Influence of vascular endothelial growth factor and radiation on gap junctional intercellular communication in glioblastoma multiforme cell lines
2017-12-15ReinhardtKrcekPaulineLatzerIrenusAntonAdamietzHelmuthlerCarstenTheiss
Reinhardt Krcek, Pauline Latzer, Irenäus Anton Adamietz, Helmut Bühler, Carsten Theiss,
1 Institute of Anatomy, Department of Cytology, Ruhr-University Bochum, Bochum, North Rhine-Westphalia, Germany
2 Department of Radiotherapy and Radio-Oncology, University Medical Centre Marienhospital, Ruhr-University Bochum, Herne, North Rhine-Westphalia, Germany
3 Institute for Molecular Oncology, Radio-Biology and Experimental Radiotherapy, University Medical Centre Marienhospital, Ruhr-University Bochum, Herne, North Rhine-Westphalia, Germany
How to cite this article:Krcek R, Latzer P, Adamietz IA, Bühler H, Theiss C (2017) Influence of vascular endothelial growth factor and radiation on gap junctional intercellular communication in glioblastoma multiforme cell lines. Neural Regen Res 12(11):1816-1822.
Introduction
Glioblastoma multiforme (GBM) is the most common primary brain tumor in adults with a poor prognosis for its patients. The etiology of this tumor is widely unknown, but there are some hints, which make dedifferentiated astrocytes and glial progenitor cells responsible (Chen et al., 2012). The current standard therapy consists of a surgical resection of the tumor followed by radiotherapy and chemotherapy with temozolomide (Stupp et al., 2005; Hottinger et al., 2014).One characteristic of the highly aggressive GBM tumor is the overproduction of vascular endothelial growth factor(VEGF), followed by an increased vascularization of the tumor (Adamson et al., 2009). Here, VEGF is involved in cell proliferation, survival, chemotaxis and permeability of endothelial cells (Senger et al., 1983; Leung et al., 1989; Ferrara et al., 1997, 2003; Gerber et al., 1998). One of the new opportunities in the treatment of GBM is the use of targeted drugs against VEGF, for instance the selective VEGF-receptor tyrosine kinase inhibitor axitinib (Kelly and Rixe, 2009; Hutson et al., 2013). Another essential column in the treatment of GBM is a fractionated radiation therapy, which can prolong survival rates of GBM patients (Stupp et al., 2005). But it is known, that radiation therapy also leads to elevated levels of VEGF expression in the tumor cells (Park et al., 2001).
To give new perspectives for significantly prolonged survival rates of GBM patients, innovative solutions and new targets in the treatment are under current investigations.However, in this context, the relevance of gap junctions in GBM is only poorly investigated. Gap junctions are small channels between adjacent cells in order to allow the exchange of small molecules like ions or second messengers(Goodenough et al., 1996, 2009). In general, gap junctions are highly important for the regulation of cell growth, metabolic transport, development and apoptosis within cells (Wei et al., 2004; King and Lampe, 2005).
Gap junctions are also known to be expressed in GBM and lower grade astrocytoma (Soroceanu et al., 2001). They appear between glioma cells (homocellular form), and between tumor cells and non-tumor cells like astrocytes (heterocellular form). The heterocellular form of gap junctions seems to be associated with an increased invasion of glioma cells into the tissue (Trosko and Chang, 2001; Lin et al., 2002). In general, one typical step in carcinogenesis is a down-regulation of gap junctional intercellular communication (GJIC) stress-ing its crucial role in tumor biology (Trosko and Chang,2001). In GBM, the role of gap junctions remains unclear,however some ideas provide the use of the gap junctional network with GJIC to perform new innovative therapies.
It has been shown in VEGF receptor positive glial cells that VEGF enhances GJIC (Wuestefeld et al., 2012). GBM also possesses VEGF receptors (Knizetova et al., 2008; Lucio-Eterovic et al., 2009; Lee et al., 2011; Francescone et al.,2012; Yao et al., 2013; Mesti et al., 2014).
Therefore, the purpose of this study was to analyze the impact of VEGF, radiation therapy and VEGF receptor blockade by axitinib on GJIC in two different human GBM cell lines.
Material and Methods
Materials
VEGF-A (Sigma-Aldrich, V4512, USA) and axitinib (Selleckchem, S1005, USA) were supplemented to fresh medium at 0.1 and 100 µg/mL, respectively (Wuestefeld et al., 2012).To investigate the effect of therapeutic radiation, cells were irradiated with 2 gray (Gy) of photon energy by the linear accelerator Elekta Synergy S (dose output of 5 Gy/min) at the university clinic Marienhospital Herne (Germany). Cell culture medium was changed 1 hour after radiation by nutrient medium as a control or complemented with addition of VEGF or axitinib.
Cell lines
U-87 MG was obtained from the American Type Culture Collection (ATCC) (Manassas, USA) and U-251 MG from CLS (Heidelberg, Germany). Cells were cultivated in Dulbecco’s modified eagle medium (DMEM) containing 4.5 g/L D-Glucose, 3.7 g/L NaHCO3, stable glutamine and Na-Pyruvat (Biochrom AG, FG 0445, Germany) and supplemented with 10% sterile fetal bovine serum (Biochrom AG, S 0115)and 1% penicillin (Sigma-Aldrich). Cells were maintained at 37°C, 5% CO2and 90% air moisture. Subcultures were made on 12 mm Ø cover slips to apply VEGF, axitinib or radiation on the confluent cell monolayer.
Immunohistochemistry
To confirm the presence of Cx43 in U-87 and U-251 actin cytoskeleton specifically, GBM cells were fixed with 4%PFA for 30 minutes followed by permeabilization with 0.3%Triton for 10 minutes. The unspecific binding sites were blocked with goat-serum (Sigma-Aldrich, G9023, 1:50 in PBS, 30 minutes). After washing, cells were incubated with anti-connexin 43 (Merck Millipore, MAB3068, USA, 1:100 in PBS) overnight, followed by incubation with FITC-coupled anti-rabbit IgG (Sigma-Aldrich, F6005, 1:200 in PBS, 1.5 hours) and subsequently treated with rhodamin-phalloidin(Sigma-Aldrich, P1951, 1:20 in PBS, 1 hour). Then bisBenzimide H 33342 trihydrochloride (Hoechst, Sigma-Aldrich,B2261, 1:1,000 in PBS, 20 minutes) was applied to counterstain the cell nuclei.
To visualize cell coupling, microinjected cells were fixed with 4% PFA plus 0.2% picrinic acid for 2 hours. After several washing steps, cells were incubated with ExtrAvidin FITC (Sigma-Aldrich, E2761, 1:100 in PBS, 2 hours) to visualize neurobiotin. To count the cells, bisBenzimide H 33342 trihydrochloride (Hoechst, Sigma-Aldrich, B2261, 1:1,000 in PBS, 20 min) was applied to stain the nucleus. Finally the cover slips were mounted on microscope slides with fluoromount mounting medium.
Microinjection
Before performing the microinjection, cells were treated with axitinib or VEGF for 1 day. Some cells also received 2 Gy irradiation one hour before VEGF or axitinib was applied.
To study GJIC cells neurobiotin was microinjected into a single cell of a confluent monolayer glioblastoma cells. The procedure was performed according to the protocol published by Theiss and Meller (2002a, b). In brief, sterile glass capillaries (Hilgenberg, 1403512, Germany), formed by the P-97 Flaming/Brown Micropipette Puller (Sutter Instrument,USA), were filled with 8% neurobiotin hydrochloride diluted in distilled water (Vector Laboratories, Camon, Germany).The procedure was performed with aid of a phase contrast microscope (Zeiss, Germany) using an average pressure of 2–3 kPa for 0.1 second. Cells were then allowed to recover for 7.5 minutes which allows neurobiotin to spread into adjacent cells. To demonstrate functional cell coupling, displayed by neurobiotin, gap junction impermeable dextran-TRITC (Sigma-Aldrich, R-8881, 6%) was injected in combination with the gap junction permeable neurobiotin (6%).
Quantitative analysis of GJIC
Microinjected cells were analyzed with the aid of a Zeiss confocal laser-scanning microscope (LSM 510, Germany)with a 10× objective (Pan Neofluar, NA, USA). The number of neurobiotin positive cells was counted.
Proliferation analysis
To perform additional proliferations experiments, 5,000 cells per well were seeded in a 96-well plate (Sarstaedt, Germany).One day later, glioma cells were treated with VEGF, radiation and/or axitinib. 24 hours after treatment, cells were incubated with the MTS reagents (Promega CellTiter 96®AQueous Non-Radioactive Cell Proliferation Assay, G5421,USA) for 2 hours. After that, absorbance at 450 nm was measured with a Multiscan Ascent 354 (Labsystems, Heidelberg, Germany). The measured values are normalized to the average of the control conditions (Krcek et al., 2017).
Statistical analysis
A Student’st-test (Statistica, StatSof t, USA) was performed to analyze the significance of the results. Error bars show the standard error.Pvalues lower than 5% were set as significant differences.
Results
Glioblastoma cell lines express Connexin43
After several passages, glioma cells were cultivated to exhibit a monolayer of confluent cells. Both human GBM cell lines,U-87 and U-251, reveal a distinct Connexin43 (Cx43) signal,partially in the cytoplasm and partially on the cell surface along the actin skeleton between contacting tumor cells(Figure 1A, B).
Neurobiotin is a specific tracer for gap junctions
To reveal that the technique of microinjection is suitable to investigate GJIC, a mixture of neurobiotin and the gap junction impermeable substance dextran-TRITC was microinjected (Figure 1C). Whereas dye spreading of neurobiotin was prominent in numerous adjacent GBM cells, the microinjected carcinoma cell turned red, indicating that dextran-TRITC remained in the microinjected cell and did not couple within other cells. In cultured GBM cells dye transfer from the microinjected cells to neighboring cells was observed in radial fashion after injection (Figure 1C).Quantitative analysis of cell coupling
In order to analyze and quantify GJIC, single GBM cells were injected with neurobiotin. At least 32 microinjected cells (n) with a minimum of 4 independent cell cultures (N)were evaluated for GJIC in each condition and cell line. In U-87 cells (Figure 2), VEGF did not modify GJIC in comparison to controls (1.087 ± 0.062,n= 51,N= 6 for control(ct) andn= 43,N= 5 for VEGF respectively,P> 0.05),whereas the VEGF receptor blocker axitinib significantly increased GJIC (1.664 ± 0.051,n= 60,N= 6 for ct andn=81,N= 9 for axitinib respectively,P< 0.001). 2 Gy irradiation also showed an increase in GJIC for U-87 cells (1.343 ±0.037,n= 57,N= 6 for ct and n = 58, N = 6 for irradiation respectively, P < 0.001) as well as irradiation in combination with VEGF (1.474 ± 0.087,n=108,N= 11 for ct andn= 74,N= 8 for radiation + VEGF respectively,P< 0.001).Moreover, irradiated cells in combination with axitinib also displayed elevated GJIC compared to controls (1.945 ± 0.08,n= 83,N= 9 for ct andn= 54,N= 6 for radiation + axitinib respectively,P< 0.001). This elevation of GJIC in irradiated cells treated with axitinib was significantly higher compared to irradiated cells (P< 0.001), irradiated cells combined with VEGF (P< 0.001) and solely axitinib treatment (P< 0.01).
In U-251 cell line (Figure 3), the addition of VEGF for 24 hours significantly increased cell coupling (1.536 ± 0.076,n= 64,N=7 for ct andn= 54,N= 6 for VEGF respectively, P< 0.001), whereas axitinib treatment had no significant effects on GJIC in comparison to controls (0.957 ± 0.038,n=64,N= 7 for ct andn= 32,N= 4 for axitinib, respectively,P> 0.05 to control,P< 0.001 to VEGF). Beside this, addition of axitinib to VEGF incubated cells also had no significant effects on GJIC comparing with controls (1.11 ± 0.051,n=64,N= 7 for ct andn= 32,N= 4 for VEGF + axitinib, respectively), but a significant decrease of GJIC in VEGF plus axitinib incubated cells compared to VEGF treated cells was obvious (1.536 ± 0.076,n= 54,N= 6 for VEGFvs.1.11 ±0.051,n= 32,N= 4 for VEGF + axitinib,P< 0.001,Figure 4).Furthermore, irradiation with 2 Gy increased GJIC in U-251 cells (1.806 ± 0.117,n= 64,N= 7 for ct andn= 32,N= 4 for radiation respectively,P< 0.001 to control), while radiation (2 Gy) combined with VEGF incubation did not further significantly enhance GJIC in comparison to radiation alone(1.859 ± 0.081,n= 32,N= 4 for radiation andn= 32,N= 4 for radiation + VEGF respectively,P> 0.05). Additionally,the increase following radiation was significantly higher compared to that of VEGF (n= 54,N= 6 for VEGF andn= 32,N= 4 for radiation, respectively,P< 0.05). While axitinib together with radiation significantly increased GJIC compared to control (1.153 ± 0.051,n= 64,N= 7 for ct andn= 36,N=4 for radiation + axitinib respectively,P< 0.05), this augmentation was significantly less pronounced than with radiation alone (1.806 ± 0.117,n= 32,N= 4 for radiationvs.1.153 ±0.051,n= 36,N= 4 for radiation + axitinib,P< 0.001).
Analysis of cell proliferation
Furthermore, we analyzed cell proliferation by the MTS assay, with at least three independent experiments per condition. In U-251 cells (Krcek et al., 2017) as well as in U-87(Figure 5A) cells there were no significant effects regarding cell proliferation by one-day treatment with VEGF, radiation or both in combination detectable. While axitinib,VEGF plus axitinib (Figure 5B) as well as the combination of radiation plus axitinib reduced cell proliferation of U-251 cells, these treatments did not affect proliferation rates of U-87 cells. Neither use of axitinib alone (0.961 ± 0.026,n= 39,P> 0.05)nor the combination of VEGF plus axitinib(0.932 ± 0.021,n= 39,P> 0.05), nor the combination of radiation and axitinib (0.977 ± 0.032,n= 30,P> 0.05) showed any significant effects.
Discussion
In the present study, we show that previous data of GJIC in astrocytes can partially be transferred to GBM. We displayed GJIC by using microinjection of neurobiotin, which is well known to pass through gap junctions due to its low molecular weight (Theiss and Meller, 2002a, b). GBM cell lines U-87 and U-251 both express Cx43 and build functional gap junctions, as demonstrated by neurobiotin transfer from the microinjected cell to adjacent cells. Other studies also revealed the expression of Cx43 in these cell lines with aid of western blot techniques (Ghosh et al., 2014; Ye et al.,2015). However, these data are partly contradictory to a previous study, in which U-251 cells were described to be Cx43 negative (Huang et al., 1998). In contrast to our experiments these authors used an antiserum against Cx43 and northern blot techniques. It is conceivable, that in this old publication a misidentified U-251 cell line was used, as it was the case in several earlier publications (Timerman et al., 2014).

Figure 1 Connexin 43 expression and gap junctional intercellular communication (GJIC) in glioblastoma multiforme cells.(A, B) Immunofluorescent staining of the human glioblastoma cell lines U-87 (A) and U-251 (B). Both cell lines express connexin 43 (Cx43) (green),partially in the cytoplasm and partially on the cell surface along the actin skeleton between contacting tumor cells (white arrows). Cell nuclei are stained with DAPI (blue). Glioblastoma cells possess a well-defined actin cytoskeleton (red). (C) In a confluent monolayer of U-251 cell line, a mixture of neurobiotin and dextran-TRITC was microinjected. Whereas there is dye spreading of neurobiotin to several adjacent glioblastoma cells, the microinjected cell turned red, indicating that dextran-TRITC remained in the microinjected cell. Scale bars: 10 μm in A, B; 50 μm in C.
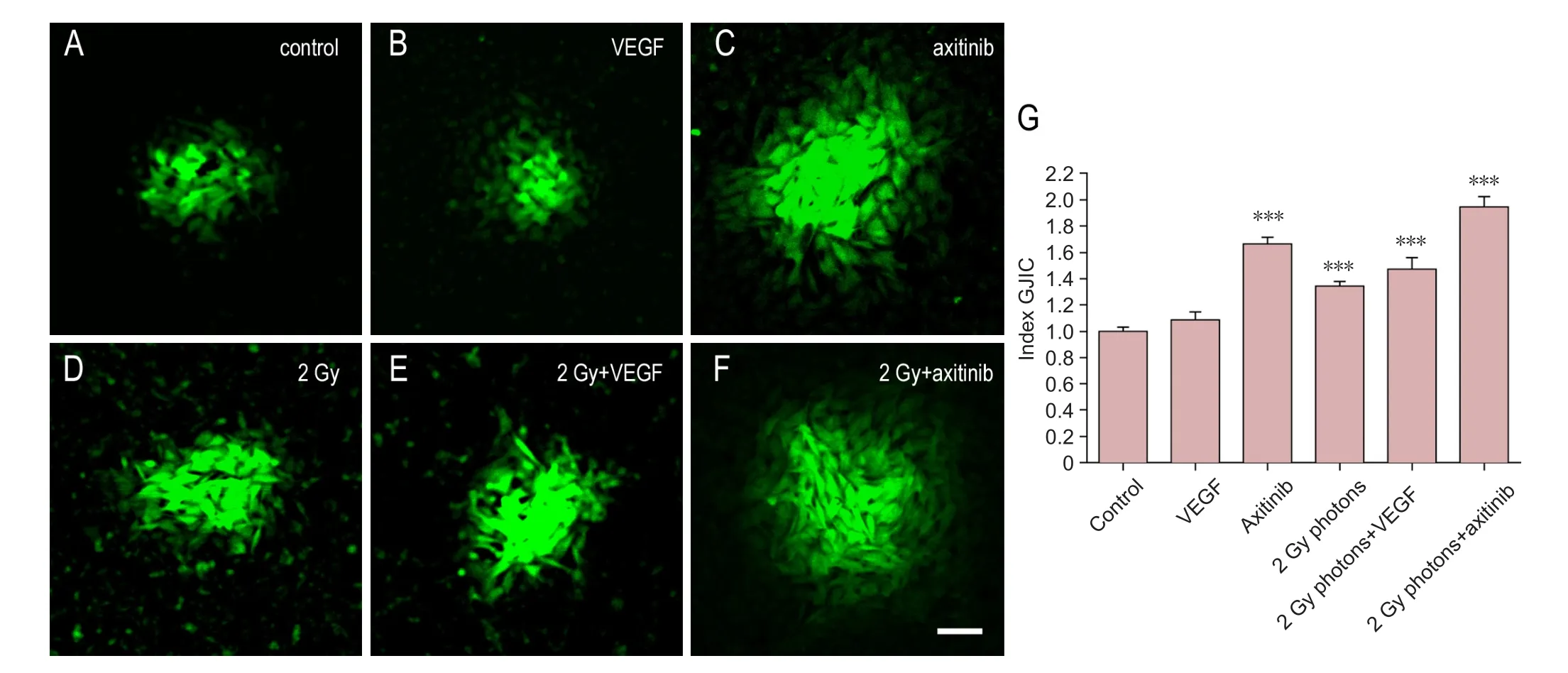
Figure 2 Influence of VEGF, the VEGF-R2 inhibitor axitinib, or irradiation with 2 Gy photons on GJIC of U-87 cell lines.(A–F) VEGF was added at 0.1 µg/mL, axitinib at 100 µg/mL. Scale bar: 50 µm. (G) Quantitative results. Data were expressed as the mean ± standard error, and analyzed by unpaired t-test. ***P < 0.001, vs. control group.
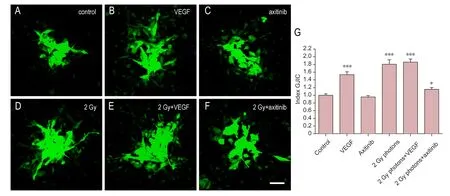
Figure 3 Influence of VEGF, the VEGF-R2 inhibitor axitinib, or irradiation with 2 Gy photons on GJIC of U-251 cell lines.(A–F) VEGF was added at 0.1 µg/mL, axitinib at 100 µg/mL. Scale bar: 50 µm. (G) Quantitative results. Data were expressed as the mean ± standard error, and analyzed by unpaired t-test. *P < 0.05, ***P < 0.001, vs. control group.
Our present data clearly indicate that one day of VEGF incubation significantly enhances GJIC in U-251, like the results obtained in cultured rat astrocytes (Wuestefeld et al.,2012). Radiation showed a similar effect with significantly increased GJIC. These enhanced cell-coupling rates after irradiation could not be further increased through an incubation with VEGF. Besides this, irradiation alone was able to enhance GJIC in comparison to VEGF treatment. However, treatment with the VEGF receptor blocker axitinib in combination with irradiation led to a significant decrease in GJIC compared to irradiated cells alone, which was nevertheless significantly higher than control. Therefore, we concluded that in U-251 cells the effects of irradiation on GJIC are partially mediated by the autocrine and paracrine release of VEGF, and partially by other irradiation related effects,possibly through activation of other receptor tyrosine kinases. Moreover, the VEGF-receptor blockade by axitinib significantly reverses the VEGF mediated increase of GJIC.
On the other hand, in U-87 GBM cells VEGF treatment did not significantly enhance GJIC. This might be due to a high endogenous VEGF expression levels in U-87 cells, as it was detected in several studies before (Gorski et al., 1999;Hovinga et al., 2005). Nevertheless, single irradiation of U-87 as well as irradiation in combination with VEGF significantly enhanced GJIC. These data are in line with another study,demonstrating GJIC inducing effect of 5 Gy irradiation in U-87 followed by a quantification of calcein diffusion (Leone et al., 2008). Surprisingly, VEGF-receptor blockade by axitinib as well as axitinib in combination with irradiation also strongly enhanced GJIC in U-87 cells. These results are contrary to the data of healthy astrocytes (Wuestefeld et al.,2012), and to the data in U-251. We assume an important role of VEGF signaling in U-87 with an unknown mechanism of the VEGF-receptor.
Although both cell lines are highly aggressive and derived from human glioblastoma, they differ in several aspects.For instance, while U-251 cells grow in a monolayer, U-87 cells rapidly build multilayer forming clusters with adjacent cells. Moreover, U-87 cells have a spindle-shape form while U-251 cells are round shaped. Additionally, another study presents differences in metabolic profiles between these two cell lines (Shao et al., 2014). It could be that U-87 cells show other reaction patterns on cellular stress, which is evoked by VEGF-receptor blockade, compared to U-251 cells.
Gap junction constituting connexins are known to have half-life times of 1–2 hours, so that they must be continuously synthesized and shifted to the cell membrane (Laird et al., 1991). In a former study, we showed that VEGF incubation had no effects on Cx43 mRNA and protein expression in astrocytes (Wuestefeld et al., 2012). It is conceivable that increased actin activity mediated by VEGF (Foehring et al.,2012; Olbrich et al., 2013, Dumpich et al., 2015) could also be accompanied by an increased shifting of connexins into the membrane. Here, VEGF stimulates phosphorylation of the tyrosine kinase receptors VEGF-receptor-1 (VEGFR-1) and VEGF-receptor-2 (VEGFR-2). VEGF effects are mostly mediated by VEGFR-2 (Ferrara et al., 2003). In endothelial cells it was demonstrated that VEGFR-2 phosphorylation, in combination with Cdc42 activation, activates the SAPK2/p38 pathway, following by MAPKAP K2 activation. Furthermore, an activation of ADF/cofilin, Arp2/3 and WASP is involved in that pathway and acts as a re-organizer of the actin cytoskeleton with increased actin dynamics (Rousseau et al., 1997; Lamalice et al., 2004). Generally, cytoplasmic gap junctions are supposed to interact with actin filaments (Larsen et al., 1979;Murray et al., 1997). In astrocytes and lens epithelial cells it has been shown that GJIC partly depends on actin dynamics(Theiss and Meller, 2002b; Giessmann et al., 2003). In GBM cells the impact of VEGF signaling is not well understood,especially the effects on actin reorganization. As irradiation is able to increase the release of VEGF (Park et al., 2001; Kil et al., 2012) we assume a VEGF-dependent stimulation to explain the elevated GJIC. Furthermore, irradiation is also able to induce the activation of multiple other pathways like for example by stimulating the epidermal growth factor receptor(EGFR) (Dent et al., 1999; Valerie et al., 2007), which was demonstrated in U-87 cells (Stahler et al., 2013). The EGFR receptor activation might have positive effects on GJIC, as proved for human kidney epithelial cells (Rivedal et al., 1996;Reuss and Unsicker, 1998).

Figure 4 Influence of VEGF and VEGF combined with the VEGF-R2 inhibitor axitinib on GJIC of U-251 cell lines.VEGF was added in a concentration of 0.1 µg/mL, axitinib of 100 µg/mL.Data were expressed as the mean ± standard error, and analyzed by unpaired t-test. ***P < 0.001, vs. control group. n.s.: Not significant;VEGF: vascular endothelial growth factor; GJIC: gap junctional intercellular communication.
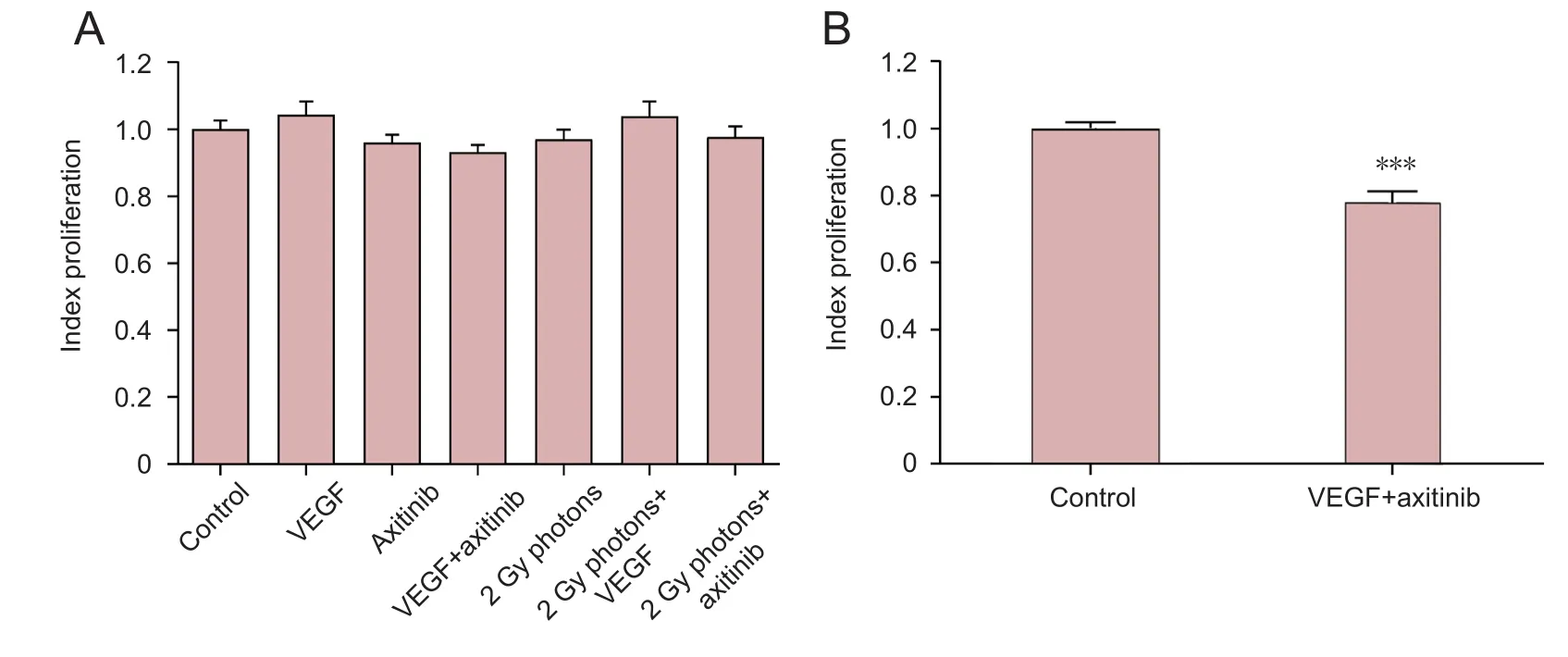
Figure 5 Influence of VEGF,the VEGF-R2 inhibitor axitinib, or irradiation with 2 Gy photons on cell proliferation of U-87 cells (A)and influence of VEGF combined with axitinib on U-251 cell proliferation (B).VEGF was added at 0.1 µg/mL,axitinib at 100 µg/mL. Data were expressed as the mean ±standard error, and analyzed by unpaired t-test.***P <0.001, vs. control group.
It is known that gap junction establishing connexins like Cx43 play an important role in tumor biology. It has been observed that high-grade glioma express less Cx43 than lowgrade glioma or astrocytes (Huang et al., 1999; Soroceanu et al., 2001; Pu et al., 2004). Furthermore, transfections with Cx43 in Cx43 deficient glioma lead to decreased cell proliferation rates and a minor malignancy of these tumors (Cirenei et al., 1998; Huang et al., 1998). These authors discussed Cx43 to act as a tumor suppressor protein. Moreover, re-expression of Cx43 induced an increase of chemotherapy-induced apoptosis with a down-regulation of the anti-apoptotic protein bcl-2 and several autocrine growth factors. These proliferation effects are probably independent of GJIC (Huang et al., 2001;Qin et al., 2003; Xia et al., 2003; Zhang et al., 2003). However,the inhibitory effect of Cx43 transfection has been addressed before, asin vitrothere is a clear dependency on the extracellular environment (Crespin et al., 2010). Nevertheless, a recent study shows that a reduction in GJIC promotes the migration of glioma cells using a 3D migration model (Aftab et al., 2015). At this point we can conclude, that Cx43 and GJIC seem to play a role in GBM, and therefore therapeutic options with a focus on GJIC are recently under investigation.For instance, an increase in GJIC could become an interesting issue in gene therapies using retroviral vectors. For these therapies, tumor cells are infected with a herpes simplex virus thymidine kinase, which makes these cells sensitive for the anti-herpetic drug ganciclovir (Culver et al., 1992; Colombo et al., 1995; Moolten and Wells, 1990). Here, gap junction mediated bystander effects are important to efficiently infect all tumor cells (Dilber et al., 1997; Cottin et al., 2011; Sun et al., 2012). Our results show that VEGF (in U-251) and radiation enhance GJIC, which suggests that targeting GJIC could be a promising strategy for patients with high VEGF levels or in combination with radiotherapy.
Author contributions:RK and PL conducted the gap junction studies and analysis. RK and CT conducted the microscopy components of the study. RK, IAA, HB and CT contributed to the concept and design of this study. The paper was written by RK and CT with contributions from all authors.
Conflicts of interest:None declared.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under identical terms.
Open peer review report:
Reviewer:Seonhee Kim, Temple University, USA.
Comments to authors:Their results suggest that VEGF signaling can increase GJIC and partially mediates the radiation effects on GJIC in U251 cells. These are new and interesting results. In this manuscript,the authors investigated the effects of VEGF and radiation on GJIC in well-established glioblastoma cell lines, U87 and U251. The authors treated confluent cultures with VEGF, VEGF receptor inhibitor, the small tyrosine kinase inhibitor axitinib, or radiation or with a combination of VEGF or axitinib with radiation. As readout of GJIC they utilized diffusion of neurobiotin after microinjection into a single cell;neurobiotin only passes through gap junctions to contacting cells. They measured the number of cells with neurobiotin expression and compared with untreated controls. They found that in U87 cells, VEGF treatment had no effects on GJIC, whereas treatment with axitinib or radiation alone or in combination increased GJIC. Results in U251 differed: VEGF and radiation alone increased GIJC, but, whereas axitinib alone had no effects, it reduced the increase due to radiation.These results suggest that VEGF signaling partially mediates the radiation effects on GJIC in U251 cells.
Adamson C, Kanu OO, Mehta AI, Di C, Lin N, Mattox AK, Bigner DD (2009)Glioblastoma multiforme: a review of where we have been and where we are going. Expert Opin Investig Drugs 18:1061-1083.
Aftab Q, Sin WC, Naus CC (2015) Reduction in gap junction intercellular communication promotes glioma migration. Oncotarget 6:11447-11464.
Chen J, McKay RM, Parada LF (2012) Malignant glioma: lessons from genomics, mouse models, and stem cells. Cell 149:36-47.
Cirenei N, Colombo BM, Mesnil M, Benedetti S, Yamasaki H, Finocchiaro G (1998) In vitro and in vivo effects of retrovirus-mediated transfer of the connexin 43 gene in malignant gliomas: consequences for HSVtk/GCV anticancer gene therapy. Gene Ther 5:1221-1226.
Colombo BM, Benedetti S, Ottolenghi S, Mora M, Pollo B, Poli G, Finocchiaro G (1995) The “bystander effect”: association of U-87 cell death with ganciclovir-mediated apoptosis of nearby cells and lack of effect in athymic mice. Hum Gene Ther 6:763-772.
Cottin S, Gould PV, Cantin L, Caruso M (2011) Gap junctions in human glioblastomas: implications for suicide gene therapy. Cancer Gene Ther 18:674-681.
Crespin S, Bechberger J, Mesnil M, Naus CC, Sin WC (2010) The carboxy-terminal tail of connexin43 gap junction protein is sufficient to mediate cytoskeleton changes in human glioma cells. J Cell Biochem 110:589-597.
Culver KW, Ram Z, Wallbridge S, Ishii H, Oldfield EH, Blaese RM (1992).In vivo gene transfer with retroviral vector-producer cells for treatment of experimental brain tumors. Science 256:1550-1552.
Dent P, Reardon DB, Park JS, Bowers G, Logsdon C, Valerie K, Schmidt-Ullrich R (1999) Radiation-induced release of transforming growth factor alpha activates the epidermal growth factor receptor and mitogen-activated protein kinase pathway in carcinoma cells, leading to increased proliferation and protection from radiation-induced cell death. Mol Biol Cell 10:2493-2506.
Dilber MS, Abedi MR, Christensson B, Björkstrand B, Kidder GM, Naus CC,Gahrton G, Smith CI (1997) Gap junctions promote the bystander effect of herpes simplex virus thymidine kinase in vivo. Cancer Res 57:1523-1528.
Dumpich M, Mannherz HG, Theiss C (2015) VEGF Signaling Regulates Cofilin and the Arp2/3-complex within the Axonal Growth Cone. Curr Neurovasc Res 12:293-307.
Ferrara N, Davis-Smyth T (1997) The biology of vascular endothelial growth factor. Endocr Rev 18:4-25.
Ferrara N, Gerber HP, LeCouter J (2003) The biology of VEGF and its receptors. Nat Med 9:669-676.
Foehring D, Brand-Saberi B, Theiss C (2012) VEGF-induced growth cone enhancement is diminished by inhibiting tyrosine-residue 1214 of VEGFR-2. Cells Tissues Organs 196:195-205.
Francescone R, Scully S, Bentley B, Yan W, Taylor SL, Oh D, Moral L, Shao R (2012) Glioblastoma-derived tumor cells induce vasculogenic mimicry through Flk-1 protein activation. J Biol Chem 287:24821-24531.
Gerber HP, Dixit V, Ferrara N (1998) Vascular endothelial growth factor induces expression of the antiapoptotic proteins Bcl-2 and A1 in vascular endothelial cells. J Biol Chem 273:13313-13316.
Ghosh S, Kumar A, Tripathi RP, Chandna S (2014) Connexin-43 regulates p38-mediated cell migration and invasion induced selectively in tumour cells by low doses of γ-radiation in an ERK-1/2-independent manner. Carcinogenesis 35:383-395.
Giessmann D, Theiss C, Breipohl W, Meller K (2003) Microinjection of actin antibodies impaired gap junctional intercellular communication in lens epithelial cells in vitro. Curr Eye Res 27:157-164.
Goodenough DA, Goliger JA, Paul DL (1996) Connexins, connexons, and intercellular communication. Annu Rev Biochem 65:475-502.
Goodenough DA, Paul DL (2009) Gap junctions. Cold Spring Harb Perspect Biol 1:a002576.
Gorski DH, Beckett MA, Jaskowiak NT, Calvin DP, Mauceri HJ, Salloum RM, Seetharam S, Koons A, Hari DM, Kufe DW, Weichselbaum RR (1999)Blockage of the vascular endothelial growth factor stress response increases the antitumor effects of ionizing radiation. Cancer Res 59:3374-3378.
Hottinger AF, Stupp R, Homicsko K (2014) Standards of care and novel approaches in the management of Glioblastoma Multiforme. Chin J Cancer 33:32-39.
Hovinga KE, Stalpers LJ, van Bree C, Donker M, Verhoeff JJ, Rodermond HM, Bosch DA, van Furth WR (2005) Radiation-enhanced vascular endothelial growth factor (VEGF) secretion in Glioblastoma multiforme cell lines--a clue to radioresistance? J Neurooncol 74:99-103.
Huang RP, Fan Y, Hossain MZ, Peng A, Zeng ZL, Boynton AL (1998) Reversion of the neoplastic phenotype of human glioblastoma cells by connexin 43 (cx43) Cancer Res 58:5089-5096.
Huang RP, Hossain MZ, Sehgal A, Boynton AL (1999) Reduced connexin43 expression in high-grade human brain glioma cells. J Surg Oncol 70:21-24.
Huang R, Liu YG, Lin Y, Fan Y, Boynton A, Yang D, Huang RP (2001) Enhanced apoptosis under low serum conditions in human glioblastoma cells by connexin 43 (Cx43) Mol Carcinog 32:128-138.
Hutson TE, Lesovoy V, Al-Shukri S, Stus VP, Lipatov ON, Bair AH, Rosbrook B, Chen C, Kim S, Vogelzang NJ (2013) Axitinib versus sorafenib as first-line therapy in patients with metastatic renal-cell carcinoma: a randomised open-label phase 3 trial. Lancet Oncol 14:1287-1294.
Kelly RJ, Rixe O (2009) Axitinib--a selective inhibitor of the vascular endothelial growth factor (VEGF) receptor. Target Oncol 4:297-305.
Kil WJ, Tofilon PJ, Camphausen K (2012) Post-radiation increase in VEGF enhances glioma cell motility in vitro. Radiat Oncol 7:25.
King TJ, Lampe PD (2005) Temporal regulation of connexin phosphorylation in embryonic and adult tissues. Biochim Biophys Acta 1719:24-35.
Knizetova P, Ehrmann J, Hlobilkova A, Vancova I, Kalita O, Kolar Z, Bartek J (2008) Autocrine regulation of Glioblastoma cell cycle progression, viability and radioresistance through the VEGF-VEGFR2 (KDR) interplay.Cell Cycle 7:2553-2561.
Krcek R, Matschke V, Theis V, Adamietz IA, Bühler H, Theiss C (2017) Vascular endothelial growth factor, irradiation, and axitinib have diverse effects on motility and proliferation of glioblastoma multiforme cells. Front Oncol 7:182.
Laird DW, Puranam KL, Revel JP (1991) Turnover and phosphorylation dynamics of connexin43 gap junction protein in cultured cardiac myocytes.Biochem J 273:67-72.
Lamalice L, Houle F, Jourdan G, Huot J (2004) Phosphorylation of tyrosine 1214 on VEGFR2 is required for VEGF-induced activation of Cdc42 upstream of SAPK2/p38. Oncogene 23:434-445.
Larsen WJ, Tung HN, Murray SA, Swenson CA (1979) Evidence for the participation ofactin microfilaments and bristle coats in the internalization of gap junction membrane. J Cell Biol 83:576-587.
Lee J, Yu H, Choi K, Choi C (2011) Differential dependency of human cancer cells on vascular Lee endothelial growth factor-mediated autocrine growth and survival. Cancer Lett 309:145-150.
Leone S, Fiore M, Lauro MG, Pino S, Cornetta T, Cozzi R (2008) Resveratrol and X rays affect gap junction intercellular communications in human glioblastoma cells. Mol Carcinog 47:587-98.
Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N (1989) Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 246:1306-1309.
Lin JH, Takano T, Cotrina ML, Arcuino G, Kang J, Liu S, Gao Q, Jiang L,Li F, Lichtenberg-Frate H, Haubrich S, Willecke K, Goldman SA, Nedergaard M (2002) Connexin 43 enhances the adhesivity and mediates the invasion of malignant glioma cells. J Neurosci 22:4302-4311.
Lucio-Eterovic AK, Piao Y, de Groot JF (2009) Mediators of glioblastoma resistance and invasion during antivascular endothelial growth factor therapy. Clin Cancer Res 15:4589-4599.
Mesti T, Savarin P, Triba MN, Le Moyec L, Ocvirk J, Banissi C, Carpentier AF (2014) Metabolic impact of anti-angiogenic agents on U87 glioma cells. PLoS One 9:e99198.
Moolten FL, Wells JM (1990) Curability of tumors bearing herpes thymidine kinase genes transferred by retroviral vectors. J Natl Cancer Inst 82:297-300.
Murray SA, Williams SY, Dillard CY, Narayanan SK, McCauley J (1997)Relationship of cytoskeletal filaments to annular gap junction expression in human adrenal cortical tumor cells in culture. Exp Cell Res 234:398-404.
Olbrich L, Foehring D, Happel P, Brand-Saberi B, Theiss C (2013) Fast rearrangement of the neuronal growth cone’s actin cytoskeleton following VEGF stimulation. Histochem Cell Biol 139:431-445.
Park JS, Qiao L, Su ZZ, Hinman D, Willoughby K, McKinstry R, Yacoub A, Duigou GJ, Young CS, Grant S, Hagan MP, Ellis E, Fisher PB, Dent P(2001) Ionizing radiation modulates vascular endothelial growth factor(VEGF) expression through multiple mitogen activated protein kinase dependent pathways. Oncogene 20:7817.
Pu P, Xia Z, Yu S, Huang Q (2004) Altered expression of Cx43 in astrocytic tumors. Clin Neurol Neurosurg 107:49-54.
Qin H, Shao Q, Igdoura SA, Alaoui-Jamali MA, Laird DW (2003) Lysosomal and proteasomal degradation play distinct roles in the life cycle of Cx43 in gap junctional intercellular communication-deficient and -competent breast tumor cells. J Biol Chem 278:30005-30014.
Reuss B, Unsicker K (1998) Regulation of gap junction communication by growth factors from non-neural cells to astroglia: a brief review. Glia 24:32-38.
Rivedal E, Mollerup S, Haugen A, Vikhamar G (1996) Modulation of gap junctional intercellular communication by EGF in human kidney epithelial cells. Carcinogenesis 17:2321-2328.
Rousseau S, Houle F, Landry J, Huot J (1997) p38 MAP kinase activation by vascular endothelial growth factor mediates actin reorganization and cell migration in human endothelial cells. Oncogene 15:2169-2177.
Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF (1983)Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science 219:983-985.
Shao W, Gu J, Huang C, Liu D, Huang H, Huang Z, Lin Z, Yang W, Liu K,Lin D, Ji T (2014) Malignancy-associated metabolic profiling of human glioma cell lines using1H NMR spectroscopy. Mol Cancer 13:197.
Soroceanu L, Manning TJ Jr, Sontheimer H (2001) Reduced expression of connexin-43 and functional gap junction coupling in human gliomas. Glia 33:107-117.
Stahler C, Roth J, Cordes N, Taucher-Scholz G, Mueller-Klieser W (2013)Impact of carbon ion irradiation on epidermal growth factor receptor signaling and glioma cell migration in comparison to conventional photon irradiation. Int J Radiat Biol 89:454-461.
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ,Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC,Ludwin SK, Gorlia T,Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO (2005) Radiotherapy plus concomitant and adjuvant temozolomide for Glioblastoma. N Engl J Med 352:987-996.
Sun P, Liu Y, Ying H, Li S (2012) Action of db-cAMP on the bystander effect and chemosensitivity through connexin 43 and Bcl-2-mediated pathways in medulloblastoma cells. Oncol Rep 28:969-976.
Theiss C, Meller K (2002a) Aluminum impairs gap junctional intercellular communication between astroglial cells in vitro. Cell Tissue Res 310:143-154.
Theiss C, Meller K (2002b) Microinjected anti-actin antibodies decrease gap junctional intercellular commmunication in cultured astrocytes. Exp Cell Res 281:197-204.
Timerman D, Yeung CM (2014) Identity confusion of glioma cell lines. Gene 536:221-222.
Trosko JE, Chang CC (2001) Mechanism of up-regulated gap junctional intercellular communication during chemoprevention and chemotherapy of cancer. Mutat Res 480-481:219-229.
Valerie K, Yacoub A, Hagan MP, Curiel DT, Fisher PB, Grant S, Dent P (2007)Radiation-induced cell signaling: inside-out and outside-in. Mol Cancer Ther 6:789-801.
Wei CJ, Xu X, Lo CW (2004) Connexins and cell signaling in development and disease. Annu Rev Cell Dev Biol 20:811-838.
Wuestefeld R, Chen J, Meller K, Brand-Saberi B, Theiss C (2012) Impact of vegf on astrocytes: analysis of gap junctional intercellular communication,proliferation, and motility. Glia 60:936-947.
Xia ZB, Pu PY, Huang Q, You YP, Wang GX, Wang CY (2003) Preliminary study on the mechanism of connexin 43 gene transfection in the control of glioma cell proliferation. Zhonghua Zhong Liu Za Zhi 25:4-8.
Yao X, Ping Y, Liu Y, Chen K, Yoshimura T, Liu M, Gong W, Chen C, Niu Q, Guo D, Zhang X, Wang JM, Bian X (2013) Vascular endothelial growth factor receptor 2 (VEGFR-2) plays a key role in vasculogenic mimicry formation, neovascularization and tumor initiation by Glioma stem-like cells.PLoS One 8:e57188.
Ye XY, Jiang QH, Hong T, Zhang ZY, Yang RJ, Huang JQ, Hu K, Peng YP(2015) Altered expression of connexin43 and phosphorylation connexin43 in glioma tumors. Int J Clin Exp Pathol. 8:4296-4306.
Zhang YW, Kaneda M, Morita I (2003) The gap junction-independent tumor-suppressing effect of connexin 43. J Biol Chem 278:44852-44856.
杂志排行
中国神经再生研究(英文版)的其它文章
- The role of general anesthetics and the mechanisms of hippocampal and extra-hippocampal dysfunctions in the genesis of postoperative cognitive dysfunction
- Saponins from Panax japonicus attenuate age-related neuroinflammation via regulation of the mitogenactivated protein kinase and nuclear factor kappa B signaling pathways
- Taking out the garbage: cathepsin D and calcineurin in neurodegeneration
- MicroRNAs as diagnostic markers and therapeutic targets for traumatic brain injury
- Interferon regulatory factor 2 binding protein 2: a new player of the innate immune response for stroke recovery
- Endogenous retinal neural stem cell reprogramming for neuronal regeneration
