The effects of microRNA-34a regulating Notch-1/NF-κB signaling pathway on lipopolysaccharide-induced human umbilical vein endothelial cells
2017-12-15YunGeManHuangYuefengMa
Yun Ge, Man Huang, Yue-feng Ma
Department of General Intensive Care Unit, The Second Affiliated Hospital, Zhejiang University School of Medicine,Hangzhou 310052, China
Original Article
The effects of microRNA-34a regulating Notch-1/NF-κB signaling pathway on lipopolysaccharide-induced human umbilical vein endothelial cells
Yun Ge, Man Huang, Yue-feng Ma
Department of General Intensive Care Unit, The Second Affiliated Hospital, Zhejiang University School of Medicine,Hangzhou 310052, China
MicroRNA-34a; Notch-1; NF-κB; Lentivirus; Human umbilical vein endothelial cells
INTRODUCTION
Sepsis, an infection-induced clinical syndrome accompanying systemic inflammation, is triggered by pathogen-associated molecular patterns, such as lipopolysaccharide (LPS) from gram-negative bacteria,and is considered as a major cause of death in the intensive care unit.[1,2]Although recent progress has been made in the understanding and management of sepsis, the mortality of sepsis is still high. It was reported that sepsis could result in about 28%–50% mortality.[3]With sepsis, the initial of inflammation damage to the endothelium, which, in turn,can result in inflammatory cascade and multi-organ failure related to disease severity and risk of death. Although the mechanism of sepsis is not fully understood, cumulative evidence has indicated that the changes of endothelium function play a central role in sepsis, and endothelium may be a target for treating sepsis.[4]Furthermore, endothelial cells are not only the passive target cells during inflammatory response but effective cells and therefor play a significant role in the microcirculation disturbance, septic shock and multiple organ dysfunction syndrome (MODS).
Notch signaling is a highly conserved pathway which plays a key role in differentiation, proliferation, synaptic plasticity,and cell survival.[5]Our previous study has suggested that Notch-1/NF-κB signaling be involved in the pathophysiological progress of sepsis.[6]Cao et al[7]and Mraz et al[8]reported that microRNA-34a (miR-34a) could regulate Notch signaling pathway on immune response, inflammation, and tumor stem cells growth and reproduction. The present study aimed to determine the effects of LPS exposure on the human umbilical vein endothelial cells (HUVEC) and intervention effects of miR-34a lentivirus regulating Notch-1/NF-κB signaling pathway on LPS-induced HUVEC.
METHODS
HUVEC culture
HUVEC (ATCC, USA) were cultured in M199 medium(Sigma, USA) supplemented with 10% fetal bovine serum, antibiotics (100 U/mL penicillin and 100 μg/mL streptomycin) and endothelial cell growth supplement(BD Biosciences, USA) at 37 °C and 5% CO2. Cells were used at the second passage.
LPS-induced HUVEC injury and invention
HUVEC were directly damaged by 1 μg/mL LPS(Sigma, USA). They were divided into four groups: NC group, HUVEC were infected with negative control lentivirus (adding 2 μL, concentration 1×109TU/mL)(Shanghai Genechem, China); OE group, HUVEC were infected with miR-34a lentivirus (adding 2 μL, 1×109TU/mL) (Shanghai Genechem, China); NC+LPS group,1 μg/mL LPS was added on the third day on the basis of NC group for 24 hours; OE+LPS group, 1 μg/mL LPS was added on the third day on the basis of OE group for 24 hours.
Fluorescent labeling of LPS and fl uorescencemicroscopy observation
Suspended HUVEC were re-cultured on a glass plate and stimulated with fluorescently labeled LPS. The HUVEC were digested with trypsin and re-cultivated in 25 mL M199 medium for 4 hours, then were directly injured with fluorescently labeled LPS (1 μg/mL) for 24 hours.Afterwards, the directly injured HUVEC were washed with 20 mL M199 medium three times to remove the residual fl uorescent labeled LPS. The membrane lipids in HUVEC were extracted and prepared as described method and lipids samples were added to lipid sample solution on a glass plate and viewed under the fl uorescent microscope(Olympus, Japan) at the 10×10 and 20×10 magnification.
ELISA assay
The levels of tumor necrosis factor-α (TNF-α),interleukin-1β (IL-1β), interleukin-6 (IL-6), and interleukin-10 (IL-10) in the cell supernatants of four groups were determined by ELISA method using ELISA kits (R&D Systems, USA), according to manufacture's instructions. Results were expressed as picograms per milligram (pg/mL) of cell supernatants.
Quantitative reverse transcription real-time polymerase chain reaction
Total RNA was extracted from HUVEC with the Qiagen RneasyR kit (Qiagen, Germany), and subjected to reverse transcription-PCR with ImProm-IITM (Promega,USA) according to the manufacturer's instructions. qPCR analysis for Notch-1 and NFκB expression was performed on a LightCycler thermocycler (Roche Diagnostics,Switzerland) by using TransStartTM SYBR Green qPCR Supermix (Qiagen, Germany). Primers used here were as follows: Notch-1, NF-κB and GAPDH. Notch-1 forward 5'-GAGGCGTGGCAGACTATGC-3', reverse 5'-CTTGTACTCCGTCAGCGTGA-3'; NF-κB forward 5'-AGGATTTCGTTTCCGTTATGT-3', reverse 5'-CCTG AGGGTAAGACTTC TTGTTC-3'.GAP DH forward 5'-TGACTTCAACAGCGACACCCA-3', reverse 5'-CACCC TGTTGCTGTAGCCAAA-3'. Results were normalized to GAPDH expression. The data represented by 2-ΔΔctwere analyzed by ABI PRISM 2.0 software. The experiment was repeated three times to verify the reliability of the result.
Western blots
Protein concentration of the supernatant was measured with a Bicinchoninic Acid Protein Assay Kit (Biyuntian Biotechnology, China). Target proteins (40 μg) were run in 10% gradient sodium dodecyl sulphate polyacrylamide electrophoresis gel and transferred to polyvinyl difluoride membranes (Millipore, USA), respectively.The membranes were incubated for 2 hours in blocking buffer (5% nonfat dry milk) at room temperature and then overnight in the buffer containing primary antibodies against Notch-1, NF-κB and actin at 4 °C. Protein bands were visualized with an ECL Western blot kit (Bio-Rad,USA). Primary antibodies were diluted as follows: 1:1 000 for anti-Notch1 (Abcam, UK), 1:5 000 for anti-GAPDH,1:1 000 for anti-NF-κB (Abcam UK).
Statistical analysis
Data were presented as mean±standard error (SE).Differences between two groups were analyzed by Student'sttest. Statistical significance was performed using one-way analysis of variance (ANOVA) followed by Fisher's least significant difference (LSD) multiplecomparisons test; aP<0.05 was considered to indicate statistical significance.
The data were analyzed with SPSS 17.0 software.
RESULTS
Fluorescein-labeled LPS incorporation into the HUVEC membrane
The direct infiltration of fl uorescein-labeled LPS on HUVEC membrane was assessed by observation with fl uorescent microscopy. It could be observed from Figure 1 that when LPS concentration was over 1 μg/mL, the whole cellular membrane surface had been infiltrated.The experimental results showed that LPS directly infiltrated the HUVEC membrane surface.
The levels of TNF-α, IL-1β, IL-6 and IL-10 assay
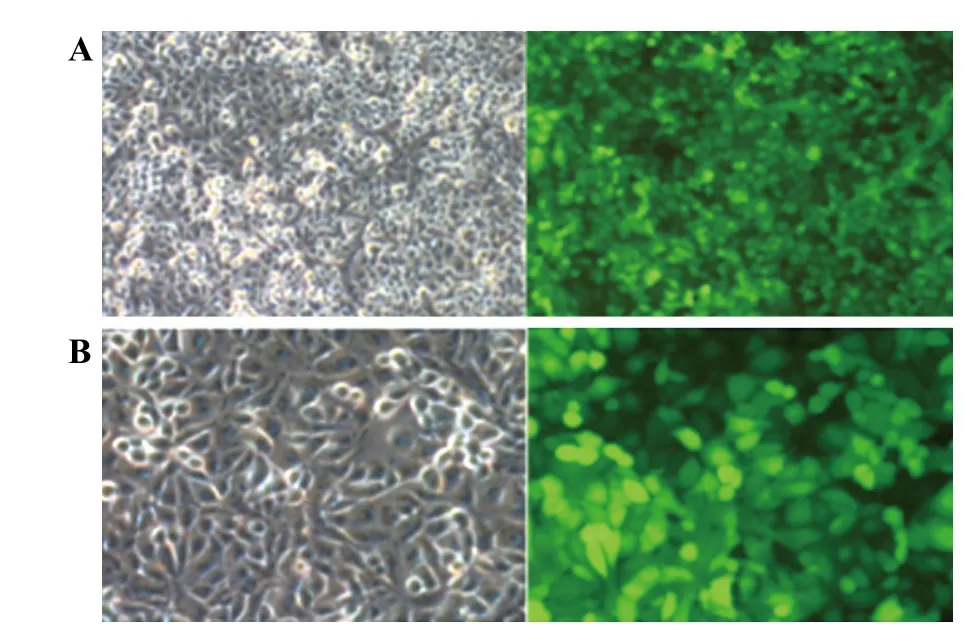
Figure 1. Fluorescent labeled in the HUVEC; A: 100×; B: 200×.
After 24 hours, the cell supernatants of TNF-α, IL-1β,IL-6 levels (P<0.01) in NC+LPS group and TNF-α (P<0.01),IL-6 (P<0.05) levels in OE+LPS group significantly increased and IL-10 level (P<0.01) in NC+LPS group significantly decreased compared to NC group (Figure 2). There was no significant difference in any indicators between OE group and NC group (P>0.05). The cellular supernatants of TNF-α level (P<0.01) in OE+LPS group after 24 hours decreased compared to NC+LPS group.No significant difference in the cell supernatants of IL-10, IL-6 and IL-1β levels was observed between OE+LPS group and NC+LPS group (P>0.05).
The mRNA expression of Notch-1 and NF-κB test
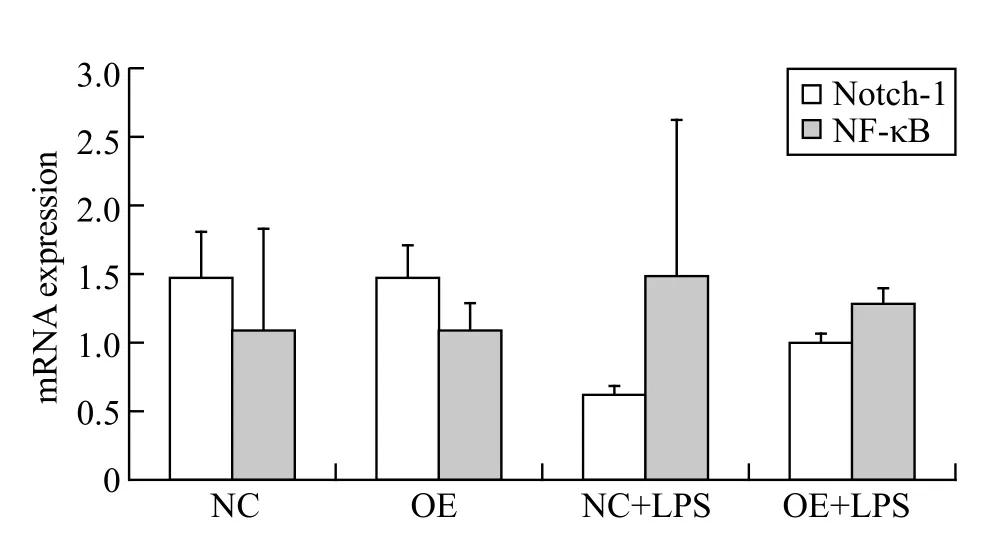
Figure 3. The mRNA expression of Notch-1 and NF-κB in the HUVEC; all results are representative of at least three independent experiments.
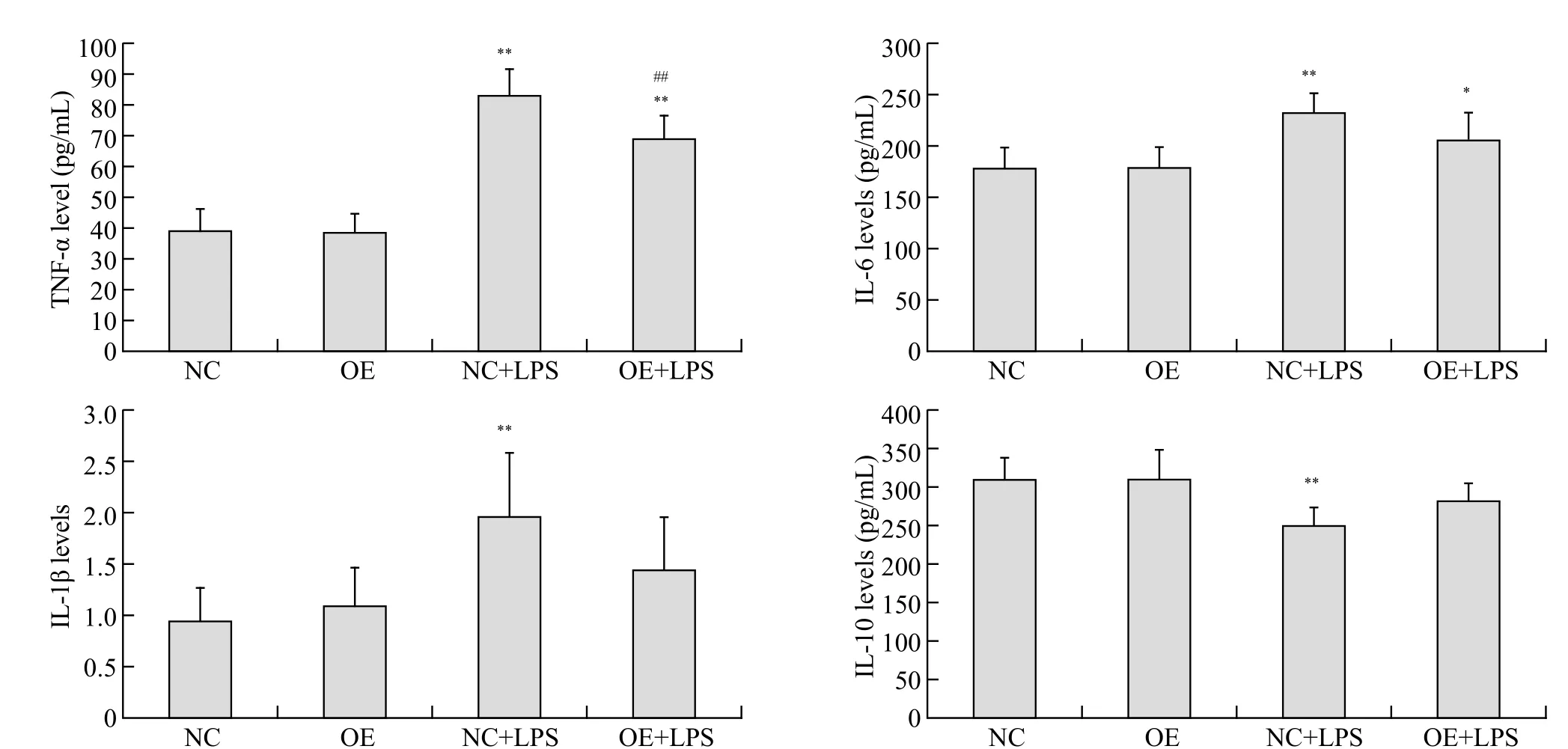
Figure 2. The levels of TNF-α, IL-1β, IL-6 and IL-10 in the cell supernatants; compared with NC group, *P<0.05, **P<0.01; compared with NC+LPS group, #P<0.05, ##P<0.01; all results are representative of at least three independent experiments.
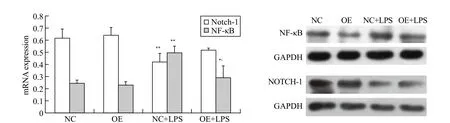
Figure#4. The protein expression of Notch-1 and NF-κB in the HUVEC; compared with NC group, *P<0.05, **P<0.01; compared with NC+LPS group, P<0.05; all results are representative of at least three independent experiments.
From Figure 3, the mean mRNA expression of Notch-1 in NC+LPS group (P>0.05) and OE+LPS group (P>0.05)was lower than that in NC group; after intervention with miR-34a lentivirus, the mean mRNA expression in OE+LPS group (P>0.05) increased compared to NC+LPS group. The mean mRNA expression of NF-κB in NC+LPS group (P>0.05) and OE+LPS group (P>0.05)was higher than that in NC group; after invention of miR-34a lentivirus, the mean mRNA expression in OE+LPS group (P>0.05) decreased compared to NC+LPS group.
The protein expression of Notch-1 and NF-κB test
From Figure 4, the protein expression of Notch-1 in NC+LPS group after 24 hours was markedly decreased(P<0.01) and the decrease of that in OE+LPS group was not statistically significant (P>0.05) compared to NC group. The protein expression of NF-κB in NC+LPS group (P<0.01) and OE+LPS group (P<0.05) after 24 hours increased compared to NC group. There was no significant difference in both indicators between NC group and OE group (P>0.05). In comparison with NC+LPS group, the protein expression of NF-κB in OE+LPS group after 24 hours decreased significantly(P<0.05). Although no significant difference in the protein expression of Notch-1 was observed between two groups, the mean protein expression in OE+LPS group was higher than that in NC+LPS group.
DISCUSSION
Sepsis is typically characterized by systemic inflammatory response or "cytokine storm" and leads to fatal multiple organ failure. In sepsis and multi-organ dysfunction syndrome, the cytokine production from innate and adaptive immune system such as tumor necrosis factor-α(TNF-α) and interleukin-1β (IL-1β) are induced by LPS.Endothelium can be the prime target for sepsis and infected endothelium may act as an initiating system for initial cytokine-mediated hyperinflammation as these cells are able to produce a wide variety of mediators (e.g., TNF-α,IL-1β, IL-6). The endothelium can in turn accelerate inflammatory cascade.[9–11]The uncontrolled release of cytokines such as TNF-α, IL-6, and IL-10 which can be activated by NF-κB pathway, which plays a key role in the development of organ dysfunction during sepsis.[12,13]As we all know, NF-κB serves as a transcription factor that regulates the cellular responses to various extracellular stress stimuli. It also initiates the upregulation of mediators such as TNF-α and IL-6 to initiate the inflammatory responses.[14,15]In the present study, after LPS simulation of HUVEC, we found that pro-inflammatory cytokines (e.g.,TNF-α, IL-1β, IL-6 levels) markedly increased after 24 hours. While, on the contrary, anti-inflammatory cytokine IL-10 level decreased significantly at the same time point.Meanwhile, the expression of NF-κB mRNA and protein had a rise, which indicated that NF-κB was activated.
Notch signaling is a well-conserved pathway involved in cell fate decisions during development of various systems, including the hematopietic, neuronal and immune systems. Notch receptor binding with its ligand will induce intramembrane proteolysis of the receptor under the action of theγ-secretase complex, and release Notch receptor intracellular domain (NICD) which translocates to the nucleus and regulates downstream target genes.[16]Recently,studies have suggested that Notch activation induce upregulation of the NICD level, which regulates transcription of many genes, and is related to neuronal apoptosis or mitochondrial dysfunction in ischemia.[17]Furthermore,modulating Notch signaling also has a profound effect on the NF-κB signaling cascade in the inflammatory and immune responses.[18]Stimulation of macrophages through LPS triggered activation of Notch-1/NF-κB signaling,which regulated gene expression patterns involved in inflammatory responses, as shown by Palaga et al.[19]However, the role of Notch-1/NF-κB signaling in regulating cytokine production in the endothelium has not been addressed. In this study, we found that the expression of Notch-1 mRNA and protein after 24 hours had a drop after stimulation of LPS, while NF-κB mRNA and protein was significantly increased after 24 hours. Activation of NF-κB in turn increased TNF-α, IL-1β, and IL-6 production. Taken together, our results indicated that Notch-1/NF-κB pathway is involved in LPS-induced HUVEC.
MicroRNAs represent an important family of small non-coding RNAs that post-transcriptionally mediate gene expression. Meanwhile, microRNAs have emerged as key gene regulators, which regulate their target genes by degrading the binding mRNAs or by suppressing the corresponding protein products. A wide variety of biological processes by which microRNAs exerts its important effects have been identified.[20,21]It was reported that microRNA-34a(miR-34a) can regulate tumor stem cell growth, immune response, and inflammation by Notch signaling pathway.[7,8]However, it is unclear whether activation of miR-34a can regulate Notch-1/NF-κB pathway in the endothelium. Our results showed that pro-inflammatory cytokines were reduced and antiinflammatory cytokines were elevated after miR-34a invention. Moreover, the mRNA and protein expression of Notch-1 had a rise and that of NF-κB was the opposite.
CONCLUSION
In conclusion, the key finding of our study is that Notch-1/NF-κB signaling plays a vital role in HUVEC damage induced by LPS. MiR-34a regulating Notch-1/NF-κB signaling pathway can attenuate inflammatory response induced by LPS in HUVEC. Our results also suggested that Notch-1/NF-κB signaling might be a potential therapy target for sepsis.
Funding: This work was supported by a grant from Natural Science Foundation of Zhejiang Province of China (LY14H150003).
Ethical approval: The ethical approval was obtained from The Second Affiliated Hospital, Zhejiang University School of Medicine,Hangzhou, China.
Conflicts of interest: All authors declare that they have no conflict of interest.
Contributors: Ge Y analyzed all data and wrote the final version of the manuscript. Ma YF participated in study design and coordination.Huang M helped to draft the manuscript.
1 Angus DC, Linde-Zwirble WT, Lidicker J. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29(7):1303–10.
2 Chen K, Zhou QX, Shan HW, Li WF, Lin ZF. Prognostic value of CD4(+)CD25(+) Tregs as a valuable biomarker for patients with sepsis in ICU. World J Emerg Med. 2015;6(1):40–3.
3 Natanson C, Esposito CJ, Banks SM. The sirens' song of confirmatory sepsis trials: selection bias and sampling error. Crit Care Med. 1998;26(12):1927–31.
4 Kotsovolis G, Kallaras K. The role of endothelium and endogenous vasoactive substances in sepsis. Hippokratia. 2010; 14(2):88–93.
5 Alberi L, Hoey SE, Brai E, Scotti AL, Marathe S. Notch signaling in the brain: In good and bad times. Ageing Res Rev.2013;12(3):801–14.
6 Huang M, Liu CH, Hu YY, Wang P, Ding M. γ-secretase inhibitor DAPT prevents neuronal death and memory impairment in sepsis associated encephalopathy in septic rats. Chin Med J(Engl). 2014;127(5):924–8.
7 Cao C, Ma T, Chai YF, Shou ST. The role of regulatory T cells in immune dysfunction during sepsis. World J Emerg Med.2015;6(1):5–9.
8 Mraz M, Dolezalova D, Plevova K, Stano Kozubik K, Mayerova V, Cerna K, et al. MicroRNA-650 expression is influenced by immunoglobulin gene rearrangement and affects the biology of chronic lymphocytic leukemia. Blood. 2012;119(9):2110–3.
9 Aird WC. Endothelium as a therapeutic target in sepsis. Curr Drug Targets. 2007;8(4):501–7.
10 Hoesel LM, Gao H, Ward PA. New insights into cellular mechanisms during sepsis. Immunol Res. 2006;34(2):133–41.
11 Mutunga M, Fulton B, Bullock R, Batchelor A, Gascoigne A,Gillespie JI, et al. Circulating endothelial cells in patients with septic shock. Am J Respir Crit Care Med. 2001;163(1):195–200.
12 Frank CZ, Carsten JK, Roberta M, Xu XP, Jin Y, Faure E, et al.Bacterial lipopolysaccharide activates nuclear factor-κB through interleukin-1 signaling mediators in cultured human dermal endothelial cells and mononuclear phagocytes. J Biol Chem.1999;274(12):7611–4.
13 Wan FY, Michael JL. The nuclear signaling of NF-κB current knowledge, new insight, and future perspectives. Cell Res. 2011;20(1): 24–33.
14 Cheng J, Montecalvo A, Kane LP. Regulation of NF-κB induction by TCR/CD28. Immunol Res. 2011;50(2–3):113–7.
15 Hayden MS, Ghosh S. NF-κB in immunobiology. Cell Res.2011;21(2):223–44.
16 Palaga T, Buranaruk C, Rengpipat S, Fauq AH, Golde TE,Kaufmann SH, et al. Notch signaling is activated by TLR stimulation and regulates macrophage functions. Eur J Immunol.2008;38(1):174–83.
17 Wei Z, Chigurupati S, Arumugam TV, Jo DG, Li H, Chan SL.Notch activation enhances the microglia-mediated inflammatory response associated with focal cerebral ischemia. Stroke.2011;42(9):2589–94.
18 Shin HM, Minter LM, Cho OH, Gottipati S, Fauq AH, Golde TE, et al. Notch1 augments NF-kappaB activity by facilitating its nuclear retention. EMBO J. 2006;25(1):129–38. Epub 2005 Dec 1.
19 Palaga T, Buranaruk C, Rengpipat S, Fauq AH, Golde TE,Kaufmann SH, et al. Notch signaling is activated by TLR stimulation and regulates macrophage functions. Eur J Immunol.2008;38(1):174–83.
20 Chaudhuri AA, So AY, Sinha N, Gibson WS, Taganov KD,O'Connell RM, et al. MicroRNA-125b potentiates macrophage activation. J Immunol. 2011;187(10):5062–8.
21 Zheng J, Jiang HY, Li J, Tang HC, Zhang XM, Wang XR, et al.MicroRNA-23b promotes tolerogenic properties of dendritic cells in vitro through inhibiting Notch1/NF-κB signaling pathways.Allergy. 2012;67(3):362–70.
Yue-feng Ma, Email: 2193017@zju.edu.cn
BACKGROUND:Notch-1/NF-κB signaling plays a key role in the cecal ligation and puncture(CLP)-induced sepsis. This study aims to investigate the intervention effects of microRNA-34a (miR-34a) lentivirus regulating Notch-1/NF-κB signaling pathway on lipopolysaccharide (LPS)-induced human umbilical vein endothelial cells (HUVEC).
METHODS:HUVEC were divided into four groups as the following: they were infected with negative control lentivirus (NC group) or miR-34a lentivirus (OE group); LPS (1 μg/mL) was added on the third day on the basis of NC group and OE group for 24 hours (NC+LPS group or OE+LPS group). The levels of TNF-α, IL-1β, IL-6, and IL-10 in the cell supernatants, and the mRNA and protein expression of Notch-1 and NF-κB in the HUVEC were evaluated.
RESULTS:After 24 hours, the levels of TNF-α, IL-1β, IL-6 in the cell supernatants and the protein expression of NF-κB from NC+LPS group were significantly higher than those of NC group,but IL-10 level and the protein expression of Notch-1 in NC+LPS group were the opposite. After intervention of miR-34a lentivirus, the cell supernatants TNF-α and the protein expression of NF-κB in OE+LPS group after 24 hours markedly decreased compared to NC+LPS group. While the cell supernatants IL-1β and IL-6 and the mRNA expression of NF-κB slightly decreased in OE+LPS group, IL-10 and the mRNA and protein expression of Notch-1 were the opposite.
CONCLUSION:miR-34a regulating Notch-1/NF-κB signaling pathway can reduce the HUVEC damage caused by LPS stimulation.
World J Emerg Med 2017;8(4):292–296
10.5847/wjem.j.1920–8642.2017.04.008
February 27, 2017
Accepted after revision July 20, 2017
杂志排行
World journal of emergency medicine的其它文章
- Instructions for Authors
- Case of morphine-induced ventricular fibrillation
- Paradoxical brain embolism followed by percutaneous atrial septal closure: Stroke in a patient's thirties highlighting some issues surrounding brain stroke in an emergency setting
- Vulvar inflammation as a manifestation of Crohn's disease
- Anticholinergic syndrome induced by toxic plants
- Procedural simulation: medical student preference and value of three task trainers for ultrasound guided regional anesthesia
