Preparation and properties of graphene oxide/polyacrylonitrile films by in-situ polymerization
2017-11-04LiFengmeiZhengYingyingLiMengzhuWangBiao
Li Fengmei, Zheng Yingying, Li Mengzhu, Wang Biao
(College of Materials Science and Engineering, State Key Laboratory for Modification of Chemical Fibers and Polymer Materials, Donghua University, Shanghai 201620)
科研快报
Preparation and properties of graphene oxide/polyacrylonitrile films by in-situ polymerization
Li Fengmei, Zheng Yingying, Li Mengzhu, Wang Biao*
(College of Materials Science and Engineering, State Key Laboratory for Modification of Chemical Fibers and Polymer Materials, Donghua University, Shanghai 201620)
A graphene oxide (GO)/polyacrylonitrile (PAN) polymer was synthesized by in-situ polymerization and was produced into GO/PAN composite film by aqueous film formation. The effect of GO amount on the monomer coversion rate(Y) and specific viscosity (ηsp) of PAN was discussed during the process of polymerization. The structure and properties of the GO/PAN compostie films were investigated. The results shown thatηspandYof PAN were improved due to the addition of GO;Ywas increased by 13.4% andηspby 77.3% as the mass fraction of GO was 2% based on pure PAN. The GO sheets were dispersed uniformly in GO/PAN composite film matrix, and there were some interactions between GO sheets and PAN chains, as compared with those of pure PAN, the crystallinity, the thermal stability and mechanical properties of GO/PAN composite film were improved;and the GO/PAN composite film had the crystallinity of 47.9%, the maximum decomposition temperature of 304 ℃, the weight maintenance rate of 49.8% at 600 ℃ and tensile strength 6.0 MPa as the mass fraction of GO was 2%.
polyacrylonitrile; graphene oxide; in-situ polymerization; aqueous film formation; structure; properties
Polyacrylonitrile (PAN) is widely used as fiber or film material in apparel, home furnishings and industrial areas[1]. The incorporation of functional fillers is an important technique to improve the properties of PAN materials[2].
Graphene has received much attention for wide potential applications such as energy storage, transparent electrodes and nanocomposites[3-5]due to its excellent thermal conductivity, mechanical and electronic transport properties. However, graphene sheets cannot be dispersed well in solvents or polymers due to the significant van der Waals force. Graphene, graphene oxide (GO) has various oxygen-containing functional groups on its surface or edges, such as hydroxyls, epoxides, diols, ketones, carboxyls, and carbonyl groups, which make GO strongly hydrophilic and dispersable in polar solvents and polymer matrices easily. Moreover, GO can be reduced by chemical or physical methods. GO is one kind of ideal nanofiller for nanocomposites[6-8].
The author synthesized GO/PAN composites by in-situ polymerization, which was produced into GO/PAN composite film by heterogeneous method. The effects of GO on the viscosity and monomer conversion rate of PAN and the structure and properties of GO/PAN composite film were discussed.
1 Experimental
1.1Materials
Acrylonitrile (AN) was provided by China Petroleum Chemical Corporation (Shanghai, China). Methyl acrylate (MA) and azobisisobutyronitrile (AIBN) were of analytical grade and purchased from Sinopharm Chemical Reagent (Shanghai, China) were distilled or recrystallized before used. Dimethyl sulfoxide (DMSO) of chemical pure grade was purchased from Shanghai Lingfeng Chemical Reagent (Shanghai, China). GO with the sheet layer size 1-10 μm was prepared from purified natural graphite by a modified Hummers method[9-11].
1.2SynthesisofGO/PANnanocomposites
GO/PAN nanocomposites containing different amounts of GO were synthesized by in-situ polymerization. A typical process of the polymerization was as followed: First, Go was accurately weighed at the mass fractions of 0,0.2%,0.5% and 2% based on the total monomers and was dispersed into a proper amount of DMSO by ultrasonic dispersion for 4 h. Secondly, AN and MA were added at the mass ratio of 90:10 and then AIBN as an initiator (0.6% of the total monomers mass) into the GO solution. The mass fraction of monomers
of polymerization dispersion sdution was 30%. The polymerization was conducted under nitrogen and 65℃ for 12 h with a stable stirring speed. The obtained polymer were designated as PAN, 0.2GP, 0.5GP and 2.0GP respectively.
The products were pressed into 150 μm thin films on glass tray, which was transferred into deionized to peel off the GO/PAN composite films.The films were washed with water several times and dried at 60 ℃ under standard atmosphere. The polymer films from PAN,0.2GP,0.5GP and 2.0GP were designated as samples 0#,1#,2#and 3#.
1.3Characterization
Monomer conversion rate (Y) was calculated by equation (1):
Y=m1/(cm0)×100%
(1)
Wherem0is the weight of liquid polymerization product,m1is the weight mass of liquid polymerization product after being washed several times and dried,cis monomer mass fraction.
Specific viscosity (ηsp) was measured with a Ubbelohde viscometer and calculated by equation (2):
ηsp=(t1-t0)/t0
(2)
wheret0andt1were the flow time of solvent and the composite dilute solutions, respectively.
The morphology of GO/PAN films was evaluated using a scanning electron microscope (SEM, JSM-5600LV, JEOL, Japan). X-ray diffraction (XRD) patterns were recorded on DMSX-2500 PC X-ray spectrometer with CuKαradiation with the wave length of 0.154 2 nm) in the range of 2θbetween 5 °-60° at the scanning rate of 5(°)/min and step angle of 0.02°. In order to analyze the interaction between GO and PAN, Raman spectra were performed using an InVia-Reflex spectrophotometer (Britain, laser wavelength 632.8 nm).
The thermal property was characterized with a synchronous thermal analyzer (Netzsch STA409PC) in nitrogen atmosphere at the heating rate of 10 ℃/min. The mechanical properties of GO/PAN films were measured by universal testing machine (i-Strentek 1510, China) at the tensile rate of 10 mm/min.
2 Results and discussion
2.1EffectofGOonin-situpolymerizationofPAN
2.1.1 Dispersibility of GO in PAN
As shown in Fig.1, the color of liquid polymerization products changed from yellow to dark brown with the increase of GO content, but the dispersion solution of GO was yellowish brown because GO was slightly reduced by heat treatment during polymerization process. Moreover, the liquid polymerization products kept homogeneous and stable without layer and deposition phenomena even after standing for 30 days, which indicated that GO can uniformly disperse in PAN solution. PAN film was white and the films with GO was gray. And the color of the films became darker and darker with the increase of GO content. The homogenous color indicated that the GO sheets were uniformly dispersed in PAN matrix.

Fig.1 Appearance of liquid polymerization product and composite films
2.1.2Yandηspof solution system
As shown in Tab.1,Yandηspwere increased with the increase of GO mass fraction.Yandηspof 2.0GP were improved by 13.4% and 77.3%, respectively, as compared with pure PAN. That might because the sheet-like structure of GO will block the movement of polymer chain segment, and the contact between long chain radicals was reduced, then the chain termination was limited.But GO sheets had a little influence on the monomers movement, so the chain propagation will not be affected. In conclusion, GO can improve theYandηspof the solution system.
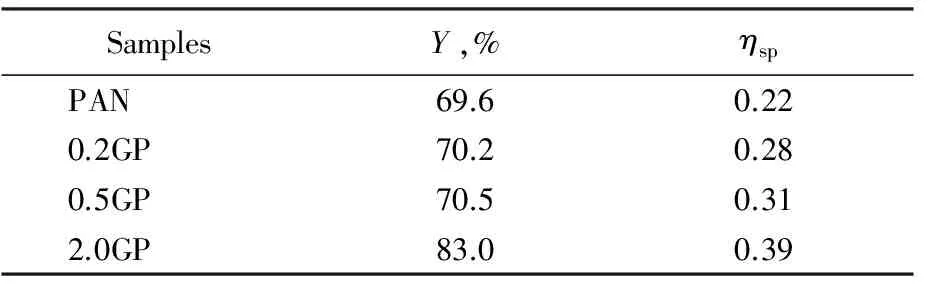
Tab.1 ηsp of solution system and Y of polymerization reaction
2.2EffectofGOonstructureofGO/PANcomposilefilms
2.2.1 SEM analysis
As presented in Fig.2, the films prepared by heterogeneous method had finger-like pores due to the double diffusion between water and solvent during the formation process. The fracture surface morphology of GO/PAN films showed that the pore surface of pure PAN film was very smooth when the pore surface of composite films with GO were rough,and the GO sheets were dispersed uniformly in PAN matrix without agglomeration.And with the increase of GO content, the surface of GO/PAN films became rougher.Moreover,the orientation of GO sheet occurred in some degree under film laying force.

Fig.2 Fracture SEM images of GO/PAN composite films
2.2.2 Raman spectra analysis
As shown in Fig.3, there were two obvious characteristic peaks,band D (1 350 cm-1)and G band (1 580 cm-1), in the Raman spectra of GO/PAN films. band D and G band were assigned to the disorder and defects in graphite structure and the ordered graphitic crystallites, respectively[12].
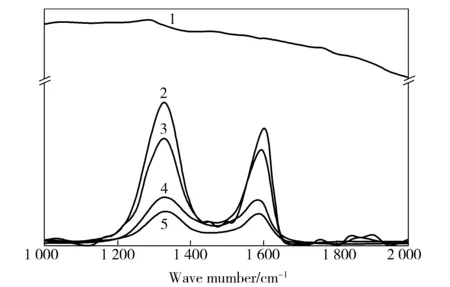
Fig.3 Raman spectra of GO/PAN films1—0#;2—1#;3—2#;4—3#;5—GO
The spectra of PAN had no obvious band D and band G, but the composites with GO had band G and band D with great intensity and width. And the intensity of band D and band G tended to be equal to those of the bands of GO with the increase of GO content. However, the up-shifts of band G for the films with GO were observed. These shifts resulted from the change of the chemical environment surrounding the carbon atoms in GO, indicating some interactions between GO sheets and PAN chains.
2.2.3 XRD analysis
As shown in Fig.4, peaks near 2θof 16.7° was corresponded to the (100) lattice plane of PAN, and a broad peak between 22°-30° was corresponded to the amorphous structure of PAN[13]. The crystalline peak position of GO/PAN composite film did not change after adding GO, which indicated that the addition of GO did not make the crystal form of PAN change.Tab.2 showed that the crystallinities of the samples were increased after adding GO. And the crystallinity of 2.0GP was increased by 14.2% as compared with pure PAN. The crystallization of PAN might be induced by GO, which was attributed to the fact that GO acted as crystallization nucleating agent in the composite film.

Fig.4 XRD spectra of GO/PAN films1—0#;2—1#;3—2#;4—3#

Tab.2 Crystallinities of GO/PAN films
2.3EffectofGOonpropertiesofGO/PANcompositefilm
As presented in Fig.5 and Tab.3, GO showed severe weight loss in nitrogen atmosphere and began to decompose below 100 ℃ because the oxygen functional groups on GO were not stable in thermal environment. And the retention rate of GO was merely 49.8% at 600 ℃. The TG curves of GO/PAN composite film showed the same tendency with the weight loss of pure PAN, but the thermal weight loss of the film was lower at the same temperature. The retention rate of sample 3#was increased by 27% at 600 ℃ as compared with that of PAN.However, the maximum decomposition temperature of GO/PAN composite film was higher than those of pure PAN and GO. The results indicated that the GO sheets loading in PAN matrix might improve the intermolecular crosslink of PAN chains during heat treatment due to GO decomposition.
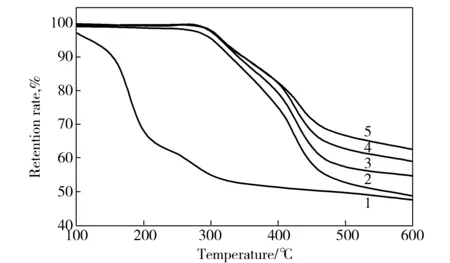
Fig.5 TG curves of GO/PAN composite films1—GO;2—0#;3—1#;4—2#;5—3#
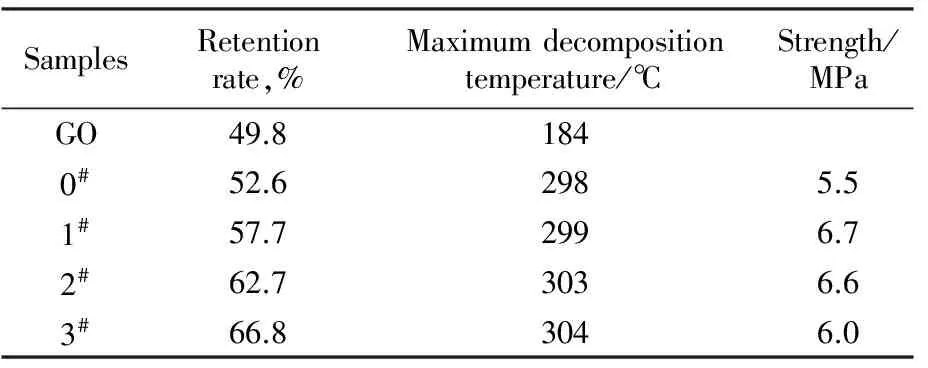
Tab.3 Thermal stability and mechanical property of GO/PAN composite films
As shown in Tab.3, the mechanical properties of GO/PAN films were improved but not notably as compared with pure PAN film due to the pore structure and the preparation process not involving drawing.
3 Conclusions
a. GO/PAN films were synthesized by in-situ polymerization. It was found thatYandηspof the composite film were improved through the addition of GO.
b. The GO sheets were dispersed uniformly in PAN matrix, and some interactions existed between GO sheets and PAN chains.
c. The crystallinity,thermal stability and mechanical properties of the composite film were increased as compared with those of pure PAN.
[1] Gries T, Rixe C, Steffens M, et al. Polyacrylic fibers[J]. Chem Fiber Int,2002, 52(4): 232-253.
[2] Nguyen H Q, Deng B. Electrospinning and in situ nitrogen doping of TiO2/PAN nanofibers with photocatalytic activation in visible lights[J]. Mater Lett, 2012, 82(9):102-104.
[3] Hernandez Y, Nicolosi V, Lotya M, et al. High-yield production of graphene by liquid-phase exfoliation of graphite[J]. Nat Nanotech, 2008, 3(9): 563-568.
[4] Tung V C, Allen M J, Yang Y, et al. High-throughput solution processing of large-scale graphene[J]. Nat Nanotech, 2009, 4(1): 25-29.
[5] Reina A, Jia X, Ho J, et al. Large area, few-layer graphene films on arbitrary substrates by chemical vapor deposition[J]. Nano Lett, 2009, 9(1): 30-35.
[6] Chua C K, Pumera M. Reduction of graphene oxide with substituted borohydrides[J]. J Mater Chem A, 2013, 1(5): 1892-1898.
[7] Fernández-Merino M J, Guardia L, Paredes J I, et al. Vitamin C is an ideal substitute for hydrazine in the reduction of graphene oxide suspensions[J]. J Phys Chem C, 2010, 114(14): 6426-6432.
[8] Sundaram R S, Gómez-Navarro C, Balasubramanian K. Electrochemical modification of grapheme[J]. Adv Mater, 2008, 20(16): 3050-3053.
[9] Hummers Jr W S, Offeman R E. Preparation of graphitic oxide[J]. J Amer Chem Soc, 1958, 80(6): 1339.
[10] Kovtyukhova N I,Ollivier P J,Martin B R,et al. Layer-by-layer assembly of ultrathin composite films from micron-sized graphite oxide sheets and polycations[J]. Chem Mater, 1999, 11(3): 771-778.
[11] Li F, Zheng Y, Wang B. Rheological behaviors of graphene oxide/polyacrylonitrile spinning solutions[J]. Mater Sci Forum, 2017, 898: 2187-2196.
[12] Lei Shuai, Zhong Shan, Wang Yu, et al. Preparation of monodisperse reduced graphene oxide/polyacrylonitrile composite and its thermal-induced structural transformation[J]. Mater Lett, 2015, 161: 108-111.
[13] Liu Jie, Zhou Peixun, Zhang Lifeng, et al. Thermo-chemical reactions occurring during the oxidative stabilization of electrospun polyacrylonitrile precursor nanofibers and the resulting structural conversions[J]. Carbon, 2009, 47(4): 1087-1095.
原位聚合法制备GO/PAN复合膜及其性能的研究
李凤美 郑迎迎 李梦竹 王彪
(东华大学材料科学与工程学院纤维材料改性国家重点实验室,上海 201620)
通过原位聚合法制备了氧化石墨烯/聚丙烯腈(GO/PAN)聚合物,采用水相成膜法制得GO/PAN复合膜,探讨了聚合过程中GO用量对PAN的单体转化率(Y)和增比黏度(ηsp)的影响,研究了GO/PAN复合膜的结构和性能。结果表明:GO的加入使PAN的ηsp和Y都有所提高;当加入GO质量分数为2%时,相对于PAN,其Y提高了13.4%,ηsp提高了77.3%;GO片层均匀地分布在GO/PAN复合膜基体当中,并且GO与PAN之间存在一定的作用力;与纯PAN相比,GO/PAN复合膜的结晶度、热稳定性和力学性能都得到一定程度提高,当GO质量分数为2%时,所制得的GO/PAN复合膜的结晶度为47.9%,最大分解温度304 ℃,600 ℃时质量保持率为49.8%,强度为6.0 MPa。
聚丙烯腈 氧化石墨烯 原位聚合 水相成膜 结构 性能
date:06-08-2017; revised date: 15- 08- 2017.
Biography: Li Fengmei( 1990-), female, Ph. D candidate, is engaged in the research of fiber modification. E-mail:mayerlee@foxmail.com.
Sinopec Group Project(32000000-16-200607-0007).
*Correspondingauthorwbiao2000@dhu.edu.cn.
TQ325+.8DocumentcodeAArticleID1001- 0041(2017)04- 0047- 04
