猪的营养应激与肠道免疫应答
2017-11-17贾舒安王旭哲
贾舒安 王旭哲
(1.新疆维吾尔自治区动物卫生监督所,新疆乌鲁木齐 530000;2.新疆石河子大学动物科技学院,新疆石河子 532000)
猪的营养应激与肠道免疫应答
贾舒安1,2王旭哲2
(1.新疆维吾尔自治区动物卫生监督所,新疆乌鲁木齐 530000;2.新疆石河子大学动物科技学院,新疆石河子 532000)
现代畜牧生产更趋向于高度密集和大规模,来提高生产效率。这种生产环境的变化会影响动物健康和加剧生长的应激。主要的应激源包括环境(空气质量和温度)、营养和感染。这些应激可以降低生长性能同时改变家畜全身和局部免疫系统的水平,包括胃肠道等。热应激会增加肠道的通透性、氧化应激和炎症反应;营养应激是由空腹、抗营养化合物、毒素作用,引起肠道紧密连接蛋白(TJ蛋白)的泄漏表达。禁食是能够抑制促炎性细胞因子,而脱氧雪腐镰刀菌烯醇能增加肠促炎性细胞因子在肠道中的淋巴细胞水平。疾病或病毒的感染如肠毒性大肠杆菌(ETEC)和病毒和猪流行性腹泻病毒可以导致肠上皮细胞屏障失效。另一方面,有研究添加乳酸杆菌或酿酒酵母可降低ETEC传染力。有研究指出,主要的应激源改变了肠道屏障的通透性和在猪粘膜系统中的促炎性细胞因子和趋化因子的基因和蛋白质的表达。但目前还不能解释,在应激条件下,猪肠道免疫系统的作用机制。在主要应激源下,肠道和全身免疫系统之间的相互作用有待进一步揭示。
营养;应激;肠道免疫;猪
肠黏膜是机体内部与外界环境之间的屏障。一般家畜和人类的肠粘膜对大部分的食物抗原消化和吸收是免疫耐受的。由于食物成分的改变,肠道黏膜形态和特征与肠道免疫细胞也在不断变化。当大量病原体通过肠道黏膜进入机体,保护性黏膜免疫迅速反应,清除病原体。然而肠道内有许多不同的共生菌,肠道免疫系统必须能够区分无害的与有害的抗原[1]。
猪肠道免疫系统在出生时处于不成熟状态,在围产期进一步发展,在5~7周时达到成年水平。肠道上皮细胞与肠道固有免疫系统关联,对食物和病原体抗原形成功能屏障。Toll 样受体(TLR)作为一类新兴的膜和胞浆受体,在识别先天免疫调节的病原体中具有重要作用。适应性免疫应答的诱导开始由专门的抗原呈递细胞加工提呈抗原[2],在小肠组织的淋巴结或肠系膜淋巴结集结[3]。IgA反应是一种自适应的肠道免疫系统最重要的部分,这需要在肠道相关淋巴组织之间的T和B淋巴细胞相互作用[4]。
家畜一直接受着各种应激,比如饮食,温度,断奶和感染等。这些应激因素的突然变化对动物健康会产生负面影响,导致生产力下降。这种应激往往影响动物的稳态,诱导全身或局部炎症反应,并造成神经内分泌的改变。由于胃肠道是由免疫系统和神经内分泌系统共同作用的[5],故降低应激能显著提高家畜肠道稳态平衡。
肠道免疫是家畜的免疫前线,关系到家畜的生长性能和健康。本文主要探讨应激对猪肠道健康和免疫状态的影响。
1 不同应激环境下猪肠道免疫功能的研究
1.1 热应激
气候变暖引起的气候变化不仅迫使热带地区的环境温度升高,而且使温带地区温度升高。因此热应激(HS)已成为养猪业健康的临界应激因子[7]。HS 每年在美国已经影响了经济损失超过3亿美元,在全球范围内数十亿美元的损失[8]。因此,在营养和免疫相关研究中,重要的是要了解是HS如何涉及动物的生长性能和健康状况。
已有报道发现,破坏紧密连接蛋白(TJ蛋白),如1/3紧密连接蛋白,会导致猪肠道上皮细胞通透性增加[9];然而,HS诱导的肠道通透性并未增加,而上调 GLUT-2蛋白表达改变的TJ[10]。但是激活SGLT 1,Na+和葡萄糖共转运,会导致通透性增加[11]。因此,HS对猪上皮细胞葡萄糖转运蛋白的表达与渗透性有直接影响。虽然,HS使通透性增加,从而血液中内毒素的浓度会超标三倍,但炎性细胞因子,如白细胞介素(IL)-1β、IL-8、肿瘤坏死因子(TNF)-α,但没有增多[10]。脂多糖(LPS)是公认的炎性细胞因子诱导剂,但研究表明,HS也可能引起动物免疫抑制,[12]或LPS没有增高,是因为①技术错误,②的结构差异[13],③微分组成[14],④脂多糖结合蛋白[15]。这很可能是HS与LPS不诱导促炎细胞因子或者是与特定的细胞因子有关,如IL-17或转化生长因子(TGF-β)。有试验表明,在HS条件下,猪肠道的热休克蛋白70和髓过氧化物酶活性,中性粒细胞活化标志物[16]会增加。这也表明,在HS诱导下,会引起猪肠道的炎性反应。
两者合计,HS可以改变猪肠道屏障功能,诱导炎症反应。如何克服HS对猪的免疫系统的全身和局部炎症的影响,有待应进一步研究。
1.2 营养应激
生长性能是养猪业生产者最关键的因素。因此,营养应激是养猪业生产者最关心的问题[1718]。一般来说,空腹对健康猪肠道的生理和免疫学均会产生负面影响。给断奶仔猪禁食或禁水24h,会提高了血液中的皮质醇水平,并抑制空肠中TNF-α的表达。但是,并没有中断TJ蛋白的表达,比如在空肠和回肠中的claudin-1、zonula occludens protein- 1(ZO-1)[19]。缺乏甘氨酸可以降低细胞增殖和蛋白质合成,会使猪肠上皮细胞凋亡加速,而且,会减少Akt通路和激活哺乳动物雷帕霉素靶蛋白(M-TOR)的激活[20]。
霉菌毒素是造成饲料污染的主要原因之一[21]。脱氧雪腐镰刀菌烯醇(DON,呕吐毒素)会影响TJ蛋白如ZO-1的表达,紧密联系蛋白和紧密联系蛋白-3,以及猪肠道上皮细胞在48h内的通透性[9,22]。DON 刺激的促炎性细胞因子如TNF-α和IL-6,IL-1β在空肠和回肠中表达[23]同时,增加淋巴细胞的数目[24]。很明显,TLR2配体可以预防猪肠道上皮细胞不受损伤[23]。伏马菌素B1(FB1)刺激10d后可增加猪空肠通透性[25],然而 DON 也只需要2天,渗透率就可达到类似水平[9]。FB1的刺激可下调IL-8的表达,而不改变其他炎性细胞因子 IL-6,IL-1β,IL-12,and TNF-α的表达[26]。与 DON 相比FB1对猪肠道上皮细胞的免疫效果相对较弱。联合饲喂黄曲霉毒素与呕吐毒素(DON)33~42d,可以增血中高浓度TNF-α与单核细胞以及白细胞的数量,而单一处理并没有出现这种现象[27,28]。值得注意的是,长期饲喂呕吐毒素和玉米赤霉烯酮,可使8-羟基脱氧鸟苷氧化应激水平增加一倍,造成DNA氧化损伤[29]。炎性细胞因子I包括L-12/Il-23和IL-1β,给猪饲喂玉米赤霉烯酮(0.1mg/kg)42d后,回肠淋巴中血管活性肠肽的含量增加[30]。同时喂玉米赤霉烯酮(0.1mg/kg),28d后,B细胞CD21 +干扰素(IFN)会减少,同时在回盲部淋巴结γ浓度增加,这可能会改变B细胞反应。此外,T-2毒素在感染鼠伤寒沙门氏菌的猪上可降低小肠上皮细胞活力。虽然T-2毒素没有改变猪
肠道上皮细胞的完整性,但是增加细菌易位[31]。通过饲喂21天低剂量T-2毒素,回肠淋巴结中CD21 + B细胞逐渐下降,此外,在T细胞细胞因子的基因表达谱在实验组和队长组中没有区别,如IL-2,IL-4 和IFN-γ[32]。
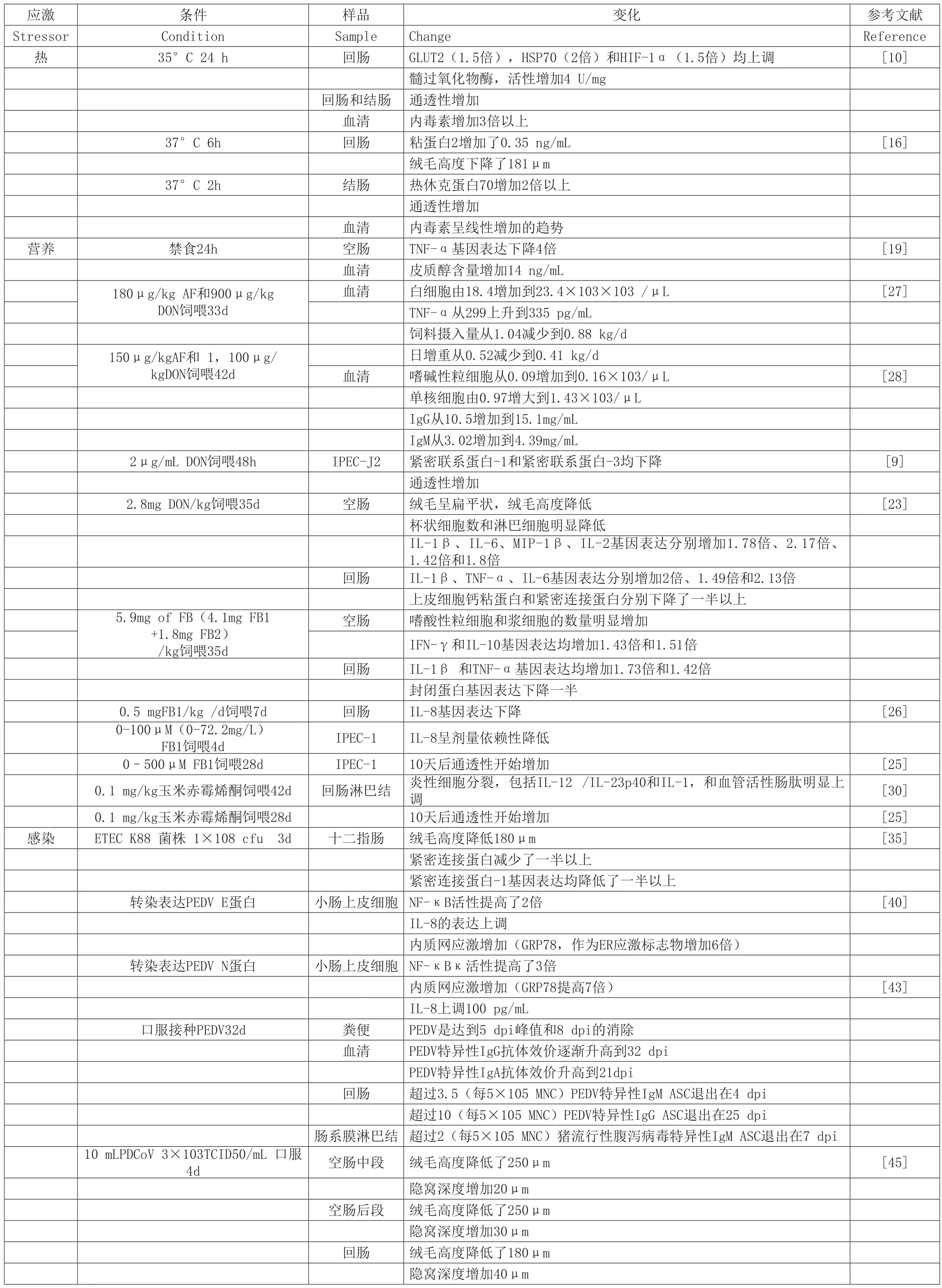
表1 主要应激对肠道免疫的影响
总之,营养缺乏或摄入污染的饲料均可引起猪的肠道炎性细胞因子的变化与功能调节障碍。营养应激作用于亚健康宿主,诱导肠道免疫调节的免疫学机制尚不清楚。
1.3 传染应激
肠道和呼吸道感染是养猪业最常见和频发的疾病。尤其是,肠道感染是已知的能抑制饲料转化率,造成家畜生产率降低的主要诱因[34,35]。不同于其他诱因,由于感染应激的不受控性,故其常造成长期经济损失。由于现在大多数集约化动物养殖场饲养密度大,一旦疾病爆发,动物群内和批次之间的传播速度非常快,此时,如若不进行大规模屠宰,是很难从现场和爆发区内将病原体完全清除。
健康家畜感染数肠道病原体大多数都是接触了感染动物的饮水、饲料或者粪便,肠道病原体进入感染家畜肠道后,通常是破坏上皮屏障的稳态,一些病原体(包括大肠杆菌)引起肠道损伤的机理是通过改变渗透压而引起分泌性腹泻,还有一些是通过上调促炎性细胞因子,产生所谓的炎症性腹泻[36]。肠道病原体可以抑制饲料的摄入和饲料的转化,激活免疫系统从而导致机体不必要的能量损失。养猪业内引起肠道疾病的主要原因有:大肠杆菌,猪流行性腹泻病毒(PEDV),猪三角冠状病毒(PDCoV),和猪传染性胃肠炎病毒(TGEV)。
大肠杆菌指的是一组革兰氏阴性肠道菌群,有些是致病的,并且它们大多数是共生的。Pathogenic E. coli,也叫产肠毒素大肠杆菌(ETEC),已知其是引起家畜脱水性腹泻,降低饲料转化率和生长性能的细菌。有报道,ETEC能缩短绒毛长度和小肠隐窝深度,它还可通过放宽上皮屏障从而抑制肠上皮细胞的TJ表达。已有研究报道通过补充植物乳杆菌[37],酿酒酵母[38],壳聚糖[39]和血管活性肠肽[40],能降低ETEC感染。
猪流行性腹泻病毒是一种冠状病毒,曾只在东亚传播,但2013以后在美国频发。一旦病毒爆发,由于没有有效治疗方案,即使使用抗生素也无效,导致养殖场损失惨重[41]。现有专家建议PEDV通过改变上皮细胞的微丝,从而增加上皮屏障对TLR2、3、9的通透性[42]。不仅上皮细胞,免疫细胞也可以对这种病毒产生免疫细胞表达TLRs。B细胞位于粘膜组织如十二指肠黏膜固有层,能产生比脾脏、血液和全身淋巴组织更多的IgG和IgA[43]。感染PEDV病毒的猪,单核细胞来源的树突状细胞产生大量的炎性细胞因子,包括IL-12,并增强T细胞增殖[44]。
PDCoV和TGEV比大肠杆菌或猪流行性腹泻病毒没有那么大的感染率,但他们仍然是影响养猪业的重要肠道冠状病毒。PDCoV曾在香港(2009)和美国(2014)发现。pdcov病理学症状包括脱水、体重减轻,相比其他病原体感染,症状不明显[45]。但是,当同时感染PEDV和pdcov是会呈协同病理。猪传染性胃肠炎病毒经常在亚洲和美国引起仔猪呕吐,严重时可引起死亡,据推测,这种病毒与猪呼吸道冠状病具有同源性[46]。
总之,当发生感染,尤其是慢性感染,会导致养猪业持续性的经济损失。鉴于目前也没有办法提高家畜对感染的耐受性,现在治疗和消灭病原体的方法只能是使用抗生素或疫苗,所以现在我们应该继续努力,来研究感染力对家畜健康和生长性能产生的影响。
2 结论
现在多种因素都阻碍家畜生长性能,威胁着家畜健康。本文重点研究了猪肠道免疫系统的主要应激因素,包括温度、营养、感染等。大量的研究集中在猪肠道的结合力检查TJ蛋白的通透性,促炎细胞因子或基因型(表1)。然而,现今我们还不能解释应激条件下猪肠道免疫系统的确切机制,加之猪的免疫系统不能直接外推到人类和小鼠上,例如,CD4CD8+T细胞在外周免疫系统[47]。在不久的将来,以下几点将成为主要研究方向:1.免疫细胞的识别和功能性研究,如小肠先天淋巴样细胞;2.免疫细胞和肠上皮细胞的交叉免疫作用;3.系统免疫应答和肠道免疫系统的改变;4.多条件刺激下,宿主肠道免疫系统的研究。
[1]Wilson AD,Haverson K,Southgate K,Bland PW,Stokes CR,Bailey M. Expression of major histocompatibility complex class II antigens on normal porcine intestinal endothelium[J].Immunology,1996,(88):98-103.
[2]Lee IK,Son YM,Ju YJ,Song SK,Gu M,Song KD,Lee HC,Woo JS,Seol JG,Park SM,Han SH,Yun CH.Survival of porcine fibroblasts enhanced by human FasL and dexamethasone-treated human dendritic cells in vitro[J]. Transpl Immunol,2014,(30):99-106.
[3]Gebert A,Rothkotter HJ,Pabst R. M cells in Peyer’s patches of the intestine[J].Int Rev Cytol,1996,(167):91-159.
[4]Cheon IS,Park SM,Lee HJ,Hong JE,Ji SY,Shim BS,Kim KH,Heo PS,Kim YY,Jung HJ,Ka H,Han SH,Song M,Yun CH. Functional characteristics of porcine peripheral T cells stimulated with IL-2 or IL-2 andPMA[J].Res Vet Sci,2014,(96):54-61.
[5]Kayama H,Takeda K. Regulation of intestinal homeostasis by innate and adaptive immunity[J].Int Immunol,2012,(24):673-680.
[6]Hayes MR,Mietlicki-Baase EG,Kanoski SE,De Jonghe BC.Incretins and amylin:Neuroendocrine communication between the gut,pancreas,and brain in control of food intake and blood glucose[J].Annu Rev Nutr,2014,(34):237-260.
[7]Upadhyay RC. Impact of climate change on livestock production and health[M].Proceeding of the ICICCA;Sri Lanka,2011:19-39.
[8]St-Pierre NR,Cobanov B,Schnitkey G. Economic Losses from Heat Stress by US Livestock Industries[J].J Dairy Sci,2003,(86):52-77.
[9]Gu MJ,Song SK,Park SM,Lee IK,Yun CH. Bacillus subtilis protects porcine intestinal barrier from deoxynivalenol via improved zonula occludens-1 expression[J].Asian Australas J Anim Sci,2014,(27):580-586.
[10]Pearce SC,Mani V,Boddicker RL,Johnson JS,Weber TE,Ross JW,Rhoads RP,Baumgard LH,Gabler NK.Heat stress reduces intestinal barrier integrity and favors intestinal glucose transport in growing pigs[J]. PLoS One,2013,(8):70215.
[11]Turner JR,Rill BK,Carlson SL,Carnes D,Kerner R,Mrsny RJ,Madara JL. Physiological regulation of epithelial tight junctions is associated with myosin light-chain phosphorylation[J].Am J Physiol-Cell Phys,1997,(273):C1378-C1385.
[12]Meng D,Hu Y,Xiao C,Wei T,Zou Q,Wang M. Chronic heat stress inhibits immune responses to H5N1 vaccination through regulating CD4(+)CD25(+)Foxp3(+)Tregs[J].Biomed Res Int,2013,(16):859.
[13]Trent MS,Stead CM,Tran AX,Hankins JV. Diversity of endotoxin and its impact on pathogenesis[J].J Endotoxin Res,2006,(12):205-223.
[14]Im J,Baik JE,Kim KW,Kang SS,Jeon JH,Park OJ,Kim HY,Kum KY,Yun CH,Han SH. Enterococcus faecalis lipoteichoic acid suppresses Aggregatibacter actinomycetemcomitans lipopolysaccharide-induced IL-8 expression in human periodontal ligament cells[J].Int Immunol,2015,(27):381-391.
[15]Lam JS,Taylor VL,Islam ST,Hao Y,Kocincova D. Genetic and Functional Diversity of Pseudomonas aeruginosa Lipopolysaccharide[J].Front Microbiol,2011,(2):118.
[16]Pearce SC,Sanz-Fernandez MV,Hollis JH,Baumgard LH,Gabler NK. Short-term exposure to heat stress attenuates appetite and intestinal integrity in growing pigs[J].J Anim Sci,2014,(92):5444-5454.
[17]Shen YB,Weaver AC,Kim SW. Effect of feed grade L-methionine on growth performance and gut health in nursery pigs compared with conventional DL-methionine[J].J Anim Sci,2014,(92):5530-5539.
[18]Zhao Y,Weaver AC,Fellner V,Payne RL,Kim SW. Amino acid fortified diets for weanling pigs replacing fish meal and whey protein concentrate:Effects on growth,immune status,and gut health[J].J Anim Sci Biotechnol,2014,(5):57.
[19]Horn N,Ruch F,Miller G,Ajuwon KM,Adeola O. Impact of acute water and feed deprivation events on growth performance,intestinal characteristics,and serum stress markers in weaned pigs[J].J Anim Sci,2014,(92):4407-4416.
[20]Wang W,Wu Z,Lin G,Hu S,Wang B,Dai Z,Wu G. Glycine stimulates protein synthesis and inhibits oxidative stress in pig small intestinal epithelial cells[J].J Nutr,2014,(144):1540-1548.
[21]Weaver AC,Campbell JM,Crenshaw JD,Polo J,Kim SW. Efficacy of dietary spray dried plasma protein to mitigate the negative effects on performance of pigs fed diets with corn naturally contaminated with multiple mycotoxins[J].J Anim Sci,2014,(92):3878-3886.
[22]Gu MJ,Song SK,Lee IK,Ko S,Han SE,Bae S,Ji SY,Park BC,Song KD,Lee HK,Han SH,Yun CH. Barrier protection via Toll-like receptor 2 signaling in porcine intestinal epithelial cells damaged by deoxynivalnol[J].Vet Res,2016,(47):25.
[23]Bracarense AP,Lucioli J,Grenier B,Drociunas Pacheco G,Moll WD,Schatzmayr G,Oswald IP. Chronic ingestion of deoxynivalenol and fumonisin,alone or in interaction,induces morphological and immunological changes in the intestine of piglets[J].Br J Nutr,2012,(107):1776-1786.
[24]Wu L,Liao P,He L,Ren W,Yin J,Duan J,Li T. Growth performance,serum biochemical profile,jejuna morphology,and the expression of nutrients transporter genes in deoxynivalenol(DON)-challenged growing pigs[J].BMC Vet Res,2015,(11):144.
[25]Bouhet S,Hourcade E,Loiseau N,Fikry A,Martinez S,Roselli M,Galtier P,Mengheri E,Oswald IP. The mycotoxin fumonisin B1 alters the proliferation and the barrier function of porcine intestinal epithelial cells[J].Toxicol Sci,2004,(77):165-171.
[26]Bouhet S,Le Dorze E,Peres S,Fairbrother JM,Oswald IP.Mycotoxin fumonisin B1 selectively downregulates the basal IL-8 expression in pig intestine:in vivo and in vitro studies[J].Food Chem Toxicol,2006,(44):1768-1773.
[27]Chaytor AC,See MT,Hansen JA,de Souza AL,Middleton TF,Kim SW. Effects of chronic exposure of diets with reduced concentrations of aflatoxin and deoxynivalenol on growth and immune status of pigs[J].J Anim Sci,2011,(89):124-135.
[28]Weaver AC,See MT,Hansen JA,Kim YB,De Souza ALP,Middleton TF,Kim SW. The use of feed additives to reduce the effects of aflatoxin and deoxynivalenol on pig growth,organ health and immune status during chronic exposure[J].Toxins,2013,(5):1261-1281.
[29]Weaver AC,See MT,Kim SW. Protective effect of two yeast based feed additives on pigs chronically exposed to deoxynivalenol and zearalenone[J].Toxins,2014,(6):3336-3353.
[30]Obremski K,Gonkowski S,Wojtacha P. Zearalenone-induced changes in the lymphoid tissue and mucosal nerve fibers in the porcine ileum[J]. Pol J Vet Sci,2015,(18):357-365.
[31]Obremski K,Wojtacha P,Podlasz P,Zmigrodzka M. The influence of experimental administration of low zearalenone doses on the expression of Th1 and Th2 cytokines and on selected subpopulations of lymphocytes in intestinal lymph nodes[J].Pol J Vet Sci,2015,(18):489-497.
[32]Verbrugghe E,Vandenbroucke V,Dhaenens M,Shearer N,Goossens J,De Saeger S,Eeckhout M,D’Herde K,Thompson A,Deforce D,Boyen F,Leyman B,Van Parys A,De Backer P,Haesebrouck F,Croubels S,Pasmans F. T-2 toxin induced Salmonella Typhimurium intoxication results in decreased Salmonella numbers in the cecum contents of pigs,despite marked effects on Salmonella-host cell interactions[J].Vet Res,2012,(43):22.
[33]Obremski K,Podlasz P,Zmigrodzka M,Winnicka A,Wozny M,Brzuzan P,Jakimiuk E,Wojtacha P,Gajecka M,Zielonka L,Gajecki M. The effect of T-2 toxin on percentages of CD4+,CD8+,CD4+ CD8+ and CD21+ly mphocytes,and mRNA expression levels of selected cytokines in porcine ileal Peyer’s patches[J].Pol J Vet Sci,2013,(16):341-349.
[34]Kiarie E,Bhandari S,Scott M,Krause DO,Nyachoti CM. Growth performance and gastrointestinal microbial ecology responses of piglets receiving Saccharomyces cerevisiae fermentation products after an oral challenge with Escherichia coli(K88)[J].J Anim Sci,2011,(89):1062-1078.
[35]Yang KM,Jiang ZY,Zheng CT,Wang L,Yang XF. Effect of Lactobacillus plantarum on diarrhea and intestinal barrier function of young piglets challenged with enterotoxigenic Escherichia coli K88[J].J Anim Sci,2014,(92):1496-1503.
[36]Fairbrother JM,Nadeau E,Gyles CL. Escherichia coli in postweaning diarrhea in pigs:An update on bacterial types,pathogenesis,and prevention strategies[J].Anim Health Res Rev,2005,(6):17-39.
[37]Yang KM,Jiang ZY,Zheng CT,Wang L,Yang XF. Effect of Lactobacillus plantarum on diarrhea and intestinal barrier function of young piglets challenged with enterotoxigenic Escherichia coli K88[J].J Anim Sci,2014,(92):1496-1503.
[38]Collier CT,Carroll JA,Ballou MA,Starkey JD,Sparks JC. Oral administration of Saccharomyces cerevisiae boulardii reduces mortality associated with immune and cortisol responses to Escherichia coli endotoxin in pigs[J].J Anim Sci,2011,(89):52-58.
[39]Xiao D,Tang Z,Yin Y,Zhang B,Hu X,Feng Z,Wang J.Effects of dietary administering chitosan on growth performance,jejunal morphology,jejunal mucosal sIgA,occludin,claudin-1 and TLR4 expression in weaned piglets challenged by enterotoxigenic Escherichia coli[J].Int Immunopharmacol,2013,(17):670-676.
[40]Xu C,Wang Y,Sun R,Qiao X,Shang X,Niu W. Modulatory effects of vasoactive intestinal peptide on intestinal mucosal immunity and microbial community of weaned piglets challenged by an enterotoxigenic Escherichia coli(K88)[J].PLoS One,2014,(9):104183.
[41]Song D,Park B. Porcine epidemic diarrhoea virus:A comprehensive review of molecular epidemiology,diagnosis,and vaccines[J].Virus Genes,2012,(44):167-175.
[42]Cao L,Ge X,Gao Y,Ren Y,Ren X,Li G. Porcine epidemic diarrhea virus infection induces NF-kappaB activation through the TLR2,TLR3,and TLR9 pathways in porcine intestinal epithelial cells[J].J Gen Virol,2015,(96):1757-1767.
[43]de Arriba ML,Carvajal A,Pozo J,Rubio P. Isotype-specific antibody-secreting cells in systemic and mucosal associated lymphoid tissues and antibody responses in serum of conventional pigs inoculated with PEDV[J].Vet Immunol Immunopathol,2002,(84):1-16.
[44]Gao Q,Zhao S,Qin T,Yin Y,Yang Q. Effects of porcine epidemic diarrhea virus on porcine monocyte-derived dendritic cells and intestinal dendritic cells[J].Vet Microbiol,2015,(179):131-141.
[45]Chen Q,Gauger P,Stafne M,Thomas J,Arruda P,Burrough E,Madson D,Brodie J,Magstadt D,Derscheid R,Welch M,Zhang J. Pathogenicity and pathogenesis of a United States porcine deltacoronavirus cell culture isolate in 5-day-old neonatal piglets[J].Virology,2015,(482):51-59.
[46]Kim L,Hayes J,Lewis P,Parwani AV,Chang KO,Saif LJ. Molecular characterization and pathogenesis of transmissible gastroenteritis coronavirus(TGEV)and porcine respiratory coronavirus(PRCV)field isolates cocirculating in a swine herd[J].Arch Virol,2000,(145):1133-1147.
[47]Cheon IS,Park SM,Lee HJ,Hong JE,Ji SY,Shim BS,Kim KH,Heo PS,Kim YY,Jung HJ,Ka H,Han SH,Song M,Yun CH. Functional characteristics of porcine peripheral T cells stimulated with IL-2 or IL-2 and PMA[J]. Res Vet Sci,2014,(96):54-61.