两个垩白突变体的鉴定及突变基因的图位克隆
2017-12-02张习春鲁菲菲吕育松罗荣剑焦桂爱邬亚文唐绍清胡培松魏祥进
张习春 鲁菲菲 吕育松 罗荣剑 焦桂爱 邬亚文 唐绍清 胡培松 魏祥进
(中国水稻研究所 水稻生物学国家重点实验室,杭州 310006; #共同第一作者;*通讯联系人,E-mail:weixiangjin@caas.cn)
两个垩白突变体的鉴定及突变基因的图位克隆
张习春#鲁菲菲#吕育松 罗荣剑 焦桂爱 邬亚文 唐绍清 胡培松 魏祥进*
(中国水稻研究所 水稻生物学国家重点实验室,杭州 310006;#共同第一作者;*通讯联系人,E-mail:weixiangjin@caas.cn)
【目的】研究两个水稻垩白突变体胚乳垩白的形成机制,为稻米品质改良提供理论基础。【方法】以从中花11的EMS突变体库中筛选出的两个稳定遗传的垩白突变体eb6、eb7为材料,对其进行农艺性状、稻米理化性质和遗传学分析,并利用eb6与南京11衍生的F2群体对控制垩白的基因进行图位克隆。同时对候选基因的表达模式及淀粉合成相关基因在突变体及野生型中表达情况进行了分析。【结果】与野生型相比,两个突变体胚乳中央部位呈现白色且不透明,淀粉复合颗粒形状不规则且排列疏松,而突变体胚乳边缘部位与野生型无异,都为半透明状,淀粉复合颗粒呈多面体且排列致密。相对于野生型,突变体 eb6、eb7成熟种子中的直链淀粉含量和胶稠度显著降低,蛋白质含量显著升高。RVA谱分析显示突变体淀粉黏滞性明显低于野生型。同时,支链淀粉聚合度分析显示突变体eb6中聚合度(DP)小于16的短链显著增加,DP为16~23的中长链显著减少。遗传分析表明突变体 eb6、eb7胚乳垩白表型由单隐性核基因控制,并且它们为一对等位突变体。利用突变体eb6与南京11杂交衍生的F2群体将突变体基因定位在第1染色体长臂上物理距离86.6 kb的区间内。该区间包含22个开放阅读框(ORF),其中ORF13编码腺苷二磷酸葡萄糖焦磷酸化酶大亚基2(OsAGPL2)。序列分析发现eb6与eb7分别在OsAGPL2第3、第7外显子上发生1个单碱基替换,并分别导致一个氨基酸替换。RT-PCR及原位杂交结果显示,OsAGPL2主要在水稻发育中的籽粒中表达。同时OsAGPL2的突变导致了多个淀粉合成相关基因在水稻籽粒灌浆过程中的表达模式发生改变。【结论】突变体 eb6、eb7籽粒胚乳出现严重垩白表型为OsAGPL2突变所致。本研究进一步证明了OsAGPL2在调控水稻籽粒胚乳中淀粉的合成、淀粉复合颗粒的形成及稻米理化性质的平衡中起着重要的作用。
垩白;淀粉合成;图位克隆;OsAGPL2;水稻
水稻是最主要的粮食作物之一,全世界超过一半的人口以水稻为主食。随着人民生活水平的提高,对稻米品质也提出了更高的要求,稻米品质的优劣直接关系到水稻品种在市场的认可度和稻米的市场竞争力。垩白是影响稻米外观品质的最重要因素之一,严重垩白不仅影响稻米的外观品质、碾磨加工品质和蒸煮食味品质,甚至会影响水稻籽粒粒重及产量[1-4]。因此,对垩白形成机制的研究对于稻米品质改良及生产都具有重要的指导意义。
垩白是稻米胚乳中白色不透明的部分,它是由于水稻籽粒灌浆不充分,胚乳中的淀粉体和蛋白质体充实不良,相互间存在空隙引起光线散射而形成的一种光学特性[5-6]。在水稻籽粒中,淀粉占胚乳中干物质含量的80%以上,因此,影响淀粉含量及颗粒形态的因素都可能导致垩白的发生。淀粉合成是一个相当复杂的过程,首先光合作用合成的葡萄糖转化为蔗糖,蔗糖通过维管束运送到籽粒中并在蔗糖转化酶(invertase)、蔗糖合酶(SuSy)、尿苷二磷酸葡萄糖焦磷酸化酶(UGPase)及己糖激酶(hexokinase)等一系列酶的作用下最终转化成葡萄糖-1-磷酸(G1P),而G1P则在腺苷二磷酸葡萄糖焦磷酸化酶(AGPase)的作用下转化为合成淀粉的底物腺苷二磷酸葡萄糖(ADPG)[7-11],在淀粉合酶(SS)、淀粉分支酶(SBE)及淀粉脱支酶(DBE)等的协同作用下最终合成直链淀粉和支链淀粉[12-14]。除了上述淀粉合成直接相关的酶外,还有很多调控子也间接影响水稻籽粒淀粉的合成[15-16]。无论是淀粉合成酶基因或调控子基因发生突变都会或多或少地影响到籽粒淀粉合成及其结构,最终导致垩白的发生;例如主要参与支链淀粉B2-B4链延伸的淀粉合成酶基因SSⅢa的突变使胚乳产生心白表型、同时粒重下降[17]。编码ADPG转运蛋白的OsBT1突变后导致总淀粉含量下降,种子表现出垩白[18]。控制水稻籽粒垩白的主效QTL chalk5编码液泡氢离子转运焦磷酸酶,通过调控细胞质中氢离子的平衡来间接影响淀粉合成,Chalk5功能缺失导致直链淀粉含量下降,胚乳出现垩白[19]。
AGPase是淀粉合成过程中的限速酶,它催化G1P和ATP转化为淀粉合成的底物ADPG。在大部分高等植物中,其活性受3-磷酸甘油酸变构激活,被无机磷酸盐抑制[20-22],其转录活性受到糖的促进,硝酸盐和磷酸盐的抑制[23-25]。研究表明,大部分原核生物中的AGPase是由单个亚基组成的同源四聚体(α4),而在植物中则是由大小亚基构成异源四聚体(α2β2)[17,26-29],越来越多的试验结果表明,大小亚基对于AGPase的催化活性以及变构调节活性都是必要的[30-32]。水稻AGPase的小亚基由OsAGPS1和OsAGPS2编码,大亚基分别由OsAGPL1、OsAGPL2、OsAGPL3、OsAGPL4编码。OsAGPS1和OsAGPL1构成了造粉体中的AGPase,主要在胚乳发育的早期大量表达。OsAGPS2具有可变剪接,分别编码了叶绿体和胚乳细胞质基质中小亚基S2a和S2b,OsAGPL2在胚乳细胞的细胞质基质中表达,OsAGPL3只在叶片中表达,而OsAGPL4在种子和叶片中表达量较低[33-34]。已有研究表明,水稻AGPase活性约90%来自于细胞质基质,仅有10%来自于质体[35-37],而且细胞质基质中表达的小亚基S2b和大亚基L2能够直接互作[38],因此OsAGPS2b和OsAGPL2对AGPase活性有决定作用。OsAGPL2在胚乳发育的中后期大量表达,该时期是籽粒灌浆、淀粉合成、稻米品质形成的重要时期,而且已报道的多个OsAGPL2的复等位突变体,无义和错义突变都使籽粒皱缩,胚乳垩白[39-41],因此OsAGPL2对籽粒的发育和稻米品质的形成具有重要的调控作用。
本研究从粳稻品种中花11的突变体库中筛选出两个胚乳中心部位白色不透明的突变体eb6、eb7,等位性测验表明其相互等位。对该两个突变体的形态学、稻米理化性质进行了详细的描述与分析,通过图位克隆发现这两个垩白突变体都在OsAGPL2不同外显子上发生单碱基替换,导致单个氨基酸的替换。本研究为揭示OsAGPL2调控水稻籽粒淀粉合成、垩白形成及籽粒发育提供了重要补充。

表1 突变体eb6基因精细定位所用分子标记Table 1. Markers for fine mapping of eb6.
1 材料与方法
1.1实验材料
垩白突变体eb6和eb7来源于粳稻品种中花11,经 EMS诱变、多代自交后,其籽粒垩白性状稳定遗传,等位性测验表明两者为一对等位突变体。利用eb6与南京11、eb7与93-11所衍生的F2群体对突变体基因进行遗传分析;以eb6和南京11的F2群体对突变基因开展定位研究。所有材料均于正季种植于中国水稻研究所富阳实验基地,种植和管理方法同大田生产。突变体和野生型完全成熟后,随机选取 15株,测定千粒重、粒型等农艺性状。每组实验重复3次,取平均值。
1.2种子灌浆过程中干物质和游离糖含量测定
在开花盛期对野生型和突变体当天开花的颖花作标记,记为开花后0 d(DAF0)。以后分别收取开花后5、10、15、20、25、30 d种子,65℃下烘干至恒重,去壳、测定干物质量。测定干物质后的样品研磨成粉,用于胚乳中蔗糖、葡萄糖、果糖含量测定,测定方法采用 Biosentec公司提供的葡萄糖-果糖-蔗糖试剂盒(酶法),用DU800UV分光光度计(Beckman)进行吸光度测定。
1.3稻米理化性质测定
总淀粉、直链淀粉、支链淀粉和胶稠度采用农业部部颁标准NY/T83-1998[42]测定,蛋白质含量参照GB/T5009.5-1995[43]指定方法,利用半微量凯氏定氮法进行测定;脂肪按照GB/T5512-2008《粮油检验:粮食中粗脂肪含量测定》索氏提取法测定;支链淀粉聚合度测定参照Han等[44]的方法。
1.4胚乳横截面扫描电镜观察
分别随机选取突变体eb6、eb7及其野生型完整的干燥种子(去壳),用金属刀片沿糙米籽粒中部外层划一圈,然后掰断,形成自然断面,以导电胶粘着于样品台上,横断面向上,利用真空镀膜仪对横断面镀金,使用HitachiTM-1000型扫描电镜对米粒横截面的不同部位进行观察和拍照[45]。
1.5eb6突变体基因的精细定位
利用本实验室保存的均匀分布于12条染色体的512对SSR引物(http://www.gramene.org/)对突变体eb6和南京11进行多态性筛选,挑选出具有多态性的SSR引物144对。从突变体和南京11衍生的F2群体中挑选糙米表型为类似突变体的垩白胚乳的分离单株,提取DNA对突变基因进行定位。首先利用340个F2垩白胚乳单株对突变体基因进行初步定位,在初步定位标记之间进一步开发13对有多态的SSR和InDel标记(表1),利用970个表现为胚乳垩白的隐性单株对突变基因进行精细定位。DNA采用CTAB法提取[41]。使用PCR快速扩增,结合非变性聚丙烯酰胺凝胶电泳及银染方法定位目的基因。
1.6 RNA提取和基因表达分析
水稻根、茎、叶和幼穗中的总RNA采用Trizol法提取,种子的总RNA采用SDS-Trizol法[46]提取。以DNaseⅠ消化处理的总RNA为模板,Oligo(dT)为引物,反转录酶催化下合成cDNA第一链。利用实时荧光定量PCR分析目的基因在野生型不同组织中及淀粉合成相关基因在突变体和野生型籽粒灌浆过程中的表达情况,内参基因为Ubiqutin(GenBank登录号为AF184280)。荧光定量PCR体系(20 μL)如下:cDNA模板1 μL,2×SYBR qPCR Mix(TOYOBO)10 μL,上下游引物(10 μmol/L)各1 μL,ddH2O 7 μL。定量PCR仪为罗氏Light Cycle 480,扩增程序如下:95℃下30 s,95℃下5 s,60℃下30 s,72℃下15 s,40个循环。以2-△△CT计算各基因的相对表达量。淀粉合成相关基因的表达引物见表2。
1.7原位杂交
取开花后9 d的籽粒,去壳后立即放入70% FAA固定液(乙醇70%,冰乙酸5%,甲醛10%,DEPC处理水15%)中固定,抽真空使样品沉于底部。使用不同浓度梯度的无水乙醇溶液对样品进行脱水处理,之后利用二甲苯对样品进行透明化处理,最后使用石蜡对样品进行包埋,每天换腊2次,4 d后包埋定型。利用石蜡切片机将样品切至8 μm厚的蜡带并置于载玻片上于42℃展片,再利用二甲苯脱蜡、不同浓度的乙醇和DEPC处理水清洗、风干后以42℃预热好的蛋白酶K溶液孵育,之后乙醇进行脱水、风干,将提前制作好的杂交溶液加于载玻片,盖上保鲜膜,42℃下杂交过夜(探针引物序列:ATG CAA TTC ATG ATG CCA TTGG和TAT CAG CAT CTG ATG TGA ACA C),之后进行免疫反应,洗脱、显色、脱水和封片,详细步骤参照Li等[47]的方法。
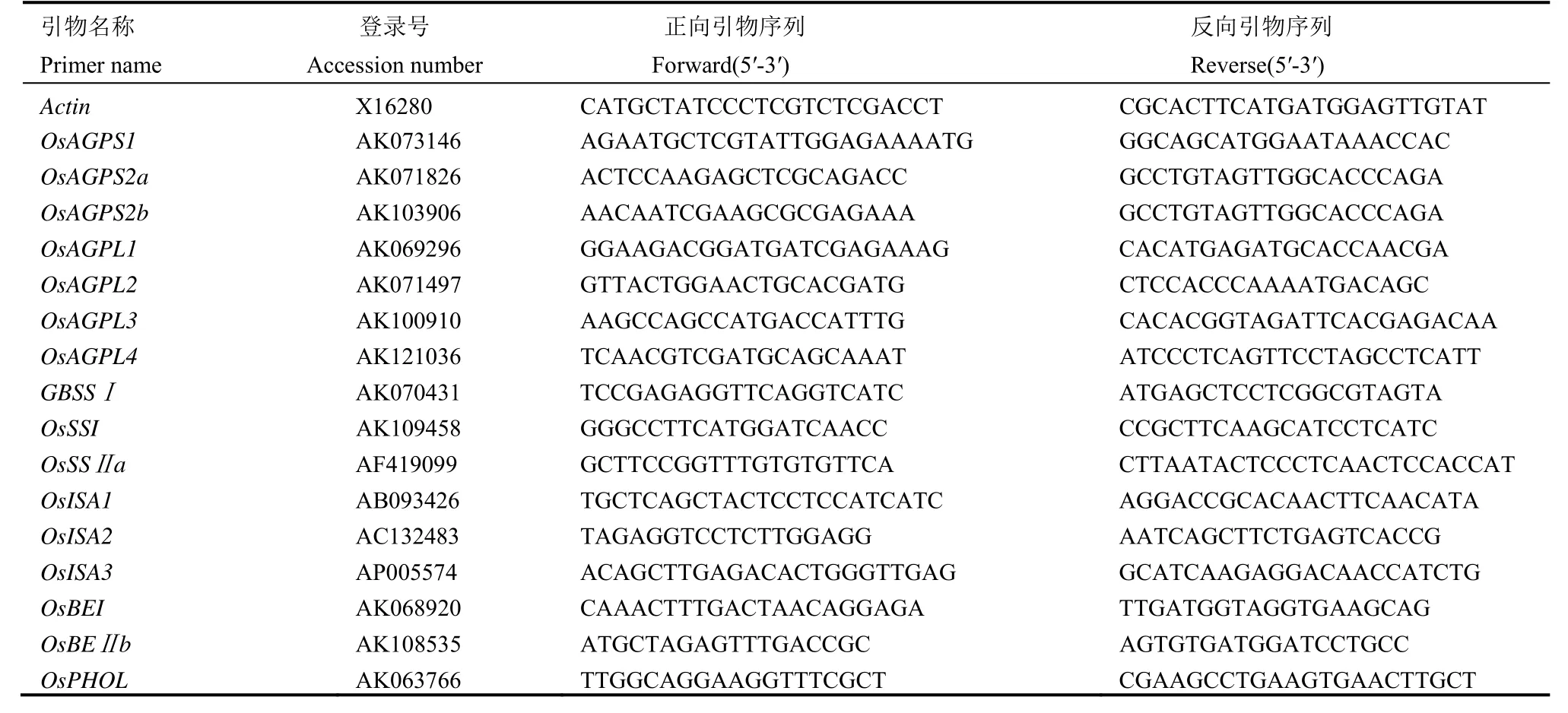
表2 淀粉合成相关基因实时荧光定量PCR分析所用的引物Table 2. Primers associated with starch synthesis used in RT-PCR.
2 结果与分析
2.1突变体eb6和eb7稻米的表型
突变体eb6和eb7成熟种子外观与野生型中花11无明显差异(图1-A),但是突变体的糙米和精米两端透明,中间部位白色不透明,表现为严重垩白,野生型种子的糙米与精米都具有较高透明度(图1-B~C)。胚乳横切面观察发现,野生型种子胚乳无论中央还是外围部位均表现为透明,突变体eb6和eb7的胚乳外围部位与野生型类似,但是中央部位表现出白色且不透明(图1-D),因此突变体eb6和eb7稻米表现为严重心白。而eb6和eb7的粒长、粒宽、粒厚及千粒重与野生型无显著差异(图 1-E~H)。除此之外,两个突变体的其他农艺性状,如株高、生育期等与野生型均无明显差异。
2.2 突变体种子的胚乳淀粉颗粒结构
对突变体和野生型成熟种子胚乳自然横断面进行电镜扫描发现,野生型胚乳横断面中央和边缘部位淀粉颗粒均呈现形状规则的多面体结构,淀粉颗粒之间排列紧密(图 2-A~C)。而突变体eb6、eb7胚乳中央部位淀粉颗粒呈球形或不规则形状,淀粉颗粒排列疏松且相互间存在较大的间歇,同时,存在大量的小的淀粉颗粒(图 2-D~G);不同于中央部位,2个突变体胚乳的边缘部位淀粉粒排列整齐,致密规则,与野生型一致(图2-F,I)。
2.3突变体与野生型理化性质差异分析
籽粒灌浆过程中干物质积累变化趋势显示,从开花后5 d(DAF5) 到种子成熟(DAF30),突变体eb7与野生型籽粒干物质积累趋势基本一致,最终籽粒干质量无显著差异(图 3-A)。分析灌浆过程籽粒中游离糖含量的变化发现,eb7与野生型在灌浆过程中蔗糖、葡萄糖和果糖的动态变化趋势基本一致,但是DAF10至灌浆结束,eb7籽粒中的蔗糖含量高于野生型(图3-B),而在开花至花后10 d期间,eb7籽粒中的葡萄糖含量和果糖含量显著高于野生型,而花后10 d,eb7与野生型一致(图3-C~D)。
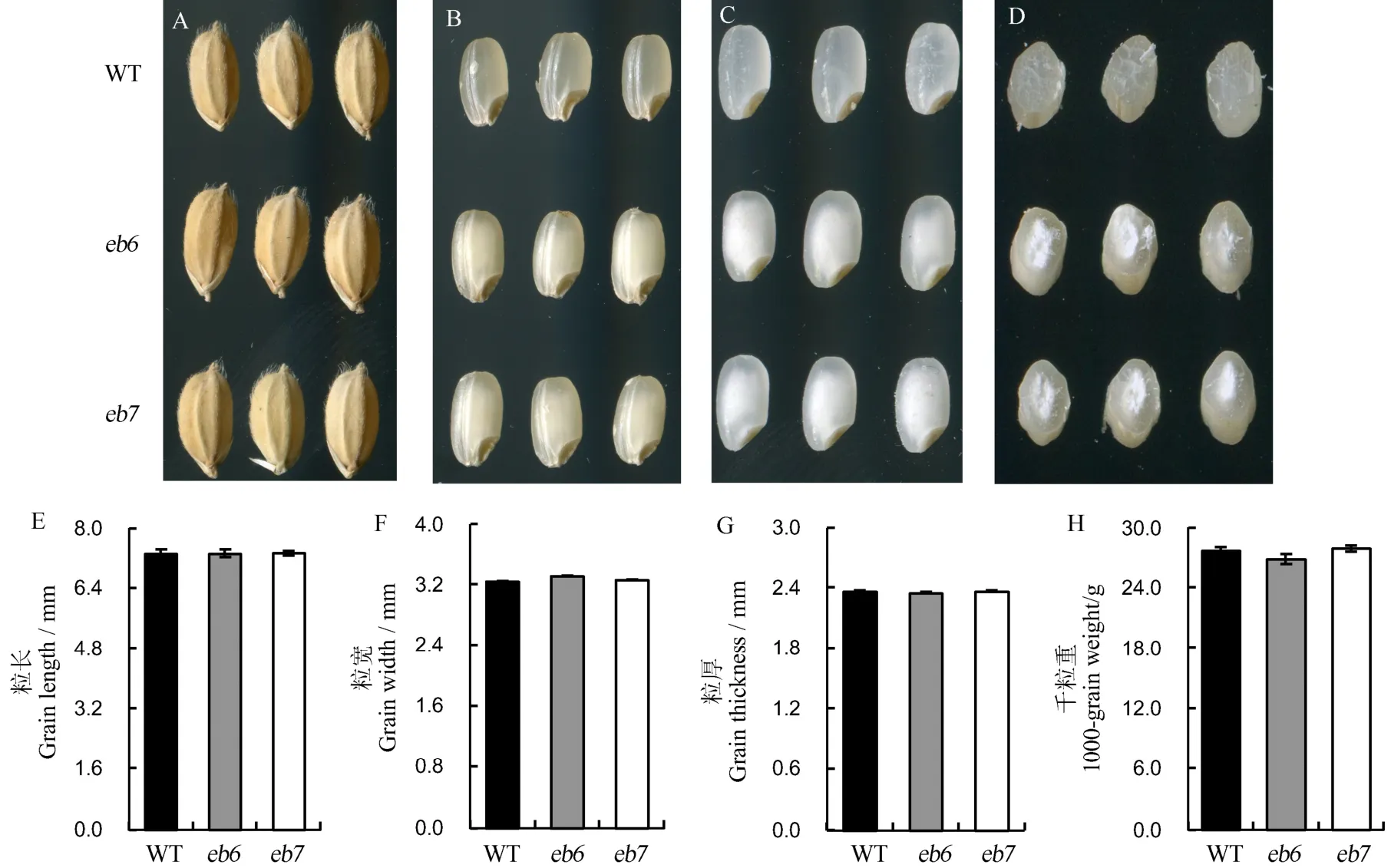
图1 突变体与野生型表型比较Fig. 1. Phenotypes of the mutants and the wild type(WT).

图2 野生型和突变体胚乳中淀粉颗粒结构观察Fig. 2. Structure of starch granules in the endosperm of the wild type and the mutants.
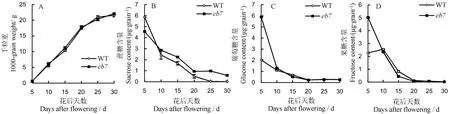
图3 突变体eb7与野生型灌浆过程中干物质和游离糖含量比较Fig. 3. Content of free sugars and dry-weight during the grain filling stage in the grain of eb7 and wild type(WT).
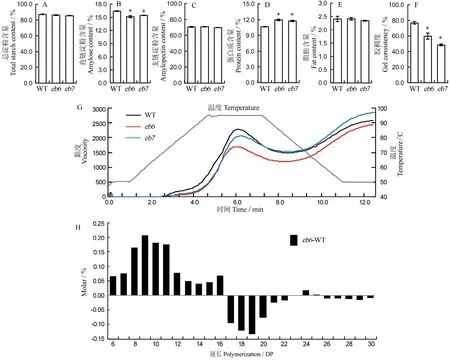
图4 突变体与野生型种子的品质特性Fig. 4. Quality properties ofseedsofmutantsand wild type(WT).
稻米理化性质分析表明,eb6、eb7的总淀粉和支链淀粉含量与野生型无显著差异,而直链淀粉含量则显著降低(图 4-A~C)。同时,突变体蛋白质含量显著高于野生型,脂肪含量未发生显著改变,但胶稠度显著降低(图 4-D~F)。此外,野生型和突变体的碱消值约为6.5,无显著差异。RVA检测显示,两个突变体的淀粉黏度下降,其中eb6下降更为显著(图4-G)。支链淀粉链长分析显示,相比野生型,突变体eb7中聚合度(DP)小于16的短链显著增加,DP为16~23的中长链显著减少(图4-H)。

表3 突变基因eb6的遗传分析Table 3. Genetic analysis of the eb6.

图5 突变体基因的精细定位及杂交F1表型Fig. 5. Fine mapping of eb6 and the appearance of hybrid seeds.
2.4突变体基因的精细定位
突变体eb6与籼稻品种南京11、93-11所衍生的F2群体胚乳表型发生分离,胚乳表现为透明的单株与表现为垩白的单株的株数分离比符合3∶1(χ2=1.72<χ20.05=3.84,χ2=1.17<χ20.05=3.84,表3),因此突变体eb6胚乳垩白表型符合单隐性核基因控制的遗传规律。从eb6与南京11的F2群体中选取340株胚乳垩白单株初步将eb6突变体基因定位在第1染色体长臂标记M4和M17之间。随后扩大定位群体至970株隐性单株,最终将eb6定位在标记M10和M11之间约86.6 kb的染色体区间(图5-A~B)。通过基因组注释网站 (http://rapdblegacy.dna.affrc.go.jp/)分析发现该区间内包含了22个开放阅读框(ORF,图5-C)。其中,ORF13基因编码AGPase的大亚基L2(OsAGPL2)。序列分析发现,eb6在OsAGPL2第3外显子的碱基C替换为碱基T,导致编码蛋白中一个异亮氨酸变成苏氨酸,并且发现eb7也在OsAGPL2第7外显子的碱基C突变为碱基T,导致编码蛋白中一个脯氨酸被替换为丝氨酸(图5-D),因此推断OsAGPL2的突变是导致eb6、eb7胚乳表现为垩白的原因。为了进一步证明该结论,我们对突变体eb6、eb7进行了等位性测验,结果表明两个突变体的正反交F1的籽粒胚乳均表现为严重垩白,胚乳淀粉复合颗粒均呈现不规则球形,排列疏松(图5-E~H)。因此,我们认为OsAGPL2的突变导致了eb6、eb7籽粒胚乳。

图6 OsAGPL2相对表达量Fig. 6. Relative expression of OsAGPL2.
2.5组织表达及原位杂交
利用实时RT-PCR分析OsAGPL2的组织表达情况,结果显示 OsAGPL2主要在发育中的种子中表达,其中在开花后12 d左右的种子中表达量最大,在叶片和幼穗中有少量表达量,而在根和茎干中基本不表达(图6-A)。对开花后9 d的籽粒进行原位杂交结果也显示,OsAGPL2在水稻籽粒胚乳和胚中都有大量表达(图6-B、C)。
2.6淀粉合成相关基因的表达分析
突变体eb6、eb7直链淀粉含量和胶稠度下降,支链淀粉链长分布明显改变,胚乳中淀粉颗粒结构异常,因此,其胚乳中淀粉合成相关基因的表达可能受到一定程度的影响。分析淀粉合成相关基因在突变体eb7和野生型开花后6、9、12和15 d的胚乳中的表达情况,结果显示,OsAGPL2的表达量在突变体中显著上调,在12 d左右表达量达到最高,腺苷葡萄腺苷葡萄糖焦磷酸化酶大亚基4(OsAGPL4)、糖焦磷酸化酶小亚基 2b(OsAGPS2b)和淀粉磷酸化酶L(OsPHOL)在eb7和野生型中的表达差异情况与 OsAGPL2类似;腺苷葡萄糖焦磷酸化酶大亚基 1(OsAGPL1)、腺苷葡萄糖焦磷酸化酶大亚基 3(OsAGPL3)、腺苷葡萄糖焦磷酸化酶小亚基2a(OsAGPS2a)、淀粉合成酶Ⅱa(OsSSⅡa)、淀粉异构酶I(OsISAI)、淀粉分支酶Ⅰ(OsBEⅠ)和淀粉分支酶Ⅱ(OsBEⅡ)在籽粒灌浆早期即开花后 6 d在eb7中显著下调;淀粉合成酶Ⅰ(OsSSⅠ)、淀粉异构酶Ⅱ(OsISAⅡ)和淀粉异构酶 III(OsISAⅢ)于开花后6~12 d内在eb7中显著下调;而在开花后6~15 d内,突变体eb7中颗粒淀粉合成酶Ⅰ(OsGBSSⅠ)的表达量始终高于野生型,腺苷葡萄糖焦磷酸化酶小亚基1(OsAGPS1)的表达量与野生型基本一致(图7)。以上结果表明,OsAGPL2的突变影响了淀粉合成相关基因的正常表达。
3 讨论

图7 野生型和突变体在种子发育过程胚乳中淀粉合成相关基因定量表达分析Fig. 7. Expression profiles of starch synthesis genes during seed development in the wild type(WT) and the mutants.
稻米品质由外观品质、加工品质、蒸煮品质和食味品质等构成。外观品质主要包括粒形、透明度、垩白率及垩白度等。稻米垩白直接关系到稻米的市场受欢迎程度,还影响着稻米的食味品质、加工品质及营养品质等。然而籽粒垩白的形成机制较为复杂,涉及到水稻源库流的协调,同时还存在基因型与环境型互作效应,因此垩白一直以来都是稻米品质研究的重点与难点[1,3,4,48-49]。本研究鉴定了两个严重心白突变体,等位性测验发现其为一对等位突变体。电镜扫描结果显示突变体垩白部分淀粉复合颗粒呈现圆球形,小颗粒数目增多,复合颗粒之间存在较大的间歇,淀粉体颗粒之间结合不紧密而引起光线折射可能突变体是垩白形成的最直接的原因。稻米垩白突变不仅影响其外观品质,通常还伴随着系列的理化性质的改变,如下调OsLTP36的表达量时胚乳会产生严重垩白,同时胚发育延迟,脂肪酸和蛋白含量减少,淀粉粒松散并且变小。粉质突变体flo7相对于其野生型,flo7籽粒外围表现为白粉状,而里层为半透明状,胚乳外围复合淀粉粒排列松散,直链淀粉含量显著降低,支链淀粉结构变化[50]。本研究结果表明,相较于野生型,两个突变体中蛋白质含量显著升高,直链淀粉含量显著降低,支链淀粉构成也发生改变,短链淀粉含量增加,中长链淀粉含量减少,胶稠度和最大黏稠度都减小(图4)。综上所述,垩白突变体eb6、eb7相对野生型不仅稻米的外观品质发生了改变,其稻米理化性质也发生了系列的改变。因此,对垩白突变体eb6、eb7的研究有助于进一步了解稻米外观、蒸煮品质及粒重的形成与调控机理。
图位克隆结果证实eb6、eb7分别是AGPase的大亚基基因OsAGPL2的第3、第7个外显子发生单碱基的替换导致单个氨基酸的改变(图 5)。AGPase是淀粉合成过的关键酶,水稻中AGPase的酶活只有10%左右来自于质体,90%左右来自于细胞质,活性高低主要由胚乳细胞质同工酶所决定[35-37],而水稻胚乳细胞质中的AGPase主要由OsAGPS2b和OsAGPL2构成。组织表达和原位杂交的结果显示OsAGPL2主要在发育中的籽粒中大量表达(图6)。RT-PCR分析显示在突变体中 OsAGPL2和OsAGPS2b表达显著上调,这可能是OsAGPL2的突变产生反馈作用使的突变体中AGPase同工酶基因包括 OsAGPL2本身发生转录上调。同时其他淀粉合成相关的基因在突变体中的表达也受到了影响,如在开花后6~15 d内,eb7中OsGBSSⅠ的表达量始终高于野生型,而 OsSSⅠ、OsSSⅡa、OsBEⅠ、OsBEⅡ、OsISAⅠ、OsISAⅡ在籽粒灌浆前期的表达量显著低于野生型。因此,OsAGPL2的突变体对淀粉合成相关基因的表达产生了重要的影响,AGPase活性的下降及其淀粉合成相关基因表达的改变最终导致了突变体籽粒胚乳中淀粉合成及淀粉复合颗粒发育异常。本研究结果进一步拓展了对OsAGPL2功能的认识,有助于更深入了解淀粉合成、垩白发生的机理。
[1] 江良荣,李义珍,王侯聪,黄育民. 稻米外观品质的研究进展与分子改良策略. 分子植物育种,2003,1(2): 243-255.Jiang L R,Li Y Z,Wang H C,Huang Y M. Research progresses on appearance quality of rice grain and strategies for its molecular improvement. Mol Plant Breed,2003,1(2): 243-255.
[2] 江户一雄,江幡守衞. 心白米.に する的研究第2.日本作物学会纪事,1959,28(1): 46-50.Nagato K,Ebata M. Studies on white-core rice kernel:II. On the physical properties of the kernel. Jpn J Crop Sci,1959,28(1): 46-50. (in Japanese)
[3] Cheng F M,Zhong L J,Wang F,Zhang G P.Differences in cooking and eating properties between chalky and translucent parts in rice grains . Food Chem,2005,90(1): 39-46.
[4] 程方民,钟连进,舒庆尧,黄华宏,石春海,吴平.早籼水稻垩白部位淀粉的蒸煮食味品质特征. 作物学报,2002,28(3): 363-368.Cheng F M,Zhong L J,Shu Q Y,Huang H H,Shi C H,Wu P. Studies on the cooking and eating quality properties in chalky milled grains of early indica rice.Acta Agron Sin,2002,28(3): 363-368. (in Chinese with English abstract)
[5] 田代亨,江幡守衞. 腹白米に する的研究第2 . 日本作物学会纪事,1974,43(1/2): 105 Tashiro T,Ebata M. Studies on white-belly rice kernel:II. Location on the panicle on occurrence of white-belly kernel. Jpn J Crop Sci,1974,43(1):105-110. (in Japanese)
[6] 程方民,胡东维,丁元树. 人工控温条件下稻米垩白形成变化及胚乳扫描结构观察. 中国水稻科学,2000,14(2): 83-87.Cheng F M,Hu D W,Ding Y S. Dynamic change of chalkiness and observation of grain endosperm structure with scanning electron microscope under controlled temperature condition. Chin J Rice Sci,2000,14(2): 83-87. (in Chinese with English abstract)
[7] Invertases S A. Primary structures,functions,and roles in plant development and sucrose partitioning. Plant Physiol,1999,121(1): 1-8.
[8] Asano T,Kunieda N,Omura Y,Ibe H,Kawasaki T,Takano M,Sato M,Furuhashi H,Mujin T,Takaiwa F,Wu C Y,Tasa Y,Satozawa T,Sakamoto M,Shimada H. Rice SPK,a calmodulin-like domain protein kinase,is required for storage product accumulation during seed development phosphorylation of sucrose synthase is a possible factor. Plant Cell,2002,14(3): 619-628.
[9] Abe T,Niiyama H,Sasahara T. Cloning of cDNA for UDP-glucose pyrophosphorylase and the expression of mRNA in rice endosperm. Theor Appl Genet,2002,105(2/3): 216-221.
[10] Cho J I,Ryoo N,Ko S,Lee S K,Lee J,Jung K H,Lee Y H,Bhoo S H,Winderickx J,An G,Hahn T R,Jeon J S. Structure,expression,and functional analysis of the hexokinase gene family in rice(Oryza sativa L.).Planta,2006,224(3): 598-611.
[11] Kawagoe Y,Kubo A,Satoh H,Takaiwa F,Nakamura Y. Roles of isoamylase and ADP-glucose pyrophosphorylase in starch granule synthesis in rice endosperm. Plant J,2005,42(2): 164-174.
[12] Kubo A,Fujita N,Harada K,Matsuda T,Satoh H,Nakamura Y. The starch-debranching enzymes isoamylase and pullulanase are both involved in amylopectin biosynthesis in rice endosperm. Plant Physiol,1999,121(2): 399-410.
[13] Hirose T,Terao T. A comprehensive expression analysis of the starch synthase gene family in rice(Oryza sativa L.) . Planta,2004,220(1): 9-16.
[14] Ball S,Guan H P,James M,Myers A,Keeling P,Mouille G,Buleon A,Colonna P,Preiss J. From glycogen to amylopectin: a model for the biogenesis of the plant starch granule. Cell,1996,86(3): 349-352.
[15] She K C,Kusano H,Koizumi K,Yamakawa H,Hakata M,Imamura T,Fukuda M,Naito N,Tsurumaki Y,Yaeshima M,Tsuge T,Matsumoto K,Kudoh M,Itoh E,Kikuchi S,Kishimoto N,Yazaki J,Ando T,Yano M,Aoyama T,Sasaki T,Satoh H,Shimada H. A novel factor FLOURY ENDOSPERM2 is involved in regulation of rice grain size and starch quality. Plant Cell,2010,22(10): 3280-3294.
[16] Wang Y H,Ren Y L,Liu X,Jiang L,Chen L M,Han X H,Jin M N,Liu S J,Liu F,Lü J,Zhou K N,Su N,Bao Y Q,Wan J M. OsRab5a regulates endomembrane organization and storage protein trafficking in rice endosperm cells. Plant J,2010,64(5): 812-824.
[17] Ryoo N,Yu C,Park C S,Baik M Y,Park I M,Cho M H,Bhoo S H;An G,Hahn T R,Jeon J S. Knockout of a starch synthase gene OsSSIIIa/Flo5 causes white-core floury endosperm in rice (Oryza sativa L.). Plant Cell Rep,2007,26(7): 1083-1095.
[18] Cakir B,Shiraishi S,Tuncel A,Matsusaka H,Satoh R,Singh S,Crofts N,Hosaka Y,Fujita N,Hwang S K,Satoh H,Okita T W. Analysis of the rice ADP-glucose transporter(OsBT1) indicates the presence of regulatory processes in the amyloplast stroma that control ADP-glucose flux into starch. Plant Physiol,2016,170(3): 1271-1283.
[19] Li Y,Fan C,Xing Y,Yun P,Luo L,Yan B,Peng B,Xie W,Wang G,Li X,Xiao J,Xu C,He Y. Chalk5 encodes a vacuolar H+-translocating pyrophosphatase influencing grain chalkiness in rice. Nat Genet,2014,46(4): 398-404.
[20] Ballicora M A,Iiglesias A A,Preiss J. ADP-glucose pyrophosphorylase: A regulatory enzyme for plant starch synthesis. Photosyn Res,2004,79(1): 1-24.
[21] Lee S K,Hwang S K,Han M,Eom J S,Kang H G,Han Y,Choi S B,Cho M H,Bhoo S H,An G,Hahn T R,Okita T W,Jeon J S. Identification of the ADP-glucose pyrophosphorylase isoforms essential for starch synthesis in the leaf and seed endosperm of rice(Oryza sativa L.). Plant Mol Biol,2007,65(4):531-556.
[22] Hwang S K,Okita T W. Understanding structurefunction relationship of ADP-glucose pyrophosphorylase by deciphering its mutant forms//Tetlow I.Starch: Origins,Structure and Metabolism. Vol.5.London UK: the Society for Experimental Biology,2012: 77-114.
[23] Harn C H,Bae J M,Lee S S,Liu J R. Presence of multiple cDNAs encoding an isoform of ADP-glucose pyrophosphorylase large subunit from sweet potato and characterization of expression levels. Plant amp; Cell Physiol,2000,41(11): 1235-1242.
[24] Akihiro T,Mizuno K,Fujimura T. Gene expression of ADP-glucose pyrophosphorylase and starch contents in rice cultured cells are cooperatively regulated by sucrose and ABA. Plantamp;Cell Physiol,2005,46(6):937-946.
[25] Nielsen T H,Krapp A,Röper-Schwarz U,Stitt M. The sugar-mediated regulation of genes encoding the small subunit of Rubisco and the regulatory subunit of ADP glucose pyrophosphorylase is modified by phosphate and nitrogen. Plant,Cell amp; Environ,1998,21(5):443-454.
[26] Haugen T H,Ishaque A,Preiss J. Biosynthesis of bacterial glycogen. Characterization of the subunit structure of Escherichia coli B glucose-1-phosphate adenylyltransferase (EC 2.7. 7.27). J Biol Chem,1976,251(24): 880-7885.
[27] Okita T W,Nakata P A,Anderosin J M,Sowokinos J,Morell M,Preiss J. The subunit structure of potato tuber ADPglucose pyrophosphorylase. Plant Physiol,1990,93(2): 785-790.
[28] Smith-White B J,Preiss J. Comparison of proteins of ADP-glucose pyrophosphorylase from diverse sources.J Mol Evol,1992,34(5): 449-464.
[29] Villand P,Olsen O A,Kleczkowski L A. Molecular characterization of multiple cDNA clones for ADP-glucose pyrophosphorylase from Arabidopsis thaliana. Plant Mol Biol,1993,23(6): 1279-1284.
[30] Cross J M,Clancy M,Shaw J R,Okita T W,Hannah L C. Both subunits of ADP-glucose pyrophosphorylase are regulatory. Plant Physiol,2004,135(1): 137-144.
[31] Georgelis N,Braun E L,Shaw J R,Hannah L C. The two AGPase subunits evolve at different rates in angiosperms,yet they are equally sensitive to activity-altering amino acid changes when expressed in bacteria. Plant Cell,2007,19(5): 1458-1472.
[32] Hwang S K,Hamada S,Okita T W. Catalytic implications of the higher plant ADP-glucose pyrophosphorylase large subunit. Phytochemistry,2007,68(4): 464-477.
[33] Ohdan T,Francisco P B,Sawadat T,Hirose T,Terao T,Satoh H,Nakamura Y. Expression profiling of genes involved in starch synthesis in sink and source organs of rice. J Exp Bot,2005,56(422): 3229-3244.
[34] Cook F R,Fahy B,Trafford K. A rice mutant lacking a large subunit of ADP-glucose pyrophosphorylase has drastically reduced starch content in the culm but normal plant morphology and yield. Funct Plant Biol,2012,39(12): 1068-1078.
[35] Sikka V K,Choi S B,Kavakli I H,Sakulsingharoj C,Gupta S,Lto H,Okita T W. Subcellular compartmentation and allosteric regulation of the rice endosperm ADPglucose pyrophosphorylase. Plant Sci,2001,161(3): 461-468.
[36] Burton R A,Johnson P E,Beckles D M,Fincher G B,Jenner H L,Naldrett M J,Naldrett M J,Denyer K.Characterization of the genes encoding the cytosolic and plastidial forms of ADP-glucose pyrophosphorylase in wheat endosperm. Plant Physiol,2002,130(3): 1464-1475.
[37] Denyer K,Dunlap F,Thorbj R T,Keeling P,Smith A M. The major form of ADP-glucose pyrophosphorylase in maize endosperm is extra-plastidial. Plant Physiol,1996,112(2): 779-785.
[38] Tang X J,Peng C,Zhang J,Cai Y,You X M,Kong F,Yan H G,Wang G X,Wang L,Jin J,Chen W W,Chen X G,Ma J,Wang P,Jiang L,Zhang W W,Wan J M.ADP-glucose pyrophosphorylase large subunit 2 is essential for storage substance accumulation and subunit interactions in rice endosperm. Plant Sci,2016,249: 70-83.
[39] Tuncel A,Kawaguchi J,Ihara Y,Matsusaka H,Nishi A,Nakamura T,Kuhara S,Hirakawa H,Nakamura Y,Cakir B,Nagamine A,Okita T W,Hwang S K,Satoh H. The rice endosperm ADP-glucose pyrophosphorylase large subunit is essential for optimal catalysis and allosteric regulation of the heterotetrameric enzyme. Plantamp;Cell Physiol,2014,55(6): 1169-1183.
[40] Zhang D P,Wu J G,Zhang Y J,Shi C H. Phenotypic and candidate gene analysis of a new floury endosperm mutant (osagpl2-3) in rice. Plant Mol Biol Rep,2012,30(6): 1303-1312.
[41] Masahiro Y,Isono Y,Satoh H,Omura T. Gene analysis of sugary and shrunken mutants of rice,Oryza sativa L. Jpn J Breeding,1984,34(1): 43-49.
[42] 中华人民共和国农业部.米质测定方法NY 147-88.北京: 中国标准出版社,2002.Ministry of Agriculture of the People’s Republic of China. Detection Method for Rice Quality NY 147-88. Beijing: China Standard Publishing House,2002.(in Chinese)
[43] 孙成效,段彬伍,谢黎虹,陈能. 利用近红外透射光谱技术同步测定糙米的多项品质指标初报. 中国水稻科学,2006,20(4): 451-454.Sun C X,Duan B W,Xie L H,Chen N. Determination of several quality characteristics of brown rice by near infrared transmission spectroscopy. Chin J Rice Sci,2006,20(4): 451-454. (in Chinese with English abstract)
[44] Han X H,Wang Y H,Liu X,Jiang L,Ren Y L,Liu F,Peng C,Li J J,Jin X M,Wu F Q,Wang J L,Guo X P,Zhang X,Cheng Z J,Wan J M. The failure to express a protein disulphide isomerase-like protein results in a floury endosperm and an endoplasmic reticulum stress response in rice. J Exp Bot,2012,63: 121-130.
[45] 康海岐,常红叶. 杂交水稻主要亲本材料的垩白性状及其胚乳结构电镜扫描. 中国农学通报,2007,23(4): 180-185.Kang H Q,Chang H Y. Study on chalkiness characters and endosperm structures of the main parents’ kernel of hybrid rice. Chin Agric Sci Bull,2007,23(4):180-185. (in Chinese with English abstract)
[46] Li Z W,Trick H N. Rapid method for high-quality RNA isolation from seed endosperm containing high levels of starch . BioTechniques,2005,38(6): 872-876.
[47] Bahaji A,Li J,Sánchez-López Á M,Fernández E B,Muñoz F J,Ovecka M,Almagro G,Montero M,Ezquer I,Etxeberria E,Romero J P. Starch biosynthesis,its regulation and biotechnological approaches to improve crop yields. Biotechnol Adv,2014,32(1): 87-106.
[48] 刘霞,付艳苹,朱晔荣,李艳萍,王勇. 水稻垩白形成的生理和遗传机制. 植物生理学通讯,2007,43(3):569-574.Liu X,Fu Y P,Zhu Y R,Li Y P,Wang Y.Physiological and genetic mechanism of rice chalkiness formation. Plant Physoil Commun,2007,43(3): 569-574. (in Chinese)
[49] 周立军,江玲,翟虎渠,万建民. 水稻垩白的研究现状与改良策略. 遗传,2009,31(6): 563-572.Zhou L J,Jiang L,Zhai H Q,Wan J M. Current status and strategies for improvement of rice grain chalkiness.Hereditas(Beijing),2009,31(6): 563-572. (in Chinese with English abstract)
[50] Zhang L,Ren Y,Lu B,Yang C Y,Feng Z M,Liu Z,Chen J,Ma W W,Wang Y,Yu X W,Wang Y L,Zhang W W,Wang Y H,Liu S J,Wu F Q,Zhang X,Guo X P,Bao Y Q,Jiang L,Wan J M. FLOURY ENDOSPERM7 encodes a regulator of starch synthesis and amyloplast development essential for peripheral endosperm development in rice. J Exp Bot,2016,67(3): 633-647.
Identification and Gene Mapping-based Clone of Two Chalkiness Mutants in Rice
ZHANG Xichun#,LU Feifei#,LÜ Yusong,LUO Rongjian,JIAO Guiai,WU Yawen,TANG Shaoqing,HU Peisong,WEI Xiangjin*
(State Key Laboratory of Rice Biology,China National Rice Research Institute,Hangzhou 310006,China; # These authors contributed equally to this work;*Corresponding author,E-mail: weixiangjin@caas.cn)
【Objective】Chalkiness affects the appearance,processing,cooking and eating quality of rice. The objective of the study was to uncover the genetic mechanism of two rice chalky endosperm mutants for improving rice quality.【Method】Two chalkiness mutants,eb6 and eb7 were identified from EMS-treated japonica rice Zhonghua 11. The agronomic traits and starch physicochemical properties of the two mutants were investigated. Genetic analysis and map-based cloning for the gene responsible for the eb6 and eb7 phenotypes were carried out with the F2population derived from the cross between eb6 and Nanjing 11,eb7 and 93-11. Furthermore,the expression pattern of candidate gene and the transcript levels of genes related to starch synthase in mutants and wild type(WT) were also investigated.【Result】The central parts of them were white and opaque,with loosely and irregularly arranged and smaller compound starch granules(SGs). Whereas,the marginal part of endosperms of WT and mutants and the central part of WT were filled with densely packed,similar sized polyhedral SGs. The amylose content and gel consistency of mutants were dramatically lower than those of WT. Simultaneously,the proportions of chains with degree of polymerization(DP) of amylopectin in the range from 6 to 16 were significantly increased,whereas the proportion of chains with DP in the range from 16 to 23 was noticeably decreased in the eb6. Genetic analysis showed that a single recessive gene controls chalkiness phenotype of mutants. Based on the F2 population derived from the cross between eb6 and Nanjing 11,the gene was finally narrowed down to a 86.6 kb physical region on chromosome 1. Within this region,one open reading frame(LOC_Os01g44220) has been annotated as large subunit of ADP-glucose pyrophosphorylase gene(OsAGPL2).Sequence analysis revealed that there was only one a nucleotide substitution in the 3rd exon of eb6 and the 7th exon of eb7,which resulted in an amino acid replacement in OsAGPL2,respectively. The qRT-PCR and in-situ hybridization assay indicated that OsAGPL2 was mainly expressed in the developing grains. Moreover,the expression patterns of many genes involved starch synthesis were intensively influenced in mutants.【Conclusion】OsAGPL2 indisputably corresponds to endosperm chalkiness of eb6 and eb7. And all results suggest that OsAGPL2 plays an important role in starch synthesis,the formation of compound starch granules and rice quality.
chalkiness; starch synthesis; mapping-based cloning; OsAGPL2; rice
10.16819/j.1001-7216.2017.7003
2017-01-06;修改稿收到日期:2017-02-13。
国家重点研发计划资助项目(2016YFD0101801);国家自然科学基金资助项目(31471472,31301303,31501280)。
Q343.5;S511.033
A
1001-7216(2017)06-0568-12
