Toxic effect of acrylamide on the development of hippocampal neurons of weaning rats
2017-11-08ShengminLaiZitingGuMengmengZhaoXixiaLiYuxinMaLiLuoJingLiu
Sheng-min Lai, Zi-ting Gu, Meng-meng Zhao, Xi-xia Li, Yu-xin Ma, Li Luo, Jing Liu
Department of Human Anatomy and Histoembryology, School of Basic Courses, Guangdong Pharmaceutical University, Guangzhou, Guangdong Province, China
How to cite this article: Lai SM, Gu ZT, Zhao MM, Li XX, Ma YX, Luo L, Liu J (2017) Toxic effect of acrylamide on the development of hippocampal neurons of weaning rats. Neural Regen Res 12(10):1648-1654.
Funding: is study was supported by the Guangdong Provincial Department of Science and Technology in China, No. 2016A020225007.
Toxic effect of acrylamide on the development of hippocampal neurons of weaning rats
Sheng-min Lai, Zi-ting Gu, Meng-meng Zhao, Xi-xia Li, Yu-xin Ma, Li Luo, Jing Liu*
Department of Human Anatomy and Histoembryology, School of Basic Courses, Guangdong Pharmaceutical University, Guangzhou, Guangdong Province, China
How to cite this article: Lai SM, Gu ZT, Zhao MM, Li XX, Ma YX, Luo L, Liu J (2017) Toxic effect of acrylamide on the development of hippocampal neurons of weaning rats. Neural Regen Res 12(10):1648-1654.
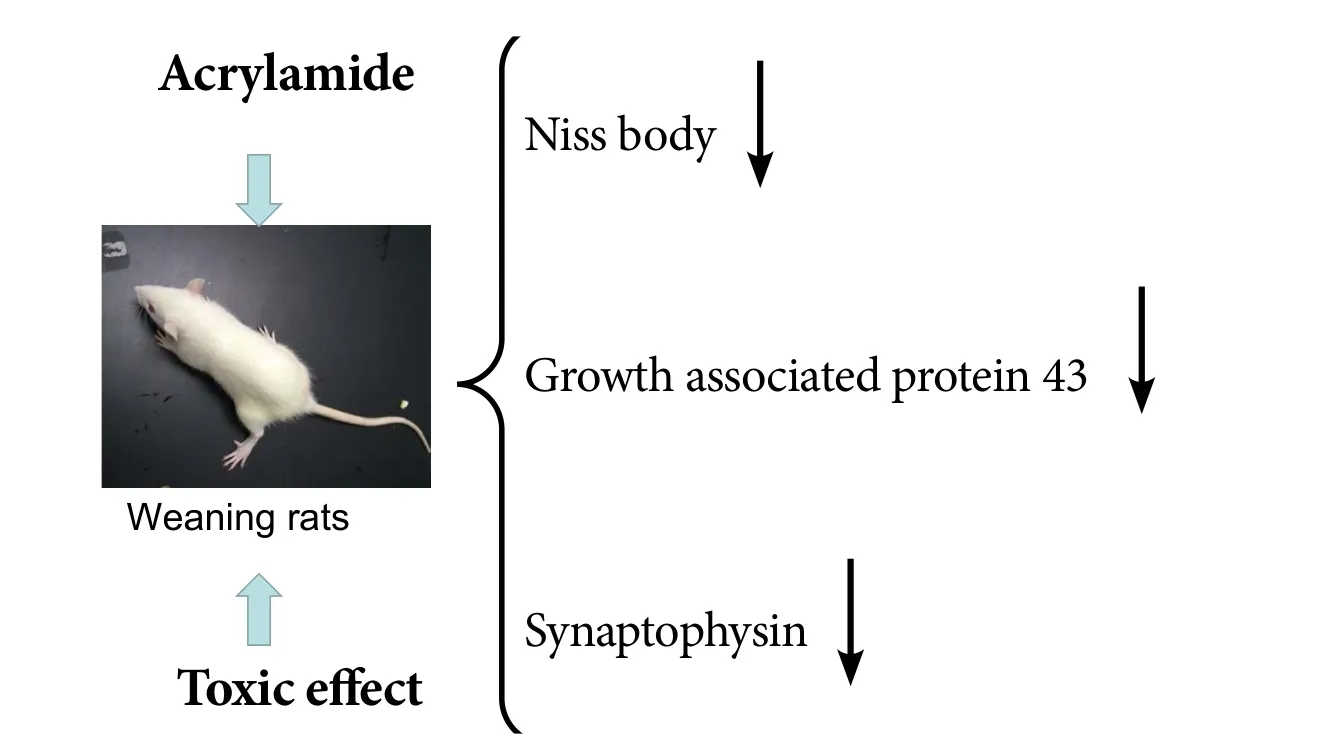
Although numerous studies have examined the neurotoxicity of acrylamide in adult animals, the effects on neuronal development in the embryonic and lactational periods are largely unknown.us, we examined the toxicity of acrylamide on neuronal development in the hippocampus of fetal rats during pregnancy. Sprague-Dawley rats were mated with male rats at a 1:1 ratio. Rats were administered 0, 5, 10 or 20 mg/kg acrylamide intragastrically from embryonic days 6–21.e gait scores were examined in pregnant rats in each group to analyze maternal toxicity. Eight weaning rats from each group were also euthanized on postnatal day 21 for follow-up studies. Nissl staining was used to observe histological change in the hippocampus. Immunohistochemistry was conducted to observe the condition of neurites,including dendrites and axons. Western blot assay was used to measure the expression levels of the specific nerve axon membrane protein,growth associated protein 43, and the presynaptic vesicle membrane specific protein, synaptophysin.e gait scores of gravid rats significantly increased, suggesting that acrylamide induced maternal motor dysfunction.e number of neurons, as well as expression of growth associated protein 43 and synaptophysin, was reduced with increasing acrylamide dose in postnatal day 21 weaning rats.ese data suggest that acrylamide exerts dose-dependent toxic effects on the growth and development of hippocampal neurons of weaning rats.
nerve regeneration; acrylamide; hippocampus; neurons; developmental toxicity; growth associated protein 43; synaptophysin;weaning rats; dentate gyrus; protein; developmental neurobiology; neural regeneration
Introduction
Acrylamide (ACR) is a water-soluble vinyl monomer used to synthesize polyacrylamide, which has broad applications in the petrochemical, water treatment, paper making, textile manufacturing and scientific research fields (Exon, 2006;Doerge et al., 2008). Although polyacrylamide is not considered toxic, ACR compounds can oen contain traces of toxic monomers (Lipworth et al., 2012). For example, monoacrylamide was reported to exhibit neurotoxicity, reproductive toxicity and carcinogenicity in various animal species (Lehning et al., 2003; Sen et al., 2015).is has drawn extensive global interest as ACR was detected during the processing of starchy food treated at a temperature > 120°C (Lingnert,et al, 2002). The average daily intake of ACR for adults is approximately 0.5 μg/kg body weight (WHO, 2002; Sansano et al., 2017). Interestingly, children may have two to three times more ACR than adults, as their relative intake may be increased by increased snacking and lower body weights(Konings et al., 2003; Svensson et al., 2003; Garey et al.,2005). Given this increasing risk of ACR exposure in chil-dren, it is important to assess the potential toxic effects of ACR on nervous system development.
In adult studies, ACR exposure is known to cause axonal neuropathy, which can affect both the central and peripheral nervous systems, and is associated with ataxia, weight loss and skeletal muscle weakness.e main pathological characteristics of ACR exposure involve distal axon swelling and degeneration. However, the effects of ACR exposure during embryonic mammalian development remain unclear (Exon,2006; Manuela et al., 2013).
Materials and Methods
Animals and experimental design
Thirty-two male and 32 female specific-pathogen-free Sprague-Dawley rats aged 5 weeks and weighing 150–180 g were provided by the Guangdong Medical Laboratory Animal Center, China (license No. SCXK (Yue) 2008-0002). All rats were maintained under controlled conditions at 24 ± 1°C and a relative humidity of 55 ± 5% in a 12-hour light/dark cycle, and were allowed free access to chow and water.
The study protocol was approved by the Animal Ethics Committee of Guangdong Pharmaceutical University of China (approval No. GDPULAC2012117).e experimental procedure followed the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals(NIH Publication No. 85-23, revised 1986). All efforts were made to minimize animal suffering and reduce the number of animals.
The female rats were mated 1:1 with males after acclimation for 1 week. We checked daily for vaginal plugs, the presence of which indicated day 0 of pregnancy.irty-two pregnant rats were randomly divided into four groups (n =8 per group) before drug administration. Rats in each group were treated by intragastric administration. Control group animals received 0.9% saline, while rats in the 5, 10 and 20 mg/kg ACR groups were treated with 5, 10 and 20 mg/kg ACR (analytical grade, 99.9%; Yongda Inc., Tianjin, China),respectively. Intragastric administration was repeated daily for 15 days from pregnancy days 6–21 (the neural tube is generated from the 6thday of pregnancy, while rats are born on the 21stday).e optimal dose of ACR was chosen based on a previous study, with modifications, which described developmental gait disorders induced by exposure to similar ACR concentrations in female rats (Takahashi et al., 2008;Ma et al., 2011; Yao et al., 2014). Aer the dams gave birth,eight pups were randomly selected from each group for follow-up experiments.e general situation of gravid rats and offspring was closely monitored, and the body weight was recorded weekly during pregnancy and in offspring. At postnatal day 21, the weaning rats were euthanized under general anesthesia, and in each group, the brains were either fixed and paraffin-embedded for immunohistochemistry (n= 8 per group) or collected for western blot assay (n = 8 per group).
Gait scores
In each pregnant rat, the gait scores were examined weekly for 5 weeks (from the 6thday of pregnancy), following a previously described method (Noble et al., 2005; Ogawa et al., 2012; Prasad and Muralidhara, 2013). In brief, rats were placed individually on an empty flat surface and observed for 3 minutes to assign subjective gait scores divided into four levels, as follows: Level 1: the rat was active and not affected (score 1); level 2: the rat was slightly affected and characterized by weakness, mild ataxia and foot splay (score 2); level 3: the rat was moderately affected and characterized by reduced activity and obvious foot splay with limb spread during ambulation (score 3); level 4: the rat was severely affected and displayed reduced activity, obvious foot splay with limb spread during ambulation, inability to support body weight, dragging of the hind-limbs and inability to rear(score 4).
Nissl staining of hippocampal neurons
Brain tissues from postnatal day 21 weaning rats were fixed in neutral formalin, dehydrated in graded ethanol and embedded in paraffin. Paraffin-embedded brain coronal sections (5 μm thick) were dewaxed with xylene and rehydrated in graded ethanol. The sections were washed three times with distilled water, and then stained with 1% toluidine blue at 60°C for 40 minutes, or with Cresyl violet at 60°C for 30 seconds. The stained sections were dehydrated in graded ethanol solutions, permeabilized with xylene, mounted with neutral balsam (Yiyang Inc., Shanghai, China) and photographed with a Zeiss microscope (Baden Wurttemberg,Germany). Optical density values of positive staining were determined and the positive expression area fraction in full field image was calculated.ese procedures were performed using ImageJ soware (National Institutes of Health,Bethesda, MD, USA).
Immunohistochemistry
Paraffin-embedded brain coronal sections (5 μm thick) from postnatal day 21 weaning rats were also used for immunohistochemistry. The sections with intact hippocampi were dewaxed with xylene and rehydrated in graded ethanol solutions, followed by heat-mediated antigen retrieval using 0.01 M citrate buffer (trisodium citrate dihydrate, citric acid, pH 6.0) in a microwave at 95°C. Endogenous peroxidase activity was quenched by incubation in 3% hydrogen peroxide in phosphate buffer saline (PBS) for 30 minutes, and sections were washed in PBS for 5 minutes. Sections were incubated for 1 hour in blocking solution (10% albumin from bovine serum) at room temperature, and then incubated overnight with rabbit SYP polyclonal antibody (A6344; ABclonal Inc.,Boston, MA, USA) or rabbit GAP-43 polyclonal antibody(A6376; ABclonal Inc.) in blocking solution at 4°C (using PBS as negative control). Sections were then washed in PBS and incubated with horseradish peroxidase AffiniPure goat anti-rabbit IgG (Earthox LLC, San Francisco, CA, USA)for 40 minutes at 37°C. Sections were washed with PBS,incubated for 2 minutes in a solution of 0.02% diaminobenzidine, rinsed in distilled water, counterstained with hematoxylin for 1 minute, mounted with neutral balsam and then photographed with a Zeiss microscope (Baden Wurttemberg, Germany).e optical density value of positive expression was measured by ImageJ soware, and the positive expression area fraction in the full field image was calculated. All procedures were performed in three different sections for each animal, and the mean value was used for analysis.
western blot assay
For western blot assay, the hippocampal tissues from postnatal day 1 rat brains were isolated and homogenized in icecold radioimmune precipitation assay lysis buffer (Beyotime Inc., Jiangsu, China).e homogenates were centrifuged at 12,000 revolutions per minute for 15 minutes at 4°C, and the supernatants then collected for protein concentration using the BCA-100 protein assay kit (KeyGen BioTECH, Nanjing,China). An equal concentration of protein (20 μg) from each sample was boiled for 10 minutes in 5 × sodium dodecyl sulfate buffer (Jetway Biotech, Guangzhou, China), and then loaded onto 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred onto polyvinylidene fluoride membranes (Bio-Rad, Hercules, CA, USA).e membranes were blocked with 5% non-fat milk powder for 60 minutes at 37°C, and then incubated overnight at 4°C with rabbit SYP polyclonal antibody (1:1,000 dilution; A6344; ABclonal Inc.) or rabbit GAP-43 polyclonal antibody (1:1,000 dilution; A6376; ABclonal Inc.).
After incubation with horseradish peroxidase AffiniPure goat anti-rabbit IgG (Earthox LLC) in a recommended dilution of 1:100,000, the relative levels of protein expression were detected with the Sper ECL Assay Kit (Earthox LLC)using rabbit anti-β-tubulin (AC008; ABclonal Inc.) as an internal control. For data analysis, quantification of relative protein levels was presented as gray values, and the ratio of target protein in each group to β-tubulin was measured.e ratio of target protein to β-tubulin in the control group was set as a reference, and this ratio was compared with the control group. Image analysis was performed with ImageJ soware.
Statistical analysis
All analyses were performed using SPSS 23.0 statistical so-ware (IBM, Armonk, NY, USA).All data are presented as the mean ± SEM (n = 8 per group). One-way analysis of variance followed by Tukey post hoc test was used for statistical analysis. Statistical significance was set to α = 0.05.
Results
Effects of body weight and gait scores induced by ACR
As shown inFigure 1A, there was no difference in body weight between the groups at the beginning of ACR exposure (P > 0.05). However, there was a significant decrease in the mean body weight in the ACR 20 mg/kg group at week 3 compared with the control group (P < 0.05). The mean body weight in the ACR 20 mg/kg group was significantly decreased from 3 weeks until the end of the experiment (P< 0.01). At the end of the experiment, the average weight of the rats in the ACR 10 mg/kg group was decreased by 15.7%compared with the control group, while the body weight of rats in the ACR 20 mg/kg group was reduced by 23.7% (P <0.01). A similar phenomenon occurred in weaning rats (P <0.01) (Figure 1B).
Results of Nissl staining on hippocampal neurons
Dose-dependent changes in hippocampal GAP-43 expression
GAP-43 is a phosphorylated protein specifically expressed on the neuronal cell membrane, and is important for adjusting neuronal responses to axonal guidance signaling in neuronal development, as well as axon growth and structure. As such, GAP-43 is a widely used molecular marker of neuronal development (Chirwa et al., 2005). There was no difference in the volume of GAP-43-immunoreactive cells between the control group and ACR 5 mg/kg group in the hippocampal dentate gyrus at postnatal day 21 (Figure 3).However, GAP-43 levels were significantly decreased in the ACR 10 mg/kg group (P < 0.05), and even further reduced in the ACR 20 mg/kg group (P < 0.01 vs. ACR 10 mg/kg group). Western blot assay results showed a similar trend(Figure 4). GAP-43 expression in ACR 20 mg/kg group was decreased significantly (P < 0.01 vs. control group).
ACR inhibited SYP expression in the hippocampal dentate gyrus
SYP is a calcium-binding glycoprotein (molecular weight of 38 kDa) that forms an abundant integral membrane protein constituent of neural synaptic vesicles (Evans et al., 2005;Rossetti et al., 2016). In control animals, SYP immunoreactivity was mainly found in the dentate gyrus granular layer of the synapses, but not in the nucleus. Image analysis showed that compared with the control group, there was a significant reduction in SYP-immunoreactive neurons in the hippocampal dentate gyrus in the ACR 20 mg/kg group (P <0.05), but not in other groups (Figure 5). Western blot assay results showed a similar trend (Figure 6). SYP expression in ACR 20 mg/kg group was decreased significantly (P < 0.01 vs. control group).
Discussion
ACR neurotoxicity has been previously reported in adult animal studies (Lopachin et al., 2012; Tian et al., 2015), which leads to axonal lesions in the central and peripheral nervous systems, with associated weight loss, skeletal muscle weakness and ataxia.e pathological features also include peripheral nerve axonal swelling and degeneration (Shi et al., 2012; He et al., 2017). In the present study, there were no obvious pathological changes in gravid rats in the ACR 5 mg/kg group,while mild ataxia appeared in the ACR 10 mg/kg group and typical ataxia and hind limb weakness were observed in the ACR 20 mg/kg group.ese findings are consistent with the clinical symptoms in patients with ACR toxicity and the pathology observed to mature neurons.
Nissl bodies are a characteristic structure in newborn neurons. Nissl bodies are mainly involved in protein synthesis, and are essential for advanced brain activity, including learning and memory (Niu et al., 2008; Cheng et al., 2010).The expression of Nissl bodies is strongly associated with neuronal function. Neurons constantly utilize proteins during excitatory transmission, which requires new protein synthesis by Nissl bodies to prevent protein depletion. In the present study, we found a decrease in the number of Nissl bodies, and they showed light staining, aer ACR exposure,reflecting partial inhibition of neuronal protein synthesis.ese findings also suggest that the toxic effects of ACR on neuronal development may be associated with reduced neuronal proliferation.us, a decrease in the number of new neurons and decreased protein synthesis may have an overall detrimental effect on newborn brain function.
To verify this hypothesis, we tested two related markers,GAP-43 and SYP. Given that the expression level of GAP-43 is related to neuronal growth (Lai et al., 2011), we used GAP-43 protein expression to assess the function of hippocampal dentate gyrus neurons. Numerous studies have also shown that GAP-43 protein is important for promoting axonal elongation and maintaining axonal morphology. Furthermore,GAP-43 protein was reported to be widely distributed at the tip of the synapse, but rarely in dendrites, suggesting that GAP-43 may modulate the transmission of neural signals.e axonal length of PC12 cells was also positively correlated with GAP-43 mRNA expression (Baetge et al., 1991; Benowitz et al., 1997), supporting a role in axonal growth. In the present study, the decreased expression of GAP-43 following ACR exposure suggests an impairment in neuronal development,including effects on axonal growth and synaptic inhibition.
SYP is a marker of synaptogenesis during embryonic developmental. SYP is widely used as a specific marker of the presynaptic membrane, and for detection of the density and distribution of synapses (Cabalka et al., 1990; Dahlqvist et al., 2004). Previous studies have also reported that increased SYP levels are associated with increased synaptic development (Leclerc et al., 1989; Liu et al., 2016). In the present study, long-term exposure to ACR may have caused reduced axonal and dendritic growth in developing hippocampal neurons, with associated functional changes. Indeed, a decrease in SYP expression may reflect a decrease in nerve transmission, which may have a detrimental effect on cognitive and limb functions (Robinson et al., 2011).
Accumulating evidence also suggests that the proliferation and differentiation of neurons during hippocampal neurogenesis can be altered by deficits in GAP-43 and SYP during development (Groves et al., 2005; Xiao et al., 2015; Sakharkar et al., 2016). Neurites, including axons and dendrites, are critical for the morphological and functional development of immature neurons. Abnormal expression of GAP-43 and SYP were previously reported to reflect central nervous system dysfunction during early neuronal development (Wang et al., 2014; Williams et al., 2016). In turn, this may result in biochemical alterations in neural metabolism and axonal transport, as previously reported following ARC exposure(Honig and Rosenberg, 2000; LoPachin et al., 2004), which may be aggravated by decreased GAP-43 expression.
In summary, we found that fetal ACR exposure during pregnancy was associated with reduced expression of GAP-43 and SYP in the hippocampus of postnatal day 21 off-spring, which may be associated with inhibition of neuronal proliferation and differentiation and synaptic function.

Figure 1 Body weight and gait scores changes induced by acrylamide (ACR) exposure.

Figure 2 Nissl staining of hippocampal neurons of postnatal day 21 weaning rats.
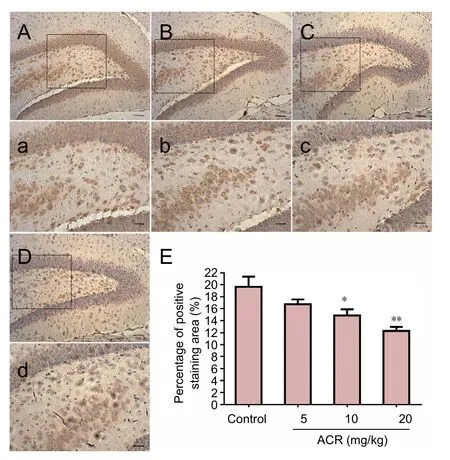
Figure 3 Percentage of positively stained area for growth associated protein 43 (GAP-43) in hippocampal neurons of postnatal day 21 weaning rats.
Acknowledgments:The authors would like to thank Guo-ying Li from Guangdong Medical Association of China for study design and De-hui Yang from Guangdong Pharmaceutical University of China for the assistance in collecting data.
Author contributions: SML and MMZ designed the study. XXL and SML performed experiment. YXM and LL analyzed data. SML and ZTG wrote the paper. JL checked the paper and advised to the content revision. All authors approved the final version of the paper.
Conflicts of interest:None declared.
Research ethics:e study protocol was approved by the Animal Ethics Committee of Guangdong Pharmaceutical University of China (approval No. gdpulac 2012117). The experimental procedure followed the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1986).
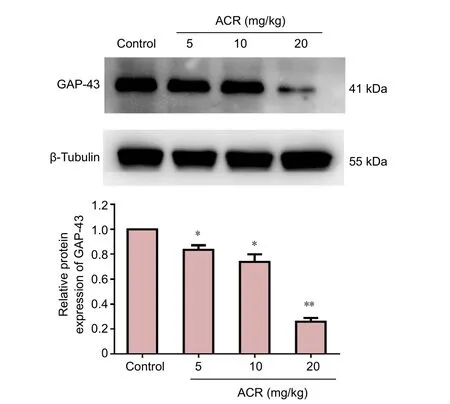
Figure 4 western blot assay of growth associated protein 43 (GAP-43)in the hippocampal dentate gyrus of postnatal day 21 weaning rats.

Figure 6 western blot assay for synaptophysin (SYP) in the hippocampal dentate gyrus of postnatal day 21 weaning rats.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by ienticate.
Peer review:Externally peer reviewed.
Open access statement:is is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under identical terms.
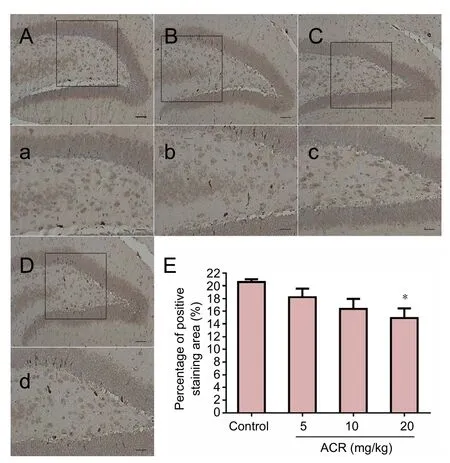
Figure 5 Percentage of synaptophysin (SYP)-immunoreactive area of hippocampal neurons in postnatal day 21 weaning rats.
Open peer reviewer: Elizabeth Hernández-Echeagaray, Universidad Nacional Autonoma de Mexico Unidad de Biomedicina, Tlalnepantla,Mexico.
Baetge EE, Hammang JP (1991) Neurite outgrowth in pc12 cells deficient in gap-43. Neuron 6:21-30.
Benowitz LI, Routtenberg A (1997) Gap-43: an intrinsic determinant of neuronal development and plasticity. Trends Neurosci 20:84.
Cabalka LM, Ritchie TC, Coulter JD (1990) Immunolocalization and quantitation of a novel nerve terminal protein in spinal cord development. J Comp Neurol 295:83-91.
Cheng O, Ostrowski RP, Liu W, Zhang JH (2010) Activation of liver x receptor reduces global ischemia brain injury by reduction of nuclear factor-κb. Neuroscience 166:1101.
Chirwa S, Aduonum A, Pizarro J, Reasor J, Kawai Y, Gonzalez M, Mcadory BS, Onaivi E, Barea-Rodriguez EJ (2005) Dopaminergic DA1 signaling couples growth-associated protein-43 and long-term potentiation in guinea pig hippocampus. Brain Res Bull 64:433-440.
Dahlqvist P, Rönnbäck A, Bergström SA, Söderström I, Olsson T (2004)Environmental enrichment reverses learning impairment in the morris water maze after focal cerebral ischemia in rats. Eur J Neurosci 19:2288-2298.
Derksen MJ, Ward NL, Hartle KD, Ivanco TL (2007) Map2 and synaptophysin protein expression following motor learning suggests dynamic regulation and distinct alterations coinciding with synaptogenesis.Neurobiol Learn Mem 87:404-415.
Doerge DR, Young JF, Chen JJ, Dinovi MJ, Henry SH (2008) Using dietary exposure and physiologically based pharmacokinetic/pharmacodynamic modeling in human risk extrapolations for acrylamide toxicity. J Agr Food Chem 56:6031-6038.
Evans GJO,Cousin MA (2005) Tyrosine phosphorylation of synaptophysin in synaptic vesicle recycling. Biochem Soc Trans 33:1350-1353.
Exon JH (2006) A review of the toxicology of acrylamide. J Toxicol Env Heal B 9:397.
Freeman LW, Wright TW (1953) Experimental observations of concussion and contusion of the spinal cord. Ann Surg 137:433-443.
Garey J, Ferguson SA, Paule MG (2005) Developmental and behavioral effects of acrylamide in Fischer 344 rats. Neurotoxicol Teratol 27:553.
He Y, Tan D, Mi Y, Zhou Q, Ji S (2017) Epigallocatechin-3-gallate attenuates cerebral cortex damage and promotes brain regeneration in acrylamide-treated rats. Food Funct 8:2275-2282
Honig LS, Rosenberg RN (2000) Apoptosis and neurologic disease. Am J Med 108:317-330.
Konings EJ, Baars AJ, van Klaveren JD, Spanjer MC, Rensen PM, Hiemstra M, van Kooij JA, Peters PW (2003) Acrylamide exposure from foods of the Dutch population and an assessment of the consequent risks. Food Chem Toxicol 41:1569-1579.
Kwon SE, Chapman ER (2011) Synaptophysin regulates the kinetics of synaptic vesicle endocytosis in central neurons. Neuron 70:847.
Lai HC, Wu MJ, Chen PY, Sheu TT, Chiu SP, Lin MH, Ho CT, Yen JH(2011) Neurotrophic effect of citrus 5-hydroxy-3,6,7,8,3′,4′-hexamethoxyflavone: promotion of neurite outgrowth via cAMP/PKA/CREB pathway in PC12 cells. PLoS One 6:e28280.
Leclerc N, Beesley PW, Brown I, Colonnier M, Gurd JW, Paladino T,Hawkes R (1989) Synaptophysin expression during synaptogenesis in the rat cerebellar cortex. J Comp Neurol 280:197-212.
Lehning EJ, Balaban CDRoss JF, Lopachin RM (2003) Acrylamide neuropathy. III. Spatiotemporal characteristics of nerve cell damage in forebrain. Neurotoxicology 24:125-136.
Li J, Wen PY, Li WW, Zhou J (2015) Upregulation effects of tanshinone iia on the expressions of neun, nissl body, and iκb and downregulation effects on the expressions of gfap and nf-κb in the brain tissues of rat models of alzheimer’s disease. Neuroreport 26:758-766.
Lingnert H, Grivas S, Jägerstad M, Skog K, Törnqvist M, Åman P (2002).Acrylamide in food: mechanisms of formation and influencing factors during heating of foods. Food Nutr Res 46:159-172.
Lipworth L, Sonderman JS, Tarone RE, Mclaughlin JK (2012) Review of epidemiologic studies of dietary acrylamide intake and the risk of cancer. Eur J Cancer Prev 21:375.
Liu SJ, Yang C, Zhang Y, Su RY, Chen JL, Jiao MM, Chen HF, Zheng N, Luo S, Chen YB, Quan SJ, Wang Q (2016) Neuroprotective effect of β-asarone against alzheimer’s disease: regulation of synaptic plasticity by increased expression of SYP and glur1. Drug Des Develer 10:1461-1469.
LoPachin RM, Gavin T (2012) Molecular mechanism of acrylamide neurotoxicity: lessons learned from organic chemistry. Environ Health Perspect 120:1650-1657.
LoPachin RM, Schwarcz AI, Gaughan CL, Mansukhani S, Das S (2004)In vivo and in vitro effects of acrylamide on synaptosomal neurotransmitter uptake and release. Neurotoxicology 25:349-363.
Ma Y, Shi J, Zheng M, Liu J, Tian S, He X, Zhang D, Li G, Zhu J (2011)Toxicological effects of acrylamide on the reproductive system of weaning male rats. Toxicol Ind Health 27:617.
Manuela P, Giulia M, Valentina P, Luisa V, Marco V, Mariano M (2013)Neurotoxicity of acrylamide in exposed workers. Int J Environ Res Public Health 10:3843-3854.
Mcphail LT, Fernandes KJ, Chan CC, Vanderluit JL, Tetzlaff W (2004).Axonal reinjury reveals the survival and re-expression of regeneration-associated genes in chronically axotomized adult mouse motoneurons. Exp Neurol 188:331-340.
Navratil V, de Chassey B, Meyniel L, Delmotte S, Gautier C, André P,Lotteau V, Rabourdin-Combe C (2009) Virhostnet: a knowledge base for the management and the analysis of proteome-wide virus–host interaction networks. Nucleic Acids Res 37:D661-668.
Niu J, Li C, Wu H, Feng X, Su Q, Li S, Zhang L, Yew DT, Cho EY, Sha O(2015) Propidium iodide (pi) stains nissl bodies and may serve as a quick marker for total neuronal cell count. Acta Histochem 117:182-187.
Niu R, Sun Z, Wang J, Cheng Z, Wang J, China S (2008) Effects of fluoride and lead on locomotor behavior and expression of nissl body in brain of adult rats. Fluoride 41:276-282.
Noble W, Planel E, Zehr C, Olm V, Meyerson J, Suleman F, Gaynor K, Wang L, Lafrancois J, Feinstein B (2005) Inhibition of glycogen synthase kinase-3 by lithium correlates with reduced tauopathy and degeneration in vivo. Proc Natl Acad Sci U S A 102:6990-6995.
Ogawa B, Wang L, Ohishi T, Taniai E, Akane H, Suzuki K, Mitsumori K, Shibutani M (2012) Reversible aberration of neurogenesis targeting late-stage progenitor cells in the hippocampal dentate gyrus of rat offspring aer maternal exposure to acrylamide. Arch Toxicol 86:779-790.
Prasad SN, Muralidhara (2013) Neuroprotective Efficacy of eugenol and isoeugenol in acrylamide-induced neuropathy in rats: behavioral and biochemical evidence. Neurochem Res 38:330-345.
Pullen AH. (1990). Morphometric evidence from c-synapses for phased nissl body response in alpha-motoneurones retrogradely intoxicated with diphtheria toxin. Brain Res 509:8-16.
Robinson PA (2011) Neural field theory of synaptic plasticity. J Theor biol 285:156-163.
Rossetti MF, Varayoud J, Lazzarino GP, Luque EH, Ramos JG (2016)Pregnancy and lactation differentially modify the transcriptional regulation of steroidogenic enzymes through DNA methylation mechanisms in the hippocampus of aged rats. Mol Cell Endocrinol 429:73-83.
Sakharkar AJ, Vetreno RP, Zhang H, Kokare DM, Crews FT, Pandey SC (2016) A role for histone acetylation mechanisms in adolescent alcohol exposure-induced deficits in hippocampal brain-derived neurotrophic factor expression and neurogenesis markers in adulthood.Brain Struct Funct 221:1-13.
Sansano M, Heredia A, Peinado I, Andrés A (2017) Dietary acrylamide:what happens during digestion. Food Chem 237:58-64.
Sen E, Tunali Y, Erkan M (2015) Testicular development of male mice offsprings exposed to acrylamide and alcohol during the gestation and lactation period. Hum Exp Toxicol 34:401-414
Shi J, Ma Y, Zheng M, Ruan Z, Liu J, Tian S, Zhang D, He X, Li G (2012)Effect of sub-acute exposure to acrylamide on GABAergic neurons and astrocytes in weaning rat cerebellum. Toxicol Ind Health 28:10.
Svensson K, Abramsson L, Becker W, Glynn A, Hellenäs KE, Lind Y,Rosén J (2003) Dietary intake of acrylamide in Sweden. Food Chem Toxicol 41:1581-1586.
Takahashi M, Shibutani M, Inoue K, Fujimoto H, Hirose M, Nishikawa A (2008) Pathological assessment of the nervous and male reproductive systems of rat offspring exposed maternally to acrylamide during the gestation and lactation periods-a preliminary study. J Toxicol Sci 33:11-24.
Tian SM , Ma YX, Shi J, Lou T Y, Liu SS, Li GY (2015) Acrylamide neurotoxicity on the cerebrum of weaning rats. Neural Regen Res 10:938-943.
Toni N, Laplagne DA, Zhao C, Lombardi G, Ribak CE, Gage FH (2008)Neurons born in the adult dentate gyrus form functional synapses with target cells. Nat Neurosci 11:901-907.
Toni N, Teng EM, Bushong EA, Aimone JB, Zhao C, Consiglio A (2007)Synapse formation on neurons born in the adult hippocampus. Nat Neurosci 10:727.
Wang H, Wang R, Xu S, Lakshmana MK (2014) Ranbp9 overexpression accelerates loss of pre and postsynaptic proteins in the AP Delta E9 transgenic mouse brain. PLoS One 9:e85484.
Williams S, Chen L, Savignac HM, Tzortzis G, Anthony DC, Burnet PW (2016) Neonatal prebiotic supplementation increases the levels of synaptophysin, glun2a-subunits and bdnf proteins in the adult rat hippocampus. Synapse 70:121-124.
WHO (2002) Health implications of acrylamide in food. Geneva, Switzerland: FAO/WHO.
Xiao F, Xu JM, Jiang XH (2015) Cx3 chemokine receptor 1 deficiency leads to reduced dendritic complexity and delayed maturation of newborn neurons in the adult mouse hippocampus. Neural Regen Res 10:772-777.
Yao X, Yan L, Yao L, GuanW, Zeng F, Cao F (2014) Acrylamide exposure impairs blood-cerebrospinal fluid barrier function. Neural Regen Res 9:555-560.
Zhao C, Teng EM, Jr SR, Ming GL, GageFH (2006) Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J Neurosci 26:3.
Graphical Abstract
Effects of acrylamide on neuronal development in weaning rats
*Correspondence to:
Jing Liu, Ph.D.,liulq1227@163.com.
orcid:
0000-0002-2416-1162
(Jing Liu)
10.4103/1673-5374.217345
Accepted: 2017-09-04
Copyedited by Wang J, Li CH, Qiu Y, Song LP, Zhao M
杂志排行
中国神经再生研究(英文版)的其它文章
- Matrix bound vesicles and miRNA cargoes are bioactive factors within extracellular matrix bioscaffolds
- Diffusion tensor tractography studies on mechanisms of recovery of injured fornix
- Using 3D bioprinting to produce mini-brain
- Beta secretase activity in peripheral nerve regeneration
- Embracing oligodendrocyte diversity in the context of perinatal injury
- On the road towards the global analysis of human synapses