Adjunctive melatonin for tardive dyskinesia in patients with schizophrenia: a meta-analysis
2017-11-29ChenHuiSUNWeiZHENGXinHuYANGDongBinCAICheeNGGaborUNGVARIHaiYanLIYuJieWUYuPingNINGYuTaoXIANG
Chen-Hui SUN, Wei ZHENG, Xin-Hu YANG, Dong-Bin CAI, Chee H. NG, Gabor S. UNGVARI,Hai-Yan LI, Yu-Jie WU, Yu-Ping NING*, Yu-Tao XIANG*
•Systematic review and meta-analysis•
Adjunctive melatonin for tardive dyskinesia in patients with schizophrenia: a meta-analysis
Chen-Hui SUN1#, Wei ZHENG2#, Xin-Hu YANG2, Dong-Bin CAI3, Chee H. NG4, Gabor S. UNGVARI5,6,Hai-Yan LI2, Yu-Jie WU2, Yu-Ping NING2*, Yu-Tao XIANG7*
Tardive dyskinesia; antipsychotic; melatonin; meta-analysis
1. Introduction
Tardive dyskinesia (TD) can occur in up to 20%of schizophrenia patients receiving long-term antipsychotics (APs)[1-4], and is associated with considerable personal suffering and social and physical disabilities.[3]TD is characterized by abnormal and involuntary movements (such as choreiform, athetoid and rhythmic) on orofacial, extremities, or even truncal region.[5]Numerous medications for TD have been examined, but there have been no well-proven treatments.[6]
The pathophysiology of APs induced TD remains controversial. Free radicals associated with low brainderived neurotrophic factor (BDNF) is probably involved in the process.[2,7,8]Several antioxidants, based on the free radical hypothesis of TD, have been studied for the therapeutic effects of TD, such as melatonin[6,9-11],vitamin B6[12,13], vitamin E[14,15], Ginkgo biloba (EGb)[4]and piracetam.[16,17]
Melatonin (N-acetyl-5-methoxytryptamine), a neurohormone that is secreted by the pineal gland,is a naturally occurring potent antioxidant in the brain.[5,18]Animal model studies[19-21]suggested that melatonin is efficacious in the improvement of abnormal movements. Some randomized controlled trials (RCTs)[6,9-11]have examined the efficacy and safety of adjunctive melatonin for schizophrenia patients with TD, but results are inconsistent.
One review[5]which examined the efficacy of melatonin for schizophrenia patients with TD based on only two RCTs[10,11]used qualitative analysis without adequate assessment of study quality.Hence, the objective of this RCT meta-analysis was to systematically assess the efficacy and safety of adjunctive melatonin therapy for TD in schizophrenia using both English and Chinese databases since the latter is not widely known internationally.
2. Methods
2.1 Search Strategy and Selection Criteria
Two authors (WZ and D-BC) independently and systematically searched English (PubMed, PsycINFO,Embase, Cochrane Library databases) and Chinese databases (Wanfang Data, Chinese National Knowledge Infrastructure (CNKI), SINOMED), with each database from their inception until June 8, 2017, using the following search terms: (Tardive Dyskinesia OR Dyskinesia*, Tardive OR Dyskinesias, Tardive OR Dystonias, Tardive) AND (Mélatonine OR Melatonin).Reference lists of all identified RCTs and relevant review articles[5]for additional studies were also handsearched.
The following selection criteria were presented using the PICOS acronym: Participants: adult schizophrenia patients with TD (≥18 years) by any diagnostic criteria. Intervention: melatonin combined with APs. Comparison: APs combined with placebo or APs monotherapy. Outcomes: the primary outcome measure was the severity of TD symptoms at endpoint assessment measured by the Abnormal Involuntary Movement Scale (AIMS).[22,23]Key secondary outcomes were cognitive function, adverse drug reactions (ADRs),and discontinuation rate. Study design: RCTs with metaanalyzable data.
The literature from the above databases was screened by two independent authors (WZ and D-BC)using EndNote X6 software. Figure 1 presents the literature screening process. Any disagreement during this process was resolved through a discussion.
2.2 Data extraction
Two authors (D-BC and X-HY) independently and systematically extracted data and conducted quality assessment of included RCTs. Any disagreement was resolved through a discussion and consensus.Data were extracted into standard forms. Whenever data were missing for the meta-analysis, first or correspondence authors were contacted by email or telephone for additional information if possible.
2.3 Statistical methods
According to the guidelines from the Preferred Reporting Items for Systematic Reviews and Meta-Analyses(PRISMA) statement[24], we used the Review Manager(RevMan) Version 5.3 (http://tech.cochrane.org/revman/)to perform this meta-analysis in cases that continuous data followed normal distribution (i.e., the mean > 2 times the standard deviation when the minimum value was 0). A sensitivity analysis was conducted by excluding skewed data. For meta-analytic pooling of continuous and dichotomous outcomes, the Inverse-Variance method and Mantel-Haenszel test were used to present weighted mean differences (WMDs) and risk ratios (RRs)with their 95% confidence intervals (CIs), respectively.Intention-to-treat (ITT) data over observed cases (OC)data were preferred when both were provided. In order to compensate for study heterogeneity, the random effect model was used in all meta-analyzable outcomes.[25]Heterogeneity was presented by I-squared statistic. When I-square was greater than 50%, sensitivity, subgroup and meta-regression analyses were conducted to detect the sources of heterogeneity. Furthermore, data of primary outcome were entered into a funnel plots or analyzed using Egger’s test[26]to test Publication bias. All analyses were two tailed, with significance level set at 0.05.
2.4 Assessment of study quality
The methodological quality of the RCTs was assessed using the Cochrane risk of bias assessment tool[27]with 7 dimension as follows: (a) random sequence generation;(b) allocation concealment; (c) blinding of the subjects and the treatment providers; (d) blinding of the result evaluators); (e) incomplete results data; (f) selective reporting; (g) other potential risks. Furthermore, the Jadad scale was used to assess study quality.[28]High and low quality of included RCTs were defined as Jadad score≥3 and <3, respectively. In addition, the overall evidence quality of all meta-analyzable outcomes was rated as“very low “, “low”, “moderate “ and “high” by two independent authors (WZ and D-BC) using the grading of recommendations assessment, development, and evaluation (GRADE) system.[29,30]
3. Results
3.1 Results of the search
Figure 1 presents a flow chart of article selection from English (n=206) and Chinese databases (n=10). Finally,4 RCTs (Castro 2011; Shamir 2000; Shamir 2001; Zhu 2010) were eligible and included for the meta-analysis(Figure 1).
3.2 The characteristics of included studies
Three double-blinded trials (Castro 2011; Shamir 2000;Shamir 2001) and one rater masked trial (Zhu 2010)compared adjunctive melatonin (n=67) and control groups (n=63) (Table 1). The weighted mean treatment duration was 9.8 weeks (range=4-12 weeks) (Table 1). Of the 4 RCTs , two RCTs (Shamir 2000; Shamir 2001) were conducted in Israel (n=41), one in China(Zhu 2010, n=76) and one in Venezuela (Castro 2011,n=13), respectively. While 24 patients in Shamir 2001 were eligible, 22 patients were described and analyzed because 2 patients were discharged from the hospital before initiation of the study.
3.3 Patient characteristics
The weighted mean age was 61.3 years (range =57.6-74.0 years) (Table 1), the percentage of males was 41.54% (range=34.2%-69.2%), and the weighted mean illness duration in 3 RCTs (Castro 2011; Shamir 2000;Shamir 2001) with available data was 28.6 years(range=24.8-31.3 years). The RCTs (n=130) were conducted in inpatient (n=119, 91.5%) and outpatient(n=3, 2.3%) and rehabilitation institution settings (n=8,6.2%).

Figure 1. Identification of included studies
3.4 Treatment characteristics
The weighted mean dosage of melatonin dosage was 9.2 mg/day (range=2.0-20.0 mg/day). One RCT(Zhu 2010) did not report details of APs use, but the remaining RCTs (Castro 2011; Shamir 2000; Shamir 2001) reported using a range of APs including levopromazine, haloperidol, clozapine, aripiprazole,olanzapine, quetiapine, risperidone, perphenazine,zuclopenthixol and chlorpromazine. Furthermore, the mean dosage of mixed APs in the 3 RCTs with available data was 262.3 mg/day in chlorpromazine dose equivalents. Only one RCT (Castro 2011) reported the study funding sources.
3.5 Risk of bias
One RCT (Shamir 2001) mentioned “random”assignment with specific description, 3 RCTs (Castro 2011; Shamir 2000; Shamir 2001) used double blind and 1 RCT (Zhu 2010) used masked assessors (Table 2).Two RCTs (Shamir 2000; Shamir 2001) were rated as low risk regarding the allocation concealment methods and the potential for selective reporting. Incomplete outcome data addressed and other sources of bias (e.g.,drug company sponsorship) were rated as low risk and unclear in all included RCTs.
3.6 Jadad scale
The weighted mean Jadad total score of the 4 RCTs was 2.4 (range=1-5). While one RCT (Zhu 2010) was classified as low quality, 75% RCTs (n=3) (Castro 2011;Shamir 2000; Shamir 2001) were rated as high quality.
3.7 Primary outcome
The improvement of TD symptoms: there were differences between the two groups in terms of the AIMS total score (4 RCTs) at the treatment endpoint. Adjunctive melatonin was superior in reducing the severity of TD symptoms (4 RCTs, n=130, WMD: -1.52 (95%CI: -3.24,0.20), p=0.08; I2=0%, Figure 2) when compared to the control group although the improvement did not reach a significant level. The results remained after removing two trials (Shamir 2001 and Zhu 2010) with non-normal distributions data (WMD: -2.70 (95%CI: -6.58,1.19,p=0.17; I2=0%). Due to the limited number of RCTs, risk of publication bias could not be examined. The overall evidence quality of the improvement of TD symptoms was rated as “Low” (table 3).

Table 1. Characteristics of the included studies

Table 2. Evaluation of risk of bias in the included studies

Table 3. Adjunctive Melatonin for Tardive Dyskinesia: GRADE assessments
3.8 Secondary outcomes
Cognitive function: One RCT (Zhu 2010) assessed the cognitive effects of the melatonin treatment (Table 4).Adjunctive melatonin outperformed the control group in the Wechsler Adult Intelligence Scale-Revised, Chinese version (WAIS-RC) [including verbal scale, performance scale and full scale total scores], Verbal Fluency Test(VFT) [including animal, fruit, and word and Repeatable Battery for the Assessment of Neuropsychological Status(RBANS) [including verbal fluency]. In terms of cognitive function assessed by the Wechsler Memory Scale (WMS),no difference was found between the two groups (total score and memory quotient of WMS).
ADRs: Only one RCT (Zhu 2010) assessed ADRs using the Treatment Emergent Symptom Scale (TESS),and no group difference was found (Table 4).
Discontinuation rate: Regarding discontinuation rate, one RCT (Shamir 2001) reported that 2 patients were discharged before the randomization.
4. Discussion
4.1 Main findings
Previously only one review[5]with 2 RCTs (n=41)involved the therapeutic effects and safety of adjunctive melatonin for schizophrenia patients suffering from TD, finding an advantage of melatonin in improving the severity of TD symptoms. In this meta-analysis (4 RCTs, n=130) based on English and Chinese databases,the results showed that adjunctive melatonin outperformed the control group in treating the severity of TD symptoms although the improvement did notreach significance level. The strengths of this metaanalysis are the inclusion of two additional RCTs[6,9]and the inclusion of Chinese databases with low quality.In addition, quality assessment of included studies using the Cochrane risk of bias[27]and Jadad scale[28]were conducted. The overall quality level of primary outcome were rated as “Low” using the GRADE approach. However, the data on the ADRs and cognitive effect were only reported in one RCT (Zhu 2010), which limits our capacity to conduct meta-analysis.

Figure 2. Adjunctive Melatonin for Tardive Dyskinesia (TD): Forest plot for the endpoint score of the Abnormal Involuntary Movement of Scale
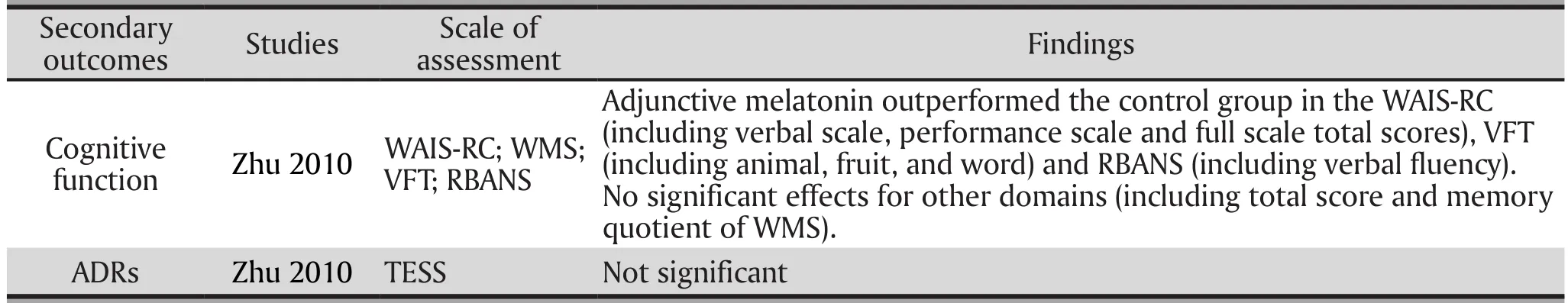
Table 4. Adjunctive Melatonin for Tardive Dyskinesia: Cognitive Function at endpoint and ADRs
Possible mechanism of melatonin in improving TD could be due to its potent antioxidant properties, which reduces oxidative stress, enhances BDNF levels and decreases the chance of neurotoxicity.[5]Furthermore,Zisapel’s study[31]suggested that melatonin inhibits dopamine release in specific areas of the mammalian central nervous system (including retina, hypothalamus,medulla-pons, and hippocampus). Thus, melatonin could modulate dopaminergic pathways associated with movement disorders including TD.
4.2 Limitations
First, the sample sizes were relatively small and some information was limited, which failed to bring about a robust result.[32]Second, the dose-response effect of adjunctive melatonin (2 to 20mg/day) on TD symptoms could not be evaluated. Third, since all included RCTs were of short duration, ranging from 4 to 12 weeks,the long-term effects of melatonin on TD need to be examined further. Finally, we made a minor correction in the methods and made it differ from the protocol of this meta-analysis, i.e., “only randomized double blinded controlled trials were included” was changed to “only randomized controlled trials were included”.
4.3 Implications
TD is irreversible, severe, and disabling, and so far no therapeutic strategies have been approved by the Food and Drug Administration (FDA). One meta-analysis of adjunctive EGb (ginkgo)[4]including 3 RCTs found adjunctive EGb could significantly reduce the severity of TD symptoms in schizophrenia. Another metaanalysis found that while adjunctive vitamin E[15]was similar with placebo in improving TD symptoms, it could significantly slow down the progression of the severity of TD symptoms in schizophrenia. Moreover, the previous systematic review[5] has explored the therapeutic effects of adjunctive melatonin, piracetam, and vitamin B6 on TD in schizophrenia. If melatonin is shown to be effective in TD, it may be a readily accessible and affordable option.However, future higher quality and larger RCTs are needed to confirm these results.
4.4 Conclusion
This meta-analysis shows that melatonin has potential for improving TD symptoms in schizophrenia. However,higher quality and larger RCTs are needed to confirm these findings, especially focusing on long-term therapeutic effects.
Funding statement
The study was supported by the University of Macau(SRG2014-00019-FHS; MYRG2015-00230-FHS;MYRG2016-00005-FHS) and the Affiliated Brain Hospital of Guangzhou Medical University (2016YFC0906302;81671334; 2014Y2-00105; 2015BAI13B02). The University of Macau and the Affiliated Brain Hospital of Guangzhou Medical University had no role in the study design, generating or interpreting the results and publication of the study.
Conflict of interest statement
The authors declare that they have no conflicts of interest concerning this paper.
Authors’ contributions
WZ and Y-TX designed the study and were assisted by X-HY and D-BC in search for papers, data extraction,and the assessment of study quality. WZ and C-HS analyzed all meta-analyzable outcomes and drafted the manuscript. H-NC, S-UG, and Y-PN made critical revisions to the manuscript. All authors approved the final version for publication.
1. Greenberg DB. Tardive dyskinesia: A task force report of the American Psychiatric Association. J Neurol Neurosurg Psychiatry. 1993; 56(5): 282-283. doi: http://doi.org/10.1136/jnnp.56.5.579-b
2. Jankelowitz SK. Treatment of neurolept-induced tardive dyskinesia. Neuropsychiatr Dis Treat. 2013; 9: 1371-1380.doi: http://doi.org/10.2147/NDT.S30767
3.McGrath JJ, Soares KV. Miscellaneous treatments for neuroleptic-induced tardive dyskinesia. Cochrane Database Syst Rev. 2000; 2: CD000208. doi: http://doi.org/10.1002/14651858.CD000208
4. Zheng W, Xiang YQ, Ng CH, Ungvari GS, Chiu HF, Xiang YT.Extract of ginkgo biloba for tardive dyskinesia: Meta-analysis of randomized controlled trials. Pharmacopsychiatry. 2016;49(3): 107-111. doi: http://doi.org/10.1055/s-0042-102884
5. Nelson LA, McGuire JM, Hausafus SN. Melatonin for the treatment of tardive dyskinesia. Ann Pharmacother. 2003;37: 1128-1131. doi: http://doi.org/10.1345/aph.1C460
6.Zhu FY, Shi XM, Zhang JX, Wei LH, Zhang XM, Ji ZF, et al.[Melatonin treatment for tardive dyskinesia: a randomized controlled study]. Zhong Hua Jing Shen Ke Za Zhi.2010; 43: 28-30. doi: http://dx.chinadoi.cn/10.3760/cma.j.issn.1006-7884.2010.01.009
7. Zhang WF, Tan YL, Zhang XY, Chan RC, Wu HR, Zhou DF.Extract of Ginkgo biloba treatment for tardive dyskinesia in schizophrenia: a randomized, double-blind, placebocontrolled trial. J Clin Psychiatry. 2011; 72(5): 615-621. doi:http://doi.org/10.4088/JCP.09m05125yel
8. Zhang XY, Zhang WF, Zhou DF, Chen DC, Xiu MH, Wu HR, et al. Brain-derived neurotrophic factor levels and its Val66Met gene polymorphism predict tardive dyskinesia treatment response to Ginkgo biloba. Biol Psychiatry. 2012; 72(8): 700-706. doi: http://doi.org/10.1016/j.biopsych.2012.04.032
9.Castro F, Carrizo E, Prieto de Rincón D, Rincón CA, Asián T, Medina-Leendertz S, et al. Effectiveness of melatonin in tardive dyskinesia. Invest Clin. 2011; pp: 252-260
10. Shamir E, Barak Y, Plopsky I, Zisapel N, Elizur A, Weizman A. Is melatonin treatment effective for tardive dyskinesia? J Clin Psychiatry. 2000; 61(8): 556-558
11. Shamir E, Barak Y, Shalman I, Laudon M, Zisapel N, Tarrasch R, et al. Melatonin treatment for tardive dyskinesia: a double-blind, placebo-controlled, crossover study. Arch Gen Psychiatry. 2001; 58(11): 1049-1052
12. Lerner V, Miodownik C, Kaptsan A, Bersudsky Y, Libov I,Sela BA, et al. Vitamin B6 treatment for tardive dyskinesia:a randomized, double-blind, placebo-controlled, crossover study. J Clin Psychiatry. 2007; 68(11): 1648-1654
13. Lerner V, Miodownik C, Kaptsan A, Cohen H, Matar M,Loewenthal U, et al. Vitamin B(6) in the treatment of tardive dyskinesia: a double-blind, placebo-controlled, crossover study. Am J Psychiatry. 2001; 158(1): 1511-1514. doi: http://doi.org/10.1176/appi.ajp.158.9.1511
14. Howland RH. Drug therapies for tardive dyskinesia: part 2.J Psychosoc Nurs Ment Health Serv. 2011; 49(7): 17-20. doi:http://doi.org/10.3928/02793695-20110602-02
15. Soares-Weiser K, Maayan N, McGrath J. Vitamin E for neuroleptic-induced tardive dyskinesia. Cochrane Database Syst Rev. 2011; 2: CD000209. doi: http://doi.org/10.1002/14651858.CD000209.pub2
16. Libov I, Miodownik C, Bersudsky Y, Dwolatzky T, Lerner V.Efficacy of piracetam in the treatment of tardive dyskinesia in schizophrenic patients: a randomized, double-blind,placebo-controlled crossover study. J Clin Psychiatry. 2007;68(7): 1031-1037
17. Malykh AG, Sadaie MR . Piracetam and piracetam-like drugs: from basic science to novel clinical applications to CNS disorders. Drugs. 2010; 70(3): 287-312. doi: http://doi.org/10.2165/11319230-000000000-00000
18. Wang YY, Zheng W, Ng CH, Ungvari GS, Wei W, Xiang YT. Meta-analysis of randomized, double-blind, placebocontrolled trials of melatonin in Alzheimer’s disease. Int J Geriatr Psychiatry. 2017; 32(1): 50-57. doi: http://doi.org/10.1002/gps.4571
19. Bazrgar M, Goudarzi I, Lashkarbolouki T, Elahdadi Salmani M. Melatonin ameliorates oxidative damage induced by maternal lead exposure in rat pups. Physiol Behav. 2015; 151: 178-188. doi: http://doi.org/10.1016/j.physbeh.2015.06.040
20. Abilio VC, Vera JA, Jr., Ferreira LS, Duarte CR, Carvalho RC,et al. Effects of melatonin on orofacial movements in rats.Psychopharmacology (Berl). 2002; 161(4): 340-347. doi:http://doi.org/10.1007/s00213-002-1081-7
21. Raghavendra V, Naidu PS, Kulkarni SK. Reversal of reserpineinduced vacuous chewing movements in rats by melatonin:involvement of peripheral benzodiazepine receptors. Brain Res. 2001; 904(1): 149-152. doi: https://doi.org/10.1016/S0006-8993(01)02455-6
22. Fan B. [The Chinese version of the Abnormal Involuntary Movement Scale (AIMS)]. Shanghai Arch Psychiatry. 1984; 2:80-81. Chinese
23. Health National Institute of Mental Health. Abnormal involuntary movement scale. In: W. Guy (Ed.). ECDEU Assessment Manual for Psychopharmacology-Revised.Rockville, MD: Department of Health, Education, and Welfare, National Institute of Mental Health. 1976; pp. 534-537
24. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group.Preferred reporting items for systematic reviews and metaanalyses: the PRISMA statement. Int J Surg. 2010; 8(5): 336-341. doi: https://doi.org/10.1016/j.ijsu.2010.02.007
25. DerSimonian R, Laird N. Meta-analysis in clinical trials.Control Clin Trials. 1986; 7(3): 177-188. doi: https://doi.org/10.1016/0197-2456(86)90046-2
26. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ.1997; 315(7109): 629-634
27. Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. 2008; John Wiley &Sons
28. Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary?Control Clin Trials. 1996; 17(1): 1-12. doi: https://doi.org/10.1016/0197-2456(95)00134-4
29. Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011; 64(4): 401-406. doi:https://doi.org/10.1016/j.jclinepi.2010.07.015
30. Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004; 328(7454): 1490. doi: https://doi.org/10.1136/bmj.328.7454.1490
31. Zisapel N. Melatonin-dopamine interactions: from basic neurochemistry to a clinical setting. Cell Mol Neurobiol.2001; 21(6): 605-616
32. Trikalinos TA, Churchill R, Ferri M, Leucht S, Tuunainen A,Wahlbeck K, et al. Effect sizes in cumulative meta-analyses of mental health randomized trials evolved over time. J Clin Epidemiol. 2004; 57(11): 1124-1130
褪黑素治疗精神分裂症患者迟发性运动障碍的meta分析
孙辰辉,郑伟,杨欣湖,蔡东滨,Ng CH,Ungvari GS,李海燕,吴玉洁,宁玉萍,项玉涛
迟发性运动障碍;抗精神病药;褪黑素;荟萃分析
Background: Tardive dyskinesia (TD) is characterized by abnormal and involuntary movements. Importantly,TD could cause considerable personal suffering and social and physical disabilities.
Aims: This meta-analysis based on randomized controlled trials (RCTs) systematically assessed the therapeutic effect and tolerability of melatonin for TD in schizophrenia.
Methods: A computerized and systematical search of both Chinese (Wanfang Data, Chinese National Knowledge Infrastructure (CNKI), SINOMED) and English (PubMed, PsycINFO, Embase, Cochrane Library databases) databases, from their inception until June 8, 2017, was conducted by two independent authors.The severity of TD symptoms were the primary outcome measure and analyzed using a random effects model by the Review Manager (RevMan) Version 5.3. Quality evaluation of included RCTs was conducted using the Cochrane risk of bias and Jadad scale. The GRADE (Grades of Recommendation, Assessment,Development, and Evaluation) system recommendation grading method was used to assess the overall quality level of meta-analytic outcomes.
Results: Four RCTs (n=130) were identified and analyzed. Three RCTs used double blind and 1 RCT used masked assessors using the Cochrane risk of bias, and 3 RCTs were rated as high quality based on Jadad scale. Compared with the control group, adjunctive melatonin was superior in reducing the severity of TD as measured by the Abnormal Involuntary Movement Scale (AIMS) (4 RCTs, n=130, weighted mean difference(WMD): -1.52 (95% confidence intervals (CI): -3.24, 0.20), p=0.08; I2=0%) although the improvement did not reach a significant level. The overall evidence quality of the improvement of TD symptoms, according to GRADE approach, was rated as “Low”. The data on the ADRs and cognitive effect were limited.
Conclusions: This meta-analysis shows that melatonin has potential for improving TD symptoms in schizophrenia. Future higher quality and larger RCTs are warranted to confirm the findings.
Review registration: CRD 42016049698 (http://www.crd.york.ac.uk/PROSPERO/)
[Shanghai Arch Psychiatry. 2017; 29(3): 129-136.
http://dx.doi.org/10.11919/j.issn.1002-0829.217046]
1Qingdao Mental Health Center, Shandong, China
2The Affiliated Brain Hospital of Guangzhou Medical University (Guangzhou Huiai Hospital), Guangzhou, China
3Clinics of Chinese Medicine, the First Clinical Medical College of Guangzhou University of Chinese Medicine, Guangzhou, China
4Department of Psychiatry, University of Melbourne, Melbourne, Victoria, Australia
5The University of Notre Dame Australia / Marian Centre, Perth, Australia
6School of Psychiatry & Clinical Neurosciences, University of Western Australia, Perth, Australia
7Unit of Psychiatry, Faculty of Health Sciences, University of Macau, Macao SAR, China
#These authors equally contributed to this work.
*correspondence: Dr. Yu-Tao Xiang. Mailing address: 3/F, Building E12, Faculty of Health Sciences, University of Macau, Avenida da Universidade, Taipa,Macau SAR, China. Postcode: 00000. E-mail: xyutly@gmail.com; Dr. Yu-Ping Ning. Mailing address: the Affiliated Brain Hospital of Guangzhou Medical University (Guangzhou Huiai Hospital), Guangzhou, China; Postcode: 510370. E-mail: ningjeny@126.com
背景:迟发性运动障碍(TD)的临床特征是异常不自主运动。TD具有严重的不可逆的致残性和社会功能损害。
目的:此荟萃分析基于随机对照试验(RCTs)文献系统评估褪黑素对精神分裂症患者迟发性运动障碍的临床疗效和安全性。
方法:两位独立评估者从以下数据库对相关的临床随机对照试验(RCT)文献进行检索(万方数据、中国知网(CNKI)、中国生物医学文摘数据库和PubMed、PsycINFO、Embase、Cochrane Library数据库) ,检索时间截止于2017年6月8日。以TD症状严重程度为主要结局指标,采用Rev Man 5.3版本进行统计分析,对RCTs的质量评估采用Cochrane风险评估偏倚和Jadad量表来评估各种偏倚的风险性。采用GRADE(Grades of Recommendation, Assessment, Development, and Evaluation) 系统推荐分级方法对meta-分析结果的整体证据质量水平进行分级评价。
结果:最终筛选确定4个RCTs(n = 130)。3个RCTs采用双盲法,1 个RCT单盲,根据Cochrane风险评估偏倚和Jadad量表显示 3个RCTs的疗效评估指标的证据质量被评定为“高质量”。与对照组相比,根据不自主运动量表(AIMS)评定褪黑素可改善TD严重程度 (4个RCTs, n = 130,加权平均差值(WMD):-1.52(95%CI: -3.24,0.20), p = 0.08; I2= 0%),但尚没有达到显著差异。根据等级方法,改善TD症状的meta分析结果的整体证据质量被评为“低”,而关于不良反应和认知损害方面则数据太少。
结论:荟萃分析表明,褪黑素或可改善精神分裂症TD症状。但仍有待今后更高质量和更大样本的RCTs验证。

Dr. Chen-Hui Sun obtained a bachelor’s degree from Jining Medical University in 2013 and a master’s degree in psychiatry and mental health from the Capital Medical University in Beijing in 2016. Since then he has been working as a resident physician at the second department of geriatrics in QingDao Mental Health Center. His main research interest is depression and schizophrenia.

Dr. Wei Zheng obtained a bachelor’s degree from Hebei Medical University in 2012 and a master’s degree in psychiatry and mental health from the Capital Medical University in Beijing in 2015. Since then he has been working as a resident physician in the Department of Psychiatry in the Guangzhou Huiai Hospital. He is currently a PhD Candidate at the Affiliated Brain Hospital of Guangzhou Medical University (Guangzhou Huiai Hospital) from 2016 year. His main research interest is depression and schizophrenia.
猜你喜欢
杂志排行
上海精神医学的其它文章
- Changes in cognitive function in patients with primary insomnia
- Peripheral SLC6A4 gene expression in obsessive-compulsive disorder in the Han Chinese population
- Dysfunction of cognition patterns measured by MATRICS Consensus Cognitive Battery (MCCB) among first episode schizophrenia patients and their biological parents
- The fantasmatic and imaginary child of the pregnant woman
- Is depression the result of immune system abnormalities?
- Autoimmune thyroiditis presenting as psychosis
