一对手性铕(Ⅲ)配合物的合成和铁电性质
2017-11-13张小朋李承辉
刘 建 张小朋 李承辉
一对手性铕(Ⅲ)配合物的合成和铁电性质
刘 建*,1,2张小朋3李承辉2
(1南京林业大学化学工程学院,江苏省生物质绿色燃料与化学品重点实验室,南京 210037)
(2南京大学化学化工学院,配位化学国家重点实验室,人工微结构科学与技术协同创新中心,南京 210023)
(3海南师范大学化学与化工学院,海南省水环境污染治理与资源化重点实验室,海口 571158)
合成并表征了一对蒎烯修饰的稀土铕(Ⅲ)配合物 Eu(TTA)3L1a(1a)和 Eu(TTA)3L1b(1b)(TTA=2-噻吩甲酰三氟丙酮,L1a=(+)-4,5-双蒎烯-2,2′-联吡啶,L1b=(-)-4,5-双蒎烯-2,2′-联吡啶)。 化合物的手性特征通过 X 射线单晶结构和圆二色光谱进行了表征。配合物1a结晶于极性空间群P1,中心Eu(Ⅲ)离子呈现出严重扭曲的四方反棱柱体的配位环境。鉴于非中心对称结构的特性,配合物1a也表现出明显的铁电性质。
配位化学;手性铕(Ⅲ)配合物;晶体结构;铁电性质
Recently,multifunctionalmaterialsbased on lanthanide complexes have received much attention due to their potential applications in optoelectronics[1-3].The introduction of chiral environments in lanthanide complexes would lead to a diversification of configuration and crystallize in a polar space group.Thus,interesting properties,such as ferroelectricity,piezoelectricity,triboluminescence,circular polarized luminescence (CPL)and second harmonic generation(SHG)would be expected[4-7].
Ferroelectric materials possessing a spontaneous electric polarization that can be reversed by the application of an external electric field,are of great interest in the field of optics and electronics[8].Compared to the typicalinorganic ABO3-type ferroelectrics,molecular-based ferroelectric materials show some advantages:largerspontaneouspolarization,facile chemical vapor deposition (CVD)and convenient structural modification.Furthermore,owing to the high coordination numbers and the interaction between a central metal ion and its ligand field (LF),the structures of lanthanide complexes could be easily distorted and spontaneous dipole moments can be induced,possibly resulting in interesting multi-ferroic properties.
In previous studies,we have studied multifunctional properties (luminescence,ferroelectricity,magnetism and so on)of chiral lanthanide complexes incorporated pinene functionalized with aromatic amine derivativesaswellassolvent-induced or temperature-induced switching of configurations and properties of these complexes[9-12].A novel Eu(Ⅲ)complex Eu(TTA)3L1a(1a)(TTA=thenoyltrifluoroacetonate,L1a=(+)-4,5-bis(pinene)-2,2′-bipyridine)with a bispinene-containing bipyridyl ligand was used as high efficient luminescent down-shifting material and lead to a significant improvement of the spectral response of c-Si PV module in the UV region[13].
As an extension of these work,the crystal structure and ferroelectric property of 1a has been studied in this paper.The compound 1a crystallizes in anoncentrosymmetricspacegroup (P1),anda distorted square-antiprism configuration exists around the central Eu(Ⅲ) ion,satisfying the prerequisites of ferroelectrics.As expected,complex 1a shows a distinct electric hysteresis loop,making it as potential multifunctional molecular materials.The enantiomer Eu(TTA)3L1b(1b)(L1b=(-)-4,5-bis(pinene)-2,2′-bipyridine)was also prepared,and chiral environments are confirmed by a mirror symmetry of circular dichroism(CD)spectra of 1a and 1b.
1 Experimental
1.1 Chemicals and synthesis
All reagents were purchased from commercial suppliers and used as received.Compounds 1a and 1b were prepared according to our published methods as follows[13]:L1aor L1b(344 mg,1 mmol)was added to a stirred solution of Eu(TTA)3·2H2O (851 mg,1 mmol)in ethanol (40 mL).The resulting mixture was stirred at room temperature for 24 h.The precipitate was collected by filtration to yield the desired product.Crystals of compound 1a suitable for X-ray crystallographic analysis were obtained by slow evaporation over a period of 3 weeks of their CH2Cl2/CH3CH2OH solution.
1.2 Chemical and physical characterizations
Single-crystalX-ray diffraction measurements were carried out on a Bruker SMART APEX CCD based on diffractometer operating at room temperature.Intensities were collected with graphite monochromatized Mo Kα radiation (λ=0.071 073 nm)operating at 50 kV and 30 mA,using ω-2θ scan mode.The data reduction was made with the Bruker SAINT package[14]. Absorption corrections were performed using the SADABS program[15].The structures were solved by direct methods and refined on F2by fullmatrix least-squares using SHELXL-97 with anisotropic displacement parameters for all non-hydrogen atoms in all two structures[16].Hydrogen atoms bonded to the carbon atomswereplaced in calculated positions and refined as riding mode,with dC-H=0.093 nm (methane)or 0.096 nm (methyl)and Uiso(H)=1.2Ueq(Cmethane)or Uiso(H)=1.5Ueq(Cmethyl).All computations were carried out using the SHELXTL-97 program package[16-17].The absorption spectra of complexes 1a and 1b in CH2Cl2solution were recorded on a Shimazu UV-3100 spectrometer.CD spectra were recorded by a Jasco J-810 spectropolarimeter.The Electric hysteresis loops were recorded by a Ferroelectric Tester Precision PremierⅡby using powder samples in pellets at room temperature.CCDC:818185,1a.
2 Results and discussion
2.1 Crystal structure
The crystal data and structure refinements for compound 1a are provided in Table 1.Similar to those europium (Ⅲ) complexes reported previously[4,12],compound 1a is a mononuclear neutral europium(Ⅲ)compound and crystalizes in triclinic system,space group P1.As shown in Fig.1,each molecule contains three β-diketonate anions,one (+)-4,5-bis(pinene)-2,2′-bipyridine,and one eight-coordinated Eu(Ⅲ) ion.Each of the three diketonate anions provides two donor O atoms to coordinate to the Eu(Ⅲ)ion.The other two coordination sites of the Eu(Ⅲ)ion are occupied by two N atomsofthe chiralbipyridine derivative to complete the eight-coordinate configuration.
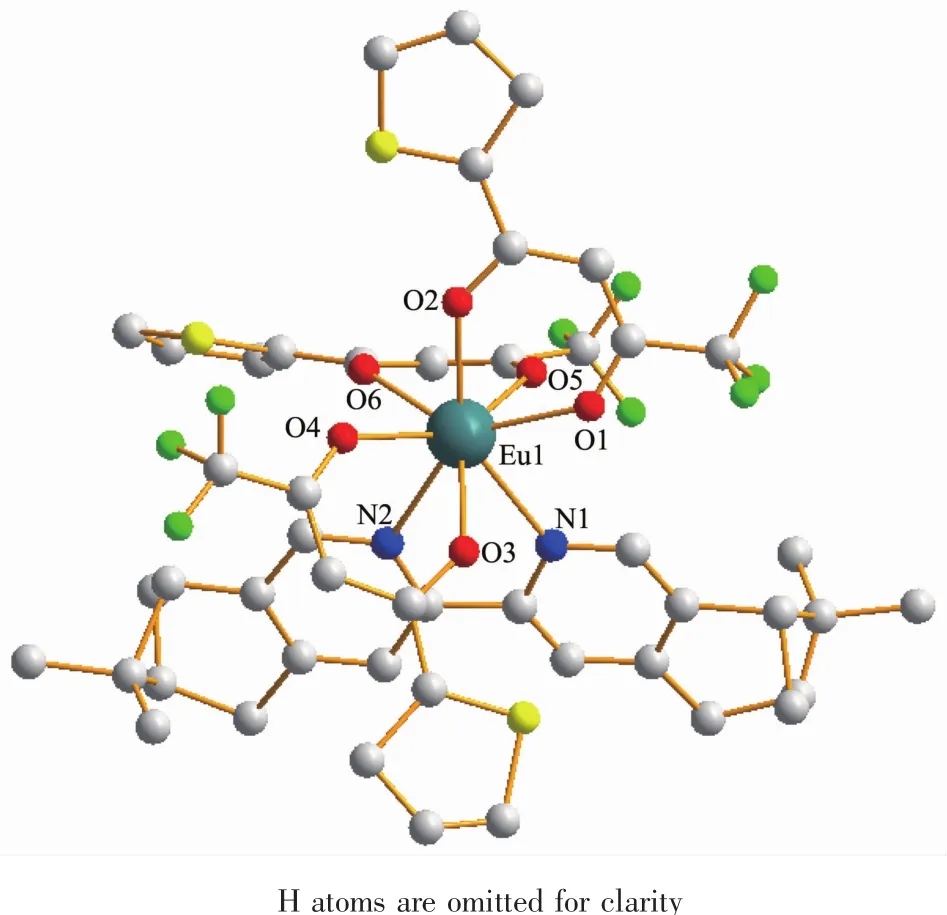
Fig.1 Molecular structure of 1a
Selected bond lengths and angles are summarized in Table 2.The Eu-O bond lengths range from 0.231 8 to 0.238 5 nm,while the two Eu-N bond lengths are 0.258 7(16)and 0.255 6(15)nm,respectively.Eight bonds with different lengths give rise to a strongly distorted square-antiprism environment around the Eu(Ⅲ) ion (Fig.2).The O1-O2-O3-O4 (bottom plane)and O5-O6-N1-N2 atoms (top plane)comprise the two square-basic planesofthe antiprism with mean deviations of 0.002 78 and 0.013 97 nm from each plane,and their dihedral angle is 3.442°.For a regularsquareantiprism,twosquareplanesare parallel to each other and one square rotates 45°from the other.However,in 1a,the top plane rotates only 41.4°from the bottom plane.

Fig.2 Coordination environments of 1a

Table 1 Crystal data and structure refinements for complex 1a

Table 2 Selected bond lengths(nm)and angles(°)for 1a
2.2 Circular dichroism (CD)spectra

Fig.3 UV-Vis and CD spectra of 1a and 1b in 2×10-5 mol·L-1dichloromethane solution
The chirality ofcomplexes 1a and 1b is confirmed by measuring circulardichroism (CD)spectra (Fig.3).Two distinct Cotton effect at 245 and 292 nm can be observed,and the spectra of 1a and 1b are almostmirror-symmetric.These features indicate that 1a and 1b are chiral and enantiomeric to each other.
2.3 Ferroelectric properties
Since complexes 1a crystalizes in a polar space group (P1),the ferroelectric properties of 1a were investigated at298 K with compressed powder samples as shown in Fig.4. The ferroelectric measurements reveal that complex 1a indeed displays an obvious ferroelectric behavior and almost reaches thesaturated polarization statuswith a remnant polarization (Pr)of ca.0.27 μC·cm-2and Ecof ca.7.2 kV·cm-1by applying an electric field of 15 kV·cm-1at 4 Hz.The saturation spontaneous polarization (Ps)is about 0.43 μC·cm-2,which exceeds the value of typical ferroelectrics NaKC4H4O6·4H2O (Rosal salt,Ps=0.25 μC·cm-2).

Fig.4 Ferroelectric hysteresis loop for complex 1a at 298 K
3 Conclusions
In summary,a couple of pinene-containing chiral europium(Ⅲ)complexes 1a and 1b were prepared.The chirality of complexes 1a and 1b is confirmed by measuring circular dichroism (CD)spectra.Complexes 1a crystalizes in P1 space group of triclinic system.The coordination polyhedron of the Eu(Ⅲ)ion in 1a can be described as a siginificantly distorted squareantiprism.The complex 1a exhibited distinct ferroelectric behaviors with a remnant polarization (Pr)of ca.0.27 μC·cm-2and Ecof ca.7.2 kV·cm-1by applying an electric field of 15 kV·cm-1at 4 Hz.
Acknowledgements:This work was supported by the Jiangsu Province Science Foundation for Youths (Grant No.BK20150569),the National Natural Science Foundation of China (Grant No.201502088),the project of Scientific and Technological Support Program in Jiangsu province (Grant No.BE2014147-2),and the Doctoral Fund of the Ministry of Education ofChina for financial support (GrantNo.20120091130002).
[1]Walton J W,Bourdolle A,Butler S J,et al.Chem.Commun.,2013,49(16):1600-1602
[2]Kotova O,Kitchen J A,Lincheneau C,et al.Chem.Eur.J.,2013,19(48):16181-16186
[3]Carr R,Di Bari L,Lo Piano S,et al.Dalton Trans.,2012,41(42):13154-13158
[4]Li D P,Li C H,Wang J,et al.Eur.J.Inorg.Chem.,2009(32):4844-4849
[5]Bozoklu G,Gateau C,Imbert D,et al.J.Am.Chem.Soc.,2012,134(20):8372-8375
[6]Harada T,Tsumatori H,Nishiyama K,et al.Inorg.Chem.,2012,51(12):6476-6485
[7]Li X L,Chen C L,Xiao H P,et al.Dalton Trans.,2013,42(43):15317-15325
[8]Hang T,Zhang W,Ye H Y,et al.Chem.Soc.Rev.,2011,40(7):3577-3598
[9]Li D P,Wang T W,Li C H,et al.Chem.Commun.,2010,46(17):2929-2931
[10]Li D P,Zhang X P,Wang T W,et al.Chem.Commun.,2011,47(24):6867-6869
[11]Liu J,Zhang X P,W T,et al.Inorg.Chem.,2012,51(16):8649-8651
[12]Li X L,Chen K,Liu Y,et al.Angew.Chem.Int.Ed.,2007,46(36):6820-6823
[13]Liu J,Wang K,Zheng W,et al.Prog.Photovoltaics Res.Appl.,2013,21(4):668-675
[14]SAINT-Plus,Ver.6.02,Bruker Analytical X-ray System,Madison,WI,1999.
[15]Sheldrick G M.SADABS,an Empirical Absorption Correction Program,Bruker Analytical X-ray Systems,Madison,WI,1996.
[16]Sheldrick G M.SHELXL-97,Program for Crystal Structure Refinement,University of Göttingen,Germany,1997.
[17]Sheldrick G M.Acta Crystallogr.Sect.A:Found.Crystallogr.,2008,64:112-122
Syntheses and Ferroelectric Properties of a Couple of Chiral Europium(Ⅲ)Complexes
LIU Jian*,1,2ZHANG Xiao-Peng3LI Cheng-Hui2
(1Jiangsu Key Lab of Biomass-based Green Fuels and Chemicals,College of Chemical Engineering,Nanjing Forestry University,Nanjing 210037,China)
(2Collaborative Innovation Center of Advanced Microstructures,State Key laboratory of Coordination Chemistry,School of Chemistry and Chemical Engineering,Nanjing University,Nanjing 210023,China)
(3Key Laboratory of Water Pollution Treatment&Resource Reuse of Hainan Province,College of Chemistry and Chemical Engineering,Hainan Normal University,Haikou 571158,China)
A couple of pinene-containing europium(Ⅲ) complexes,Eu(TTA)3L1a(1a)and Eu(TTA)3L1b(1b)(TTA=thenoyltrifluoroacetonate,L1a=(+)-4,5-bis(pinene)-2,2′-bipyridine,L1b=(-)-4,5-bis(pinene)-2,2′-bipyridine),were prepared and characterized.The chiral characterization was confirmed by single crystal X-ray diffraction and distinct electronic circular dichroism (CD)signals.Complex 1a crystallizes in the polar space group P1,and a strongly distorted square-antiprism environment around the Eu(Ⅲ)ion is exhibited.Due to the non-centrosymmetric structure,distinct ferroelectric behavior of complex 1a was observed as expected.CCDC:818185,1a.
coordination chemistry;chiral europium(Ⅲ)complexes;crystal structure;ferroelectric properties
O614.33+8
A
1001-4861(2017)11-2060-05
10.11862/CJIC.2017.256
2017-09-20。收修改稿日期:2017-10-16。
江苏省自然科学青年基金(No.BK20150569)、国家自然科学青年基金(No.201502088)、江苏省科技支撑计划(No.BE2014147-2)、国家教育部博士点专项基金(No.20120091130002)资助项目。
*通信联系人。E-mail:liu.jian@nju.edu.cn
