鲑鱼降钙素及甲状旁腺素(1-34)对卵巢切除大鼠椎体骨密度及骨组织形态计量学影响的比较
2017-12-23罗鹏远梁少博田发明
骆 阳,齐 璨,罗鹏远,梁少博,田发明,张 柳
(1.河北医科大学第三医院创伤急救中心,河北 石家庄 050051;2.华北理工大学医学实验中心,河北 唐山 063000;3.华北理工大学附属医院骨科,河北 唐山 063000)
·论著·
鲑鱼降钙素及甲状旁腺素(1-34)对卵巢切除大鼠椎体骨密度及骨组织形态计量学影响的比较
骆 阳1,齐 璨1,罗鹏远1,梁少博1,田发明2,张 柳3
(1.河北医科大学第三医院创伤急救中心,河北 石家庄 050051;2.华北理工大学医学实验中心,河北 唐山 063000;3.华北理工大学附属医院骨科,河北 唐山 063000)
目的比较鲑鱼降钙素(salmon calcitonin,CT )及甲状旁腺素( parathyroid hormone,PTH)(1-34)对卵巢切除大鼠腰椎骨密度(bone mineral density,BMD)及骨组织形态计量学的影响。方法40只3月龄雌性Sprague-Dawley(SD)大鼠,随机分为4组,每组10只:假手术(Sham)组;双侧卵巢切除(OVX)组;双侧卵巢切除+甲状旁腺素(1-34)(OVX+PTH)组;双侧7卵巢切除+鲑鱼降钙素(OVX+CT)组。OVX+CT组及OVX+PTH组于卵巢切除术后3个月分别给予皮下注射CT或PTH(1-34)。连续给药3个月后处死所有大鼠并收集标本。取L4椎体进行BMD检测。L5椎体进行Gemisa染色及骨组织形态计量学检测。结果卵巢切除术后6个月,OVX组BMD显著低于Sham组(P<0.05),而OVX+CT组及OVX+PTH组椎体BMD显著高于OVX组(P<0.05),且OVX+PTH组BMD显著高于OVX+CT组(P<0.05)。OVX组BV/TV低于Sham组,OVX+CT组高于OVX组,OVX+PTH组BV/TV高于Sham组、OVX组、OVX+CT组,差异均有统计学意义(P<0.05)。OVX组、OVX+CT组Tb.Th低于Sham组,OVX+PTH组高于Sham组、OVX组、OVX+CT组,差异均有统计学意义(P<0.05)。OVX组Tb.Sp高于Sham组,OVX+CT组和OVX+PTH组低于OVX组,差异均有统计学意义(P<0.05)。各组大鼠Tb.N差异无统计学意义(P>0.05)。结论CT及PTH(1-34)可显著增加卵巢切除大鼠腰椎BMD并抑制骨重建的高转换状态,且PTH(1-34)作用要优于CT。
骨质疏松;骨密度;甲状旁腺;降钙素
10.3969/j.issn.1007-3205.2017.12.008
骨质疏松是以骨量减少,骨显微结构破坏,骨脆性增加,生物力学结构改变,易发生骨折为特征的一种全身性骨病,骨质疏松是老年人尤其是绝经后妇女的常见病及多发病[1-3]。随着人口老龄化进程的加速,骨质疏松及其所引起的骨折作为当前的一个严重的社会问题而备受关注。骨质疏松药物主要分为骨吸收抑制剂[降钙素(calcitonin,CT)、双膦酸盐、雌激素等]和刺激骨形成的药物[甲状旁腺素(parathyroid hormone,PTH)、氟化物等]两大类[4-5]。CT通过抑制骨吸收防止骨量下降[6-8]。PTH可促进成骨活性、抑制成骨细胞凋亡、增加新骨形成[9-13]。本研究通过卵巢切除术建立骨质疏松大鼠模型,应用CT及PTH(1-34)进行干预,通过骨密度(bone mineral density,BMD)及骨组织形态计量学检测,比较CT及PTH(1-34)对卵巢切除大鼠椎体骨量及骨重建的影响。
1 材 料 与 方 法
1.1动物来源及分组 3月龄雌性Sprague-Dawley(SD)大鼠40只,无特定病原体(specific pathogen free,SPF)级,平均体质量(260±30) g。动物购自北京维通利华实验动物技术有限公司。随机分为4组(每组10只):假手术(Sham)组;卵巢切除组(OVX);卵巢切除+降钙素组(OVX+CT)及卵巢切除+甲状旁腺素(OVX+PTH)组。所有大鼠均分笼饲养,自由摄食水。室温(24±2) ℃,自然光照,动物饲料购自北京维通利华实验动物技术有限公司。
1.2骨质疏松大鼠模型制备 用10%的水合氯醛按3 mL/kg剂量对大鼠腹腔麻醉,取侧卧位,以后方髂骨嵴上2 cm、脊柱旁1 cm处为中心备皮,碘伏消毒术区,铺手术巾。以后方髂骨嵴上2 cm、脊柱旁1 cm处纵行切开,依次切开皮肤、皮下组织及筋膜组织,钝性分离肌层,暴露腹腔。于切口下方脂肪组织中暴露卵巢及输卵管(成熟卵巢为淡红色菜花状,黄豆大小,与输卵管相连,表面有不规则结节状卵泡),用镊子将卵巢及其周围的软组织提起后,丝线结扎周围组织及输卵管,切除卵巢,将切除卵巢后的组织还纳回腹腔,对手术切口进行逐层缝合,关闭腹腔。应用碘伏对切口周围进行消毒。对侧卵巢切除依照此方法进行切除。Sham组只行背部切开手术,即暴露卵巢但不切除。
1.3药物干预 卵巢切除术后3个月,OVX+CT组及OVX+PTH组皮下注射鲑鱼降钙素(瑞士诺华公司)16 U/kg,2 d 1次或甲状旁腺素(1-34)(美国Sigma公司)30 μg/kg每天1次。连续给药3个月后以过量麻醉的方法处死所有大鼠,取L4椎体进行BMD测量。L5椎体进行Gemisa染色及骨组织形态计量学检测。
1.4检测方法
1.4.1椎体BMD检测 应用QDR Discovery双能X线吸收骨密度测量仪(Hologic, Bedford, MA, USA)对椎体进行BMD检测,应用小动物扫描模式进行扫描。扫描结束后,用仪器自选工具选定所测椎体的兴趣区,读出每个标本的BMD值,并记录[14]。
1.4.2椎体Gemisa染色及骨组织形态计量学检测 将L5椎体置于70%酒精固定液中固定2 d,剔除椎体表面的韧带及肌肉组织,并沿正中矢状位剖开椎体,然后进行梯度脱水。置于包埋剂(成分为甲基丙烯酸甲脂、邻苯二甲基二丁脂、过氧化苯甲酰)中充分浸透。已浸透的标本置于10 mL大小的玻璃瓶中,倒入新鲜配置的浸液约5 mL后,调整椎体于瓶底中间位置。再放入42 ℃烤箱内聚合凝固。包埋块凝固后,用锯修理包埋块,以适合于切片机的大小,硬组织切片机进行切片,厚度为8 μm。组织切片进行Gemisa染色,应用Leica DMLB2荧光/光学显微镜及Leica DC300数码摄像系统对椎体硬组织切片进行测量,大鼠椎体骨组织形态计量学测算范围为距骺板1~4 mm,两侧皮质骨内膜之间的松质骨,应用Leica QWin病理图象分析软件进行椎体骨组织形态计量学参数测定,经公式计算得出骨小梁相对体积(percent trabecular area,BV/TV)、骨小梁厚度(trabecular thickness,Tb.Th)、骨小梁数量(trabecular number,Tb.N)、骨小梁分离度(trabecular separation,Tb.Sp)。
1.5统计学方法 应用SPSS 15.0统计学软件分析数据。各组数据经Shapiro-Wilk正态性检验和Bartlett方差齐性检验后,组间比较采用单因素方差分析,两两比较采用LSD-t检验。P<0.05为差异有统计学意义。
2 结 果
2.1椎体BMD检测结果 卵巢切除术后6个月,OVX组大鼠椎体BMD显著低于Sham组(P<0.05),而OVX+PTH组及OVX+CT组大鼠椎体BMD与OVX组相比显著增高(P<0.05),且OVX+PTH组大鼠椎体BMD显著高于OVX+CT组(P<0.05)。值得注意的是,OVX+PTH组椎体BMD显著高于Sham组(P<0.05)。见表1。
表1卵巢切除术后6个月各组大鼠腰椎骨密度结果
Table1BMDvaluesamongthethreegroupsat6monthsafteroperation
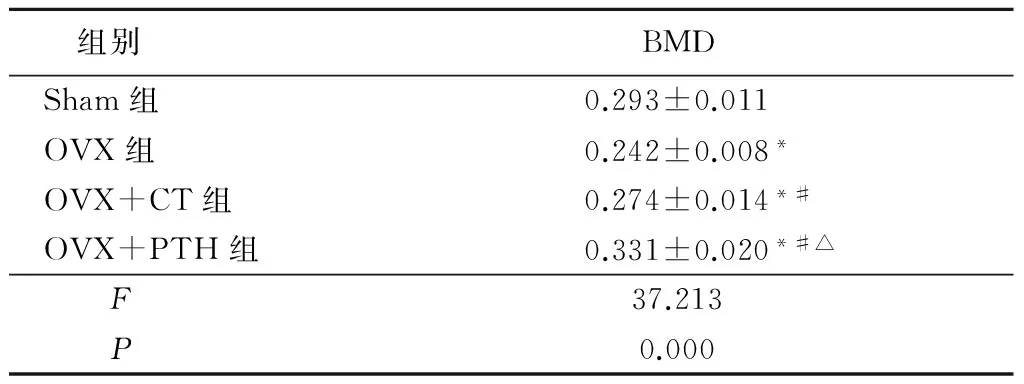
组别BMDSham组0.293±0.011OVX组0.242±0.008*OVX+CT组0.274±0.014*#OVX+PTH组0.331±0.020*#△F37.213P0.000
*P<0.05与Sham组比较 #P<0.05与OVX组比较 △P<0.05与OVX+CT组比较(LSD-t检验)
2.2椎体骨组织形态结果 各组大鼠L5椎体组织切片经Gemisa染色后可见:卵巢切除术后6个月,OVX组松质骨骨小梁宽度较Sham组减少,骨小梁排列稀疏;而PTH(1-34)及CT干预后,OVX+PTH组及OVX+CT组较OVX组松质骨骨小梁排列整齐,骨小梁宽度明显增加(图1)。

图1各组大鼠椎体(Gemisa染色×20)
A.Sham组;B.OVX组;C.OVX+CT组;D.OVX+PTH组
Figure1lumbarvertebraineachgroup(Gemisastaining×20)
2.3椎体骨组织形态计量学检测结果 各组大鼠Tb.N差异无统计学意义(P>0.05)。OVX组BV/TV低于Sham组,OVX+CT组高于OVX组,OVX+PTH组BV/TV高于Sham组、OVX组、OVX+CT组,差异均有统计学意义(P<0.05)。OVX组、OVX+CT组Tb.Th低于Sham组,OVX+PTH组高于Sham组、OVX组、OVX+CT组,差异均有统计学意义(P<0.05)。OVX组Tb.Sp高于Sham组,OVX+CT组和OVX+PTH组低于OVX组,差异均有统计学意义(P<0.05)。见表2。

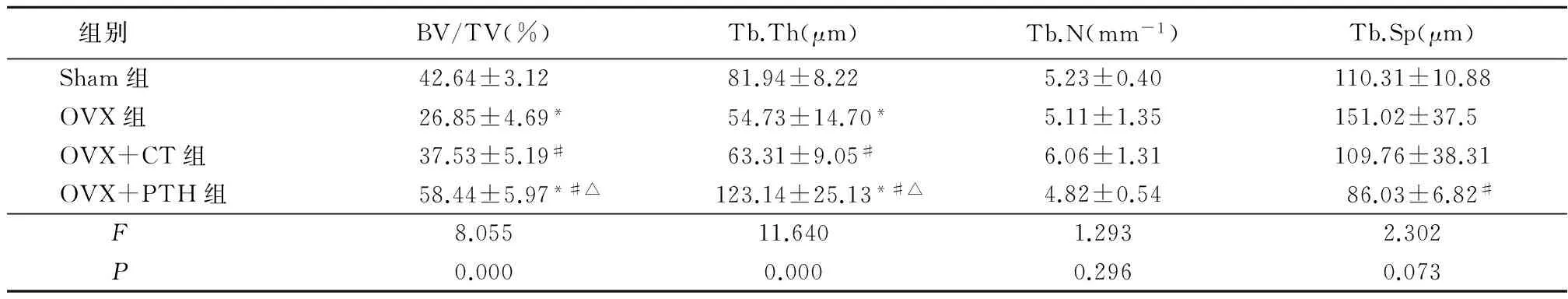
组别 BV/TV(%)Tb.Th(μm)Tb.N(mm-1)Tb.Sp(μm)Sham组42.64±3.1281.94±8.225.23±0.40110.31±10.88OVX组26.85±4.69*54.73±14.70*5.11±1.35151.02±37.5OVX+CT组37.53±5.19#63.31±9.05#6.06±1.31109.76±38.31OVX+PTH组58.44±5.97*#△123.14±25.13*#△4.82±0.5486.03±6.82#F8.05511.6401.2932.302P0.0000.0000.2960.073
*P<0.05与Sham组比较 #P<0.05 与OVX组比较 △P<0.05与OVX+CT组比较 (LSD-t检验)
3 讨 论
雌激素缺乏是绝经后女性骨质疏松的主要原因,雌激素缺乏引起骨转换率增高,骨吸收大于骨形成,骨小梁数量减少,骨小梁变细,乃至断裂,骨小梁难以承受原来的载荷,导致骨强度下降,易于发生骨折,这将严重影响患者的生活质量[15]。卵巢切除大鼠是公认的模拟人类绝经后骨质疏松的经典动物模型。卵巢切除所导致的大鼠骨量降低与人类绝经后骨质疏松有相似之处[16]。本课题组前期研究结果显示,行双侧卵巢切除术后12周可成功建立骨质疏松大鼠模型[17-21]。因此,本研究通过双侧卵巢切除术建立骨质疏松大鼠模型,并应用CT及PTH对其进行干预,比较CT及PTH对卵巢切除大鼠椎体骨量及骨代谢的影响。
目前治疗骨质疏松的药物主要有两大类:一类为骨吸收抑制剂如CT,主要通过抑制破骨细胞增殖和骨吸收,降低骨转化率,增强成骨细胞的活性和增殖分化,从而减少骨丢失,使机体骨骼组织钙、磷等物质沉积增加;另一类为促进骨形成类药物如PTH(1-34),主要通过刺激骨形成增加BMD,对抑制骨吸收作用不明显[22]。Wronski等[23]和Zhang等[24]研究结果显示,给予卵巢切除大鼠PTH 16 U/kg,2 d 1次或PTH(1-34)每天30 μg/kg 可有效维持卵巢切除大鼠松质骨骨量。因此,本研究选择此剂量进行对比研究。
骨组织的强度除了与骨组织结构有关外,更主要的是与骨量相关[25]。骨量与骨组织强度呈正相关性,骨组织强度的60%~80%由骨量决定,而 BMD 是全面评价骨量和诊断骨质疏松的可靠指标,也是衡量临床骨质疏松症治疗效果的评价指标[26-28]。本研究结果显示,卵巢切除术后3个月,分别给予CT及PTH(1-34)治疗3个月后,OVX+PTH及OVX+CT组大鼠腰椎BMD显著均高于OVX组(P<0.05),且OVX+PTH组腰椎BMD显著高于OVX+CT组(P<0.05)。说明PTH(1-34)及CT均可提高卵巢切除大鼠腰椎BMD,且PTH(1-34)改善骨质疏松作用较CT作用强。
骨组织的病理改变也是评价骨质疏松的重要参考指标[29]。本研究发现卵巢切除术后6个月,Gemisa染色结果显示OVX组与Sham组相比椎体显微结构明显退变,骨小梁稀疏或断裂。而OVX+CT组及OVX+PTH组与OVX组相比,椎体骨小梁增粗,骨小梁之间的连接较多。表明CT及PTH可以明显改善骨组织的显微结构,发挥治疗骨质疏松的作用。
骨显微结构直接决定骨骼局部的力学强度,而骨组织形态计量技术是目前了解骨显微结构变化的最好的方法。骨显微结构的改变是导致骨质疏松及发生骨质疏松性骨折的重要病理环节。骨组织形态计量学指标既是直接反映骨形成与骨吸收的重要指标,也是评估骨转换状态和骨质疏松程度、诊断分型及确定疗效的重要指标。骨组织形态计量学方法可以精确地反映出骨重建过程的变化规律和特点,并有可能就此提出某一时段用药治疗的方针,同时也能对研究对象所处的状态作出正确的评价。因此,本研究进一步观察了CT及PTH对卵巢切除大鼠椎体骨组织形态计量学的影响。卵巢切除术后6个月,OVX组BV/TV及TB.Th显著低于Sham组(P<0.05),说明卵巢切除后雌激素缺乏引起骨小梁变薄、变细,骨小梁组织形态发生了改变。而经过CT及PTH(1-34)治疗后,OVX+CT组及OVX+PTH组BV/TV、TB.Th显著高于OVX组(P<0.05),且OVX+PTH组BV/TV、TB.Th显著高于OVX+CT组(P<0.05),说明CT及PTH(1-34)可有效降低卵巢切除大鼠椎体骨转换率,减少椎体骨量的丢失,而PTH(1-34)对于降低卵巢切除大鼠椎体骨转换率、增加椎体骨量的作用明显优于CT。
综上所述,基于本研究所用药物的剂量及干预周期,PTH对于增加卵巢切除大鼠腰椎BMD、抑制椎体的高转换作用的治疗效果明显优于CT。
[1] Fonseca H,Moreira-Goncalves D,Coriolano HJ,et al. Bone quality:the determinants of bone strength and fragility[J]. Sports Med,2014,44(1):37-53.
[2] Rachner TD,Khosla S,Hofbauer LC. Osteoporosis:now and the future[J]. Lancet,2011,377(9773):1276-1287.
[3] 陈小伟,余卫清,季必池,等.鲑鱼降钙素联合阿仑膦酸钠治疗糖尿病伴骨质疏松的疗效观察[J].河北医科大学学报,2013,34(6):702-704.
[4] Khosla S. The bone and beyond:a shift in calcium[J]. Nat Med,2011,17(4):430-431.
[5] Baron R,Hesse E. Update on bone anabolics in osteoporosis treatment:rationale,current status,and perspectives[J]. J Clin Endocrinol Metab,2012,97(2):311-325.
[6] Atbinici H,Sipahiolu S,Aksoy N,et al. Effects of salmon calcitonin treatment on serum and synovial fluid bone formation and resorption markers in osteoporosis patients[J]. Acta Orthop Traumatol Turc,2015,49(2):160-165.
[7] Wei J,Wang J,Gong Y,et al. Effectiveness of combined salmon calcitonin and aspirin therapy for osteoporosis in ovariectomized rats[J]. Mol Med Rep,2015,12(2):1717-1726.
[8] Bhandari KH,Asghar W,Newa M,et al. Evaluation of bone targeting salmon calcitonin analogues in rats developing osteoporosis and adjuvant arthritis[J]. Curr Drug Deliv,2015,12(1):98-107.
[9] Tao ZS,Zhou WS,Tu KK,et al. The effects of combined human parathyroid hormone(1-34) and simvastatin treatment on osseous integration of hydroxyapatite-coated titanium implants in the femur of ovariectomized rats[J]. Injury,2015,46(11):2164-2169.
[10] Chen YJ,Wang SP,Cheng FC,et al. Intermittent parathyroid hormone improve bone microarchitecture of the mandible and femoral head in ovariectomized rats[J]. BMC Musculoskelet Disord,2017,18(1):171.
[11] Meakin LB,Todd H,Delisser PJ,et al. Parathyroid hormone's enhancement of bones' osteogenic response to loading is affected by ageing in a dose-and time-dependent manner[J]. Bone,2017,98:59-67.
[12] Bilezikian JP,Matsumoto T,Bellido T,et al. Targeting bone remodeling for the treatment of osteoporosis:summary of the proceedings of an ASBMR workshop[J]. J Bone Miner Res,2009,24(3):373-385.
[13] von Mühlen DG,Greendale GA,Garland CF,et al. Vitamin D,parathyroid hormone levels and bone mineral density in community-dwelling older women:the Rancho Bernardo Study[J]. Osteoporos Int,2005,16(12):1721-1726.
[14] Liu CC,Tian FM,Zhou Z,et al. Protective effect of calcitonin on lumbar fusion-induced adjacent-segment disc degeneration in ovariectomized rat[J]. BMC Musculoskelet Disord,2015,16:342.
[15] Lirani-Galvão AP,Lazaretti-Castro M. Physical approach for prevention and treatment of osteoporosis[J]. Arq Bras Endocrinol Metabol,2010,54(2):171-178.
[16] Wronski TJ,Dann LM,Horner SL. Time course of vertebral osteopenia in ovariectomized rats[J]. Bone,1989,10(4):295-301.
[17] 李震,张柳,穆树林,等.仙灵骨葆预防卵巢切除大鼠的腰椎间盘退变[J].中国组织工程研究与临床康复,2010,14(15):2722-2726.
[18] 穆树林,张柳,田发明,等.甲状旁腺激素1-34、阿仑膦酸钠、辛伐他汀治疗大鼠骨质疏松效果的比较[J].中国组织工程研究,2016,20(46):6854-6860.
[19] Tian FM,Li SY,Yang K,et al. Orally administered simvastatin partially preserves lumbar vertebral bone mass but not integrity of intervertebral discs in ovariectomized rats[J]. Exp Ther Med,2017,13(3):877-884.
[20] Tian FM,Yang K,Wang WY,et al. Calcitonin suppresses intervertebral disk degeneration and preserves lumbar vertebral bone mineral density and bone strength in ovariectomized rats[J]. Osteoporos Int,2015,26(12):2853-2861.
[21] Song H,Luo Y,Wang W,et al. Effects of alendronate on lumbar intervertebral disc degeneration with bone loss in ovariectomized rats[J]. Spine J,2017,17(7):995-1003.
[22] 林亮,刘思源,韩永台.骨质疏松症的药物治疗进展[J].河北医科大学学报,2008,29(1):134-136.
[23] Wronski TJ,Yen CF,Burton KW,et al. Skeletal effects of calcitonin in ovariectomized rats[J]. Endocrinology,1991,129(4):2246-2250.
[24] Zhang L,Takahashi HE,Tanizaw T,et al. Maintenance of bone mineral density of femoral cortex in ovariectomized rats after withdrawal of concurrent administration of human parathyroid hormone(1-34) and incadronate disodium (YM175)[J]. J Bone Miner Metab,1997,15(4):206-212.
[25] 韩利华,王全平,刘建,等.卵巢切除大鼠皮质骨骨密度与生物力学特性[J].第四军医大学学报,2001,22(11):1023-1025.
[26] Afzelius P,Garding MM,Molsted S. Dual-energy X-ray absorptiometry of both hips helps appropriate diagnosis of low bone mineral density and osteoporosis[J]. Diagnostics (Basel),2017,7(3):E41.
[27] Rothman MS,Lewiecki EM,Miller PD. Bone density testing is the best way to monitor osteoporosis treatment[J]. Am J Med,2017,130(10):1133-1134.
[28] Cui R,Zhou L,Li Z,et al. Assessment risk of osteoporosis in Chinese people:relationship among body mass index,serum lipid profiles,blood glucose,and bone mineral density[J]. Clin Interv Aging,2016,11:887-895.
[29] Pavel OR,Popescu M,Novac L,et al. Postmenopausal osteoporosis-clinical,biological and histopathological aspects[J]. Rom J Morphol Embryol,2016,57(1):121-130.
Comparisonoftheeffectsofsalmoncalcitoninandparathyroidhormone(1-34)onbonemineraldensityandbonehistomorphometryofvertebrainovariectomizedrats
LUO Yang1, QI Can1, LUO Peng-yuan1, LIANG Shao-bo1, TIAN Fa-ming2, ZHANG Liu3
(1.DepartmentofOrthopedicSurgery,theThirdHospitalofHebeiMedicalUniversity,Shijiazhuang050051,China; 2.MedicalResearchCenter,theNorthChinaUniversityofScienceandTechnology,Tangshan063000,China; 3.DepartmentofOrthopaedicSurgery,theAffiliatedHospitalofNorthChinaUniversityofScienceandTechnology,Tangshan063000,China)
ObjectiveTo compare the effects of salmon calcitonin(CT) and parathyroid hormone(PTH)(1-34) on bone mineral density and bone histomorphometry of vertebra in ovariectomized rats.MethodsForty female Sprague-Dawley rats aged 3 months were randomly divided into four groups(with 10 rats each). All rats but those in the Sham group
ovariectomy and treated by vehicle(OVX), salmon calcitonin(OVX+CT) and PTH(1-34)(OVX+PTH). After animals were sacrificed at 6 months post-OVX, the L4-5spinal segments were harvested. Bone mineral density(BMD) and histomorphometry analysis were performed to evaluate the bone mass and microstructural changes in the lumbar vertebral bodies.Histological analysis with Gemisa staining was used to identify the characteristics of the degenerative discs.ResultsSix months after OVX, the BMD values of L4vertebral bodies in the OVX group were significantly decreased, compared with the Sham group(P<0.05).The BMD values in the OVX+CT group and OVX+PTH group were significantly increased,compared with the OVX group(P<0.05), and the BMD values in the OVX+PTH group were significantly increased than that in the OVX+CT group(P<0.05). The BV/TV values of L5vertebral bodies in the OVX group were significantly decreased, compared with the Sham group, the BV/TV values in the OVX+CT group were significantly increased than that in the OVX group, the BV/TV values in the OVX+PTH group were significantly increased, compared with the Sham, OVX and OVX+CT group(P<0.05). The Tb.Th values in the OVX group and OVX+CT group were significantly decreased, compared with the Sham group, the Tb.Th values in the OVX+PTH group were significantly increased than that in the Sham group, OVX group and OVX+CT group(P<0.05). The values of Tb.Sp in the OVX group were higher than that in the Sham group, OVX+CT group and OVX+PTH group(P<0.05). There are no significantly changes of Tb.N values in each groups(P>0.05).ConclusionBoth salmon calcitonin and parathyroid hormone(1-34) can improve the bone mass and suppress the high bone turn-over in OVX rats. But the effect of parathyroid hormone(1-34) to suppress the bone resorption and improve the bone mass is better than that of salmon calcitonin.
osteoporosis; bone density; parathyroid hormone; calcitonin
2017-07-31;
2017-08-23
河北省医学科学研究重点课题(20170137)
骆阳(1982-),男,河北石家庄人,河北医科大学第三医院主治医师,医学博士,从事骨外科疾病诊治研究。
R681.1
A
1007-3205(2017)12-1392-05
许卓文)
