摩擦力和样品厚度对压痕法测量生物试样弹性的影响
2017-12-01严旭高琦程茜周红生徐峥
严旭,高琦,程茜,周红生,徐峥
摩擦力和样品厚度对压痕法测量生物试样弹性的影响
严旭1,高琦2,程茜1,周红生2,徐峥1
(1. 同济大学物理科学与工程学院,上海 200092;2. 中国科学院声学研究所东海研究站,上海 201815)
生物试样的弹性测量可为生物体疾病的早期诊断和治疗提供依据。利用压痕法对生物试样的弹性进行了测量,并用有限元软件对压痕过程进行了模拟。研究发现,试样厚度对弹性测量存在影响,试样厚度越大,测量结果越接近试样真实的杨氏模量。当试样厚度为压痕深度的75倍时,测量误差仅为0.74 %。又研究了压头速度对弹性测量结果的影响。研究发现,当压头速度较大时,由于摩擦力的作用,测量结果与试样弹性的真实值之间存在一定的差异。在模拟过程中添加摩擦力可准确反演试样的弹性,误差在5 %以下。
压痕法;弹性;有限元;摩擦力
0 引言
生物体内大到器官小到细胞均为生物软组织。这些组织时刻受到不同大小的内力和外力的作用,并保持弹性。一旦组织的弹性改变时,就意味着组织的损伤或者病变,如癌症[1-4]、动脉粥样硬化[5]、青光眼[6]等都会导致生物组织弹性发生改变。在细胞层面,研究者们发现细胞形态[7-9]改变,或细胞发生迁移[8,10-11]、增殖[9,12-14]或分化[10,15-17]时,其弹性将改变。因此,弹性对于表征生物体的活性和理解生物组织的生理功能均非常重要。
目前,生物组织的弹性可以通过超声弹性成像技术[18-21]、磁共振弹性成像技术[22-26]等进行测量,但这些测量均为定性测量,无法反映生物组织的真实弹性。为得到生物组织弹性的定量结果,一般可使用压缩法[27-28]、静态拉伸法[29-31]、压痕法[32-35]等。其中压痕法因为操作简单、对测量试样无损、测试精度高等优点而被广泛应用。但压痕法测量弹性是基于半无限大试样建立的理论,当试样厚度有限时,尤其是当压痕深度与试样厚度可比拟时,试样底面的反射会给测量结果带来误差。另外,压痕法要求测试的速度必须很慢,当压头速度较大时,由于粘滞力和摩擦力等的作用会给弹性测量结果带来误差。而现在很多研究工作已不局限于对试样单点弹性的测量,而是测量整个生物试样的弹性分布[36],这样的测量在现有方法基础上需要耗费大量的时间。因此建立一种快速且准确的弹性测量方法非常必要。
本文利用实验和有限元仿真研究了试样厚度对压痕法测量弹性结果的影响。挑选了恰当厚度的试样并通过改变压头速度,分析了摩擦力对弹性测量结果的影响,并给出了存在摩擦力作用时反演生物试样弹性的方法。
1 实验方法和材料
图1为压痕法测量生物试样弹性的实验装置示意图(1(a)~1(c))与仪器实物图(1(d))。实验装置由激光器、伺服电机、样品台、悬臂梁、压头组成。悬臂梁一端连接伺服电机,一端连接压头。压头直径为5 mm,悬臂梁的长度为45 mm。实验前,为得到激光位移补偿,需校准仪器,利用探针下压载物台上的钢块,得出行程-变形量的曲线,根据曲线调整电机行程。实验过程中,伺服电机驱动悬臂梁向下运动,代表电机的位移,当压头触碰到试样表面时,悬臂梁发生弯曲,激光器发射的光照射到悬臂梁上反射的光路发生改变,激光仪检测到反射光路的变化,软件记录下电机位移和激光位移。根据测得的数据,可通过赫兹模型计算得到弹性。

(a) 测试试样的侧视图(b) 悬臂梁俯视图 (c) 悬臂梁侧视图
当半径为的球形压头压在半无限大的介质上,力与压痕深度的关系[37]满足:

*表示有效弹性,表达式为

为得到试样的真实弹性,使用有限元模拟压痕过程。如图2所示,根据实物构建二维轴对称模型,固定试样的轴以及其底部,设置压头与试样表面接触。为了确保计算结果的准确,在模型的接触区域细分了网格。
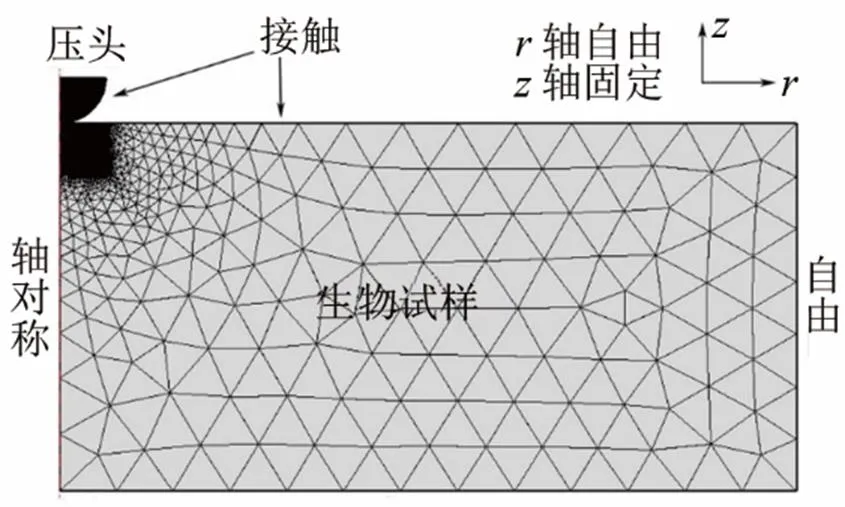
图2 Comsol模拟压痕过程的网格以及边界条件
2 结果与讨论
Cox等人发现,在压头速度非常低时,反馈力仅与试样弹性有关[39]。实验中压头速度设置为0.03 mm/s。图3是压痕过程(进针、静止和退针)中试样反馈力与时间的关系曲线。从图3可以发现,在该速度下进针和退针曲线具有很好的对称性,并且在中间静止段没有受到其他力的作用,表明试样的粘滞作用可忽略。
当试样厚度大于10倍的压痕深度时,可以忽略试样底部反馈力的作用,本实验采用的压痕深度为0.6 mm。选择5、15、45 mm厚度的生物试样,分别研究了在这些厚度条件下,压痕法由于试样厚度造成的误差。首先研究了不同厚度试样(5、15、45 mm)对弹性测量结果的影响。如图4所示,在压痕刚产生时,实验测得力曲线与赫兹模型计算得到结果符合得较好。随着压痕深度的增加,实验测得的结果逐渐偏离赫兹模型计算结果,当试样厚度越小时,测得的反馈力越大。这是因为赫兹模型建立在半无限大厚度试样上,对有限厚度试样,存在载物台的反作用力,当试样厚度越小时,反作用力越大,因此实验与赫兹模型的计算结果偏差也越大。另外,当试样与压头的接触面积变大,赫兹模型的假设(接触半径<<压头半径)不再满足,这也会导致实验力曲线与理论计算结果的偏差增大。
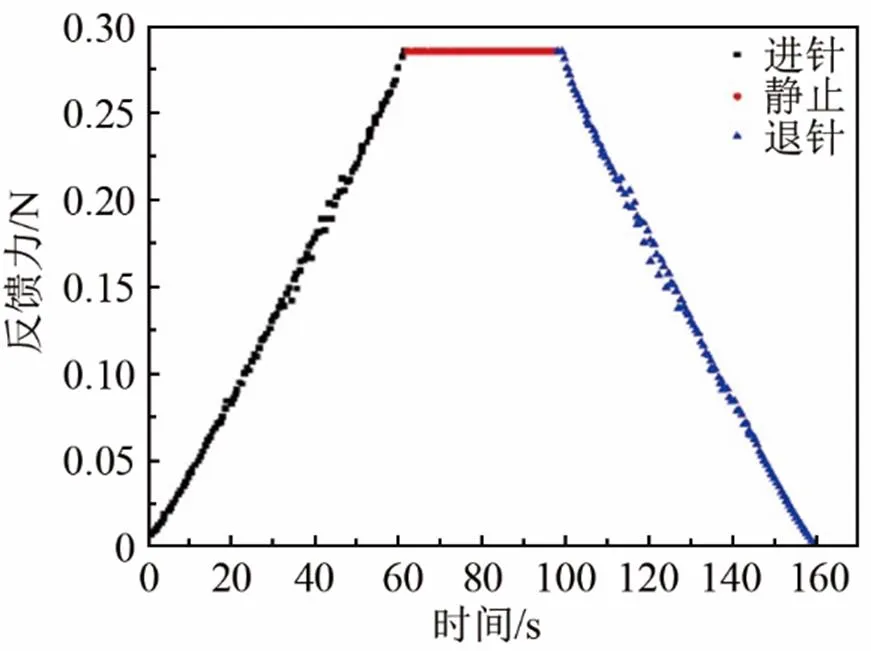
图3 时间与反馈力关系图

图4 实验测出的不同厚度的力曲线及理论力曲线
将力-压痕深度关系通过式(1)和(2)可反演试样的杨氏模量,图5给出了不同厚度生物试样测出的杨氏模量,5 mm和15 mm厚度的生物试样计算出的杨氏模量分别为95.06、94.45 kPa,误差分别为8.02%、7.33%。计算得到的45 mm厚度的生物试样的弹性为88.65 kPa,有限元法计算得出的生物试样的弹性为88 kPa,误差仅为0.74%。
我们又研究了压头速度对弹性反演的影响,不同压头速度测出的杨氏模量如图6所示。为了减少载物台对压头的反作用力,选择45 mm厚度的试样进行实验。分别研究了压头以0.03、0.09、0.12、0.2 mm/s速度撤针测量得到的杨氏模量,结果如表1所示。由表1可知,压头速度越快,反演得到的杨氏模量误差越大。因此,用赫兹模型反演试样的杨氏模量需考虑摩擦力的作用。由表1的结果可知,试样与压头间的摩擦力与压头速度有关。因此假设摩擦力表达式为
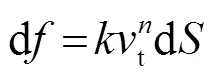
积分后得到
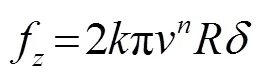
式中:为单位面积阻尼系数;表示压头半径;为压头速度沿接触面的切向速度分量;为压头速度;为压痕深度;为常数。随后可根据实验数值计算出。

图5 不同厚度试样的杨氏模量

图6 不同压头速度测出的杨氏模量图
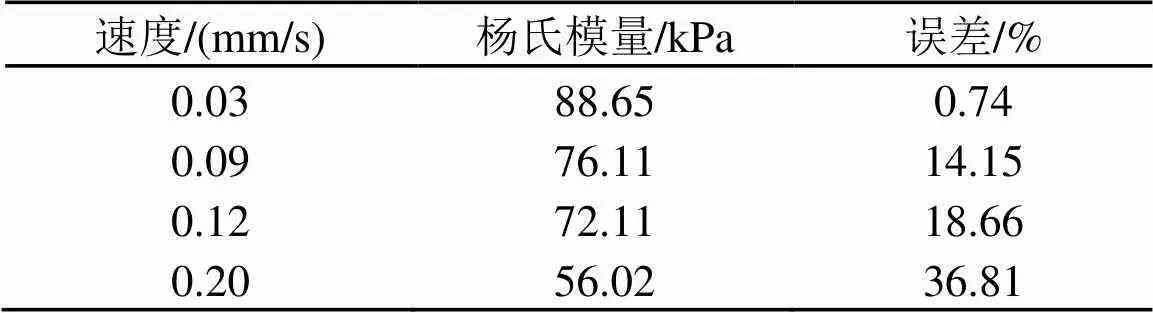
表1 不同速度测出的45 mm试样的杨氏模量
通过模拟结果与实验结果对比,本文计算出为2.05×107 kg/(m2∙s),当1时,计算与实验结果符合最好。图7分别给出了在压头速度为0.03、0.09、0.12 mm/s和0.20 mm/s时,力曲线的实验值与未考虑摩擦和考虑摩擦后的计算值对比。从图7中发现,当压头速度很小(低于0.03 mm/s)时(图7(a)),考虑摩擦和不考虑摩擦计算出的力曲线几乎重合,这表明速度很小(低于0.03 mm/s)时可以忽略摩擦力的作用。此外,图3中低速(0.03 mm/s)的进针曲线与退针曲线相对称,说明速度很小时可忽略粘滞力的作用。当速度渐渐增大时,摩擦力的作用变得明显。在退针过程中受摩擦力作用使反馈力减小,当压头速度为0.20 mm/s时,由于摩擦力作用使实验和赫兹模型得到的力曲线相差很远。此时反演得到的杨氏模量与试样的真实值相差达到36.81 %(见表1)。在考虑摩擦的情况下,可以看到实验测量值与赫兹模型符合较好。

(a) 压头速度为0.03 mm/s
(b) 压头速度为0.09 mm/s

(c) 压头速度为0.12 mm/s
(d) 压头速度为0.20 mm/s
图7 压头速度分别为0.03、0.09、0.12、0.20 mm/s时力曲线的实验值、未考虑以及考虑摩擦力之后的计算值
Fig.7 The force curves of experimental value and the calculated values without and with considering frictional force for indentation speeds of 0.03, 0.09, 0.12 and 0.20 mm/s
表2为考虑摩擦力后反演的杨氏模量。发现在考虑摩擦的情况下四种压头速度得到的杨氏模量很接近,与杨氏模量的真实值相差均在5 %以下。

表2 考虑摩擦力后计算出的杨氏模量
3 结论
本文利用压痕实验测出生物试样的力曲线及弹性,同时利用有限元法计算了理想状态下生物试样的力曲线,并给出了有摩擦力作用下的弹性反演方法。实验表明试样厚度对弹性测量存在影响,当试样厚度远大于压痕深度时,测量误差仅为0.47 %。在此基础上研究了有摩擦力作用下的弹性测量方法,根据公式(4)可将测量误差减小到5 %以下。在以后利用压痕法测量生物试样的弹性时,可通过降低速度、缩小压头的尺寸达到减少摩擦的效果。
4 致谢
感谢钱梦騄教授对本文中摩擦力公式及实验手段改进的悉心指导。
[1] Kawano S, Kojima M, Higuchi Y, et al. Assessment of elasticity of colorectal cancer tissue, clinical utility, pathological and phenotypical relevance[J]. Cancer Science, 2015, 106(9): 1232-1239.
[2] Miyanaga N, Akaza H, Yamakawa M, et al. Tissue elasticity imaging for diagnosis of prostate cancer: a preliminary report[J]. International Journal of Urology, 2006, 13(12): 1514-1518.
[3] Aboumarzouk O M, Ogston S, Huang Z, et al. Diagnostic accuracy of transrectal elastosonography (TRES) imaging for the diagnosis of prostate cancer: a systematic review and meta-analysis[J]. BJU International, 2012, 110(10): 1414-1423.
[4] Samani A, Zubovits J, Plewes D. Elastic moduli of normal and pathological human breast tissues: an inversion-technique-based investigation of 169 samples[J]. Physics in Medicine and Biology, 2007, 52(6): 1565-1576.
[5] Claridge M W, Bate G R, Hoskins P R, et al. Measurement of arterial stiffness in subjects with vascular disease: Are vessel wall changes more sensitive than increase in intima–media thickness[J]. Atherosclerosis, 2009, 205(2): 477-480.
[6] Last J A, Pan T, Ding Y, et al. Elastic modulus determination of normal and glaucomatous human trabecular meshwork[J]. Investigative Ophthalmology & Visual Science, 2011, 52(5): 2147-2152.
[7] Chou S Y, Cheng C M, Leduc P R. Composite polymer systems with control of local substrate elasticity and their effect on cytoskeletal and morphological characteristics of adherent cells[J]. Biomaterials, 2009, 30(18): 3136-3142.
[8] Isenberg B C, Dimilla P A, Walker M, et al. Vascular smooth muscle cell durotaxis depends on substrate stiffness gradient strength[J]. Biophysical Journal, 2009, 97(5): 1313-1322.
[9] Liao S W, Yu T B, Guan Z. De novo design of saccharide-peptide hydrogels as synthetic scaffolds for tailored cell responses[J]. Journal of the American Chemical Society, 2009, 131(48): 17638-17646.
[10] Chen C C, Hsieh P C H, Wang G M, et al. The influence of surface morphology and rigidity of the substrata on cell motility[J]. Materials Letters, 2009, 63(21): 1872-1875.
[11] Chen Y M, Ogawa R, Kakugo A, et al. Dynamic cell behavior on synthetic hydrogels with different charge densities[J]. Soft Matter, 2009, 5(9): 1804-1811.
[12] Leipzig N D, Shoichet M S. The effect of substrate stiffness on adult neural stem cell behavior[J]. Biomaterials, 2009, 30(36): 6867-6878.
[13] Hadjipanayi E, Mudera V, Brown R A. Close dependence of fibroblast proliferation on collagen scaffold matrix stiffness[J]. Journal of Tissue Engineering & Regenerative Medicine, 2009, 3(2): 77-84.
[14] Ghosh K, Pan Z, Guan E, et al. Cell adaptation to a physiologically relevant ECM mimic with different viscoelastic properties[J]. Biomaterials, 2007, 28(4): 671-679.
[15] Kloxin A M, Benton J A, Anseth K S. In situ elasticity modulation with dynamic substrates to direct cell phenotype[J]. Biomaterials, 2010, 31(1): 1-8.
[16] Moussallem M D, Olenych S G, Scott S L, et al. Smooth muscle cell phenotype modulation and contraction on native and cross-linked polyelectrolyte multilayers[J]. Biomacromolecules, 2009, 10(11): 3062-3068.
[17] Banerjee A, Arha M, Choudhary S, et al. The influence of hydrogel modulus on the proliferation and differentiation of encapsulated neural stem cells[J]. Biomaterials, 2009, 30(27): 4695-4699.
[18] Gao L, Parker K J, Lerner R M, et al. Imaging of the elastic properties of tissue-A review[J]. Ultrasound in medicine & biology, 1996, 22(8): 959-977.
[19] Chen E J, Novakofski J, Jenkins W K, et al. Young's modulus measurements of soft tissues with application to elasticity imaging[J]. IEEE Transactions on Ultrasonics, Ferroelectrics, and Frequency Control, 1996, 43(1): 191-194.
[20] 他得安, 陈启敏. 非均匀软组织声散射模型的研究概况[J]. 声学技术, 1999, 18(2): 91-94. TA De’an, CHEN Qimin. Review the ultrasonic scattering model for inhomogeneous soft tissue[J]. Technical Acoustics, 1999, 18(2): 91-94.
[21] 他得安, 陈启敏. 基于AR倒谱法研究人体脾组织微结构的变化[J]. 声学技术, 2000, 19(3): 118-120. TA De’an, CHEN Qimin. Study on the variation of microstructures of human spleen by AR cepstrum[J]. Technical Acoustics, 2000, 19(3): 118-120.
[22] Vappou J. Magnetic resonance- and ultrasound imaging-based elasticity imaging methods: a review.[J]. Critical Reviews in Biomedical Engineering, 2012, 40(2): 121-134.
[23] Garteiser P, Doblas S, Daire J L, et al. MR elastography of liver tumours: value of viscoelastic properties for tumour characteriza-tion[J]. European Radiology, 2012, 22(10): 2169-2177.
[24] McGee K P, Mariappan Y K, Hubmayr R D, et al. Magnetic resonance assessment of parenchymal elasticity in normal and edematous, ventilator-injured lung[J]. Journal of Applied Physiology, 2012, 113(4): 666-676.
[25] Nightingale K. Acoustic radiation force impulse (ARFI) imaging: a review[J]. Current Medical Imaging Reviews, 2011, 7(4): 328-339.
[26] Palmeri M L, Wang M H, Dahl J J, et al. Quantifying hepatic shear modulus in vivo using acoustic radiation force[J]. Ultrasound in Medicine & Biology, 2008, 34(4): 546-558.
[27] ZHOU H S, WANG T Y, XU Z, et al. Measurement of the elasticity of biological soft tissue of finite thickness[J]. Chinese Physics Letters, 2016, 33(12): 96-100.
[28] Barnes S L, Lyshchik A, Washington M K, et al. Development of a mechanical testing assay for fibrotic murine liver[J]. Medical Physics, 2007, 34(11): 4439-4450.
[29] Harrison S M, Bush M B, Petros P E. Towards a novel tensile elastometer for soft tissue[J]. International Journal of Mechanical Sciences, 2008, 50(4): 626-640.
[30] Vappou J, Hou G Y, Marquet F, et al. Non-contact, ultrasound-based indentation method for measuring elastic properties of biological tissues using Harmonic Motion Imaging(HMI)[J]. Physics in Medicine and Biology, 2015, 60(7): 2853-2868.
[31] McKee C T, Last J A, Russell P, et al. Indentation versus tensile measurements of Young's modulus for soft biological tissues[J]. Tissue Engineering Part B: Reviews, 2011, 17(3): 155-164.
[32] Egorov V, Tsyuryupa S, Kanilo S, et al. Soft tissue elastometer[J]. Medical Engineering & Physics, 2008, 30(2): 206-212.
[33] Yao W, Yoshida K, Fernandez M, et al. Measuring the compressive viscoelastic mechanical properties of human cervical tissue using indentation[J]. Journal of the Mechanical Behavior of Biomedical Materials, 2014, 34(6): 18-26.
[34] Jiang Y, Li G, Qian L X, et al. Measuring the linear and nonlinear elastic properties of brain tissue with shear waves and inverse analysis[J]. Biomechanics and Modeling in Mechanobiology, 2015, 14(5): 1119-1128.
[35] 徐峥, 段俊丽, 钱梦騄, 等. 扫描探针显微技术测量血管内皮细胞的弹性[J]. 声学技术, 2016, 35(3): 239-242. XU Zheng, DUAN Junli, QIAN Menglu, et al. Elasticity measurement of endothelial cells by scanning probe microscopy[J]. Technical Acoustics, 2016, 35(3): 239-242.
[36] Liu F, Tschumperlin D J. Micro-mechanical characterization of lung tissue using atomic force microscopy[J]. Journal of Visualized Experiments Jove, 2011, 54(54): 2911-2911.
[37] Codan B, Favero G D, Martinelli V, et al. Exploring the elasticity and adhesion behavior of cardiac fibroblasts by atomic force microscopy indentation[J]. Materials Science & Engineering C, 2014, 40(949): 427-434..
[38] Fung, Y. C., Richard Skalak. Biomechanics: Mechanical properties of living tissues[M]. Springer-Verlag, 1981: 231-298.
[39] Cox M A J, Gawlitta D, Driessen N J B, et al. The non-linear mechanical properties of soft engineered biological tissues determined by finite spherical indentation[J]. Computer Methods in Biomechanics and Biomedical Engineering, 2008, 11(5): 585-592.
The effects of friction and specimen thickness on the measurement of biological specimen elasticity with indentation method
YAN Xu1, GAO Qi2, CHENG Qian1, ZHOU Hong-sheng2, XU Zheng1
(1. Institute of Acoustics, Tongji University, Shanghai 200092, China;2. Shanghai acoustics laboratory, Chinese Academy of Sciences, Shanghai 201815, China)
The measurement of elasticity of biological samples can provide the basis for the early diagnosis and treatment of diseases. In this paper, the elasticity of biological samples is measured by indentation method. The indentation process is simulated by finite element software. Results indicate that the thickness of the sample influences the measurement of the elasticity. The measurement error is 0.74 % when the thickness of the specimen is 75 times of the indentation depth. The effect of indentation velocity on elasticity measurement has also been studied. It is found that there is a certain difference between the measured result and the true Young’s modulus due to the effect of friction when the indentation velocity is high. The elasticity of the sample can be accurately calculated when the friction has been taken into account and the error of the calculated modulus is 5 % or less relative to the true modulus.
indentation method; elasticity; finite element; friction force
O429
A
1000-3630(2017)-05-0410-05
10.16300/j.cnki.1000-3630.2017.05.003
2017-05-10;
2017-07-18
国家自然科学基金资助项目(11404245、11374231、11674249)、国家重点研发计划项目(2016YFA0100800、2012YQ150213)
严旭(1993-), 男, 江苏泰州人, 硕士研究生, 研究方向为生物医学超声。
徐峥, E-mail: gotoxvzheng@tongji.edu.cn
