, Pate J S. 2000. An appraisal of recent field measurements of symbiotic N2fixation by annual legumes.Field Crops Research,65, 211–228.
Wang P, Zhou D W, Zhang B T. 2009. Coexistence and inter-speci fic competition in grass-legume mixture. Acta Ecologica Sinica,29, 2560–2567. (in Chinese)
Willey R W, Reddy M S. 1980. A competitive ratio for quantifying competition between intercrops. Experimental Agriculture,16, 117–125.
Xiao Y B, Li L, Zhang F S. 2005. The interspeci fic nitrogen facilitation and the subsequent nitrogen transfer between the intercropped wheat and fababean. Scientia Agricultura Sinica,38, 965–973. (in Chinese)
Xie K Y, Zhang Y J, Li X L, He F, Wan L Q, Wang D, Qin Y. 2015.Competition and coexistence of alfalfa (Medicago sativa L.) and smooth brome (Bromus inermis Layss.) in mixture.Scientia Agricultura Sinica,48, 3767–3778. (in Chinese)
Zhu S X, Yang Z Z. 1992. Research on the advantages of alfalfa and old mans wheat sown. Scientia Agricultura Sinica,25,63–68. (in Chinese)
23 January, 2017 Accepted 4 August, 2017
TANG Qiu-xiang, Mobile: +86-15299175098, E-mail: 790058828@qq.com; Correspondence REN Tian-zhi, E-mail: rentianzhi@caas.cn
© 2018 CAAS. Publishing services by Elsevier B.V. All rights reserved.
10.1016/S2095-3119(17)61772-6
Section editor ZHANG Wei-li
Managing editor SUN Lu-juan
杂志排行
Journal of Integrative Agriculture的其它文章
- Phenolic and flavonoid contents of mandarin (Citrus reticulata Blanco)fruit tissues and their antioxidant capacity as evaluated by DPPH and ABTS methods
- Effects of different drying methods on quality, bacterial viability and storage stability of probiotic enriched apple snacks
- Nitrous oxide emissions following seasonal freeze-thaw events from arable soils in Northeast China
- Effects of long-term organic fertilization on soil microbiologic characteristics, yield and sustainable production of winter wheat
- Construction of Salmonella Pullorum ghost by co-expression of lysis gene E and the antimicrobial peptide SMAP29 and evaluation of its immune ef ficacy in speci fic-pathogen-free chicks
- Transcriptome analysis of salt-responsive genes and SSR marker exploration in Carex rigescens using RNA-seq
