人髓核细胞分离、传代培养方法的建立及意义
2017-10-10李大鹏吴燕黄永辉蒋璐孙继芾岳佳伟
李大鹏,吴燕,黄永辉,蒋璐,孙继芾,岳佳伟
(1江苏大学附属医院,江苏镇江212001;2江苏大学)
·论著·
人髓核细胞分离、传代培养方法的建立及意义
李大鹏1,吴燕2,黄永辉1,蒋璐2,孙继芾1,岳佳伟1
(1江苏大学附属医院,江苏镇江212001;2江苏大学)
目的建立一种体外分离、培养人髓核细胞(NPCs)的新方法,并探讨其意义。方法采用0.2% Ⅱ型胶原酶消化法分离人正常NPCs,单层贴壁法进行传代培养,倒置显微镜下观察细胞形态。取第1、2、3代NPCs,采用CCK-8法检测细胞增殖能力(以吸光度值表示),连续检测12天。取第2代NPCs,采用免疫组化法检测Ⅱ型胶原(Col-2)、细胞角蛋白18(KRT-18)、KRT-19、低氧诱导因子1α(HIF-1α)、葡萄糖转运子1(GLUT-1)、Sox-9、聚集蛋白聚糖(ACAN)、CD24阳性表达率。结果分离的NPCs呈多角形或短梭形,类似于软骨细胞;第4代以后,NPCs细胞突起延长,呈长梭形,生长缓慢,出现老化现象。NPCs生长曲线呈S形:1~2天细胞生长缓慢;3~7天细胞生长迅速,进入对数生长期;8天后进入平台期,细胞增殖缓慢。连续培养12天,第1、2、3代NPCs相同时间点吸光度值比较差异均无统计学意义(P均>0.05)。第2代NPCs中Col-2、KRT-18、KRT-19、HIF-1α、GLUT-1、Sox-9、ACAN、CD24阳性表达率均≥85.6%(85.6%~91.2%)。结论单纯0.2% Ⅱ型胶原酶消化法及单层贴壁法可分离、培养人NPCs,并经免疫组化染色鉴定证实;传代3代以内的NPCs细胞形态及增殖能力均无明显改变,可作为椎间盘退变相关研究的种子细胞。
髓核细胞;Ⅱ型胶原酶消化法;单层贴壁法;细胞培养;椎间盘退变
Abstract:ObjectiveTo establish a new method for isolation and culture of human nucleus pulposus cells (NPCs) in vitro and to explore its significance.MethodsHuman normal NPCs were isolated by type Ⅱ collagenase digestion, then were cultured and expanded in monolayer. Cell morphology was observed by inverted microscope; the proliferation ability (expressed as absorbance value) of passage 1, passage 2 and passage 3 NPCs were detected by CCK-8 assay for consecutive 12 days; the positive expression rates of type Ⅱ collagen (Col-2), cytokeratin 18 (KRT-18), KRT-19, hypoxia-inducible factor-1α(HIF-1α), glucose transporter 1 (GLUT-1), Sox-9, aggrecan (ACAN), and CD24 in passage 2 NPCs were detected by immunohistochemistry.ResultsThe isolate human NPCs were polygonal or short spindle, similar to chondrocytes. However, the nucleus pulposus cells after passage 4 grew slowly, the protrusion of cells extended, and the cells were long spindle shape, all of these indicated the cells were aging. The growth curve of NPCs was S-shaped, cells grew slowly in 1-2 days, then grew into the logarithmic growth phase in 3-7 days, and after 8 days the cells proliferated slowly and entered the plateau. After consecutive 12-day culture, the difference of absorbance values at 450 nm among passage 1, passage 2 and passage 3 NPCs was not statistically significant at the same time point (allP>0.05). Immunohistochemical staining showed: the positive rates of Col-2, KRT-18, KRT-19, GLUT-1, HIF-1α, Sox-9, ACAN, and CD24 in passage 2 NPCs were no less than 85.6% (85.6%-91.2%).ConclusionsHuman NPCs can be effectively isolated by 0.2% type Ⅱ collagenase digestion method, and can be cultured by monolayer adherence method. The cells we cultured were identified as nucleus pulposus cells by immunohistochemistry. There are no significant changes in the morphological and proliferation abilities of NPCs within 3 generations, and could be used as seed cells for study of intervertebral disc degeneration.
Keywords: nucleus pulposus cells; type Ⅱ collagenase digestion; monolayer adherence; cell culture; intervertebral disc degeneration
下腰痛的主要原因之一为椎间盘退变[1,2]。椎间盘在结构上由纤维环、软骨终板及髓核三部分组成[3,4]。目前研究认为,椎间盘退变始于髓核,髓核细胞(NPCs)的过早老化与凋亡使其数量减少、功能降低,使得聚集蛋白聚糖(ACAN)、Ⅱ型胶原(Col-2)等功能性细胞外基质的合成与分泌减少[5],进而引起髓核水分含量降低并失去其凝胶状态,最终导致椎间盘生物学功能减退与丧失。椎间盘退变的生物学治疗方法包括基因治疗、细胞因子、干细胞移植、组织工程等[6,7],可恢复NPCs的数量及功能,延缓甚至逆转椎间盘退变。2016年7~10月,本研究建立一种体外分离、培养人NPCs的新方法,并对其生物学特性进行鉴定,以期为椎间盘退变病因、机制及治疗的相关基础研究提供稳定的细胞来源。
1 材料与方法
1.1 材料 主要试剂:髓核细胞培养基(NPCM,美国Sciencell公司),Col-2(美国Gibco公司),胎牛血清(美国Sciencell公司),青霉素-链霉素(美国Sciencell公司),CCK-8试剂盒(上海翊圣生物科技有限公司),Col-2、细胞角蛋白18(KRT-18)抗体、KRT-19抗体、低氧诱导因子1α(HIF-1α)抗体(武汉博士德生物工程有限公司),鼠抗人葡萄糖转运子1(GLUT-1)抗体、鼠抗人Sox-9抗体、鼠抗人CD24抗体、鼠抗人ACAN抗体(美国Santa Cruz公司),SABC-POD(小鼠/兔IgG)免疫组化试剂盒(武汉博士德生物工程有限公司)。主要仪器:细胞培养超净台(苏州净化设备有限公司),二氧化碳培养箱(美国Thermo公司),显微镜(德国Zeiss公司)。
1.2 人NPCs的分离及传代培养 选择1例因L2椎体爆裂骨折行L1/2髓核切除术及椎间融合术的患者(男性,20岁),术中根据Gries评分标准[8]未见椎间盘退变。将切取的髓核组织置于超净台上,PBS反复冲洗至组织无血污,去除纤维环组织,并将髓核组织剪碎至1 mm3大小的组织块。加入不含胎牛血清的NPCM,1 500 r/min离心5 min,弃上清。加入0.2% Ⅱ型胶原酶10 mL,37 ℃恒温震荡消化4 h,1 500 r/min离心5 min,弃上清。加入含2%胎牛血清的NPCM 5 mL重悬细胞,以1×105个/mL接种至培养瓶中。37 ℃、5% CO2、饱和湿度条件下静置培养3天,加入NPCM 5 mL;培养7天时第1次换液,待细胞融合至90%时进行传代培养。本研究通过江苏大学附属医院医学伦理委员会审核,并与患者签订知情同意书。
1.3 NPCs生长情况观察 ①贴壁情况及细胞形态:观察细胞传代培养后的贴壁情况,200倍倒置显微镜下观察细胞形态。②细胞增殖能力:采用CCK-8法。取第1、2、3代生长状态良好的NPCs,消化收集细胞,以1×104~2×104个/mL接种至96孔板,每孔100 μL。每天固定时间加入CCK-8溶液,每孔10 μL,孵育4 h后采用酶标仪检测450 nm波长处的吸光度值,以吸光度值表示细胞增殖能力。实验重复3次,连续检测12天。
1.4 NPCs鉴定 采用免疫组化法。取第2代NPCs,加入胰蛋白酶消化,以1×105个/mL接种于24孔板, 每孔250 μL,细胞贴壁并融合至70%左右,给予4%多聚甲醛固定30 min。30% H2O2+纯甲醇(1∶50混合)室温浸泡30 min,蒸馏水冲洗2次;加入5% BSA封闭液,室温条件下孵育20 min,甩去多余液体;滴加抗Col-2一抗,4 ℃过夜,PBS冲洗2 min×3次;滴加生物素化山羊抗小鼠(或兔)IgG,20~37 ℃条件下孵育20 min,PBS冲洗2 min×3次;滴加SABC试剂,37 ℃条件下孵育20 min,PBS冲洗5 min×4次;DAB显色,蒸馏水洗涤。200倍显微镜下观察染色情况及阳性表达位置,以细胞质或者细胞核染出现棕黄色视为阳性表达。每孔随机选取3个视野,阳性表达率=阳性细胞数/总细胞数×100%,取平均值。参照上述方法分别检测并计算细胞KRT-18、KRT-19、GLUT-1、HIF-1α、Sox-9、ACAN、CD24阳性表达率。
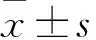
2 结果
2.1 NPCs生长情况 原代NPCs贴壁缓慢,培养4~5天开始有细胞贴壁,14天左右基本铺满瓶底;细胞形态呈多角形或短梭形,类似于软骨细胞。传代后细胞贴壁较快,12 h内即可贴壁生长,细胞生长迅速,仍呈短梭形;第4代以后,NPCs细胞突起延长,呈长梭形,生长缓慢,出现老化现象。
2.2 NPCs增殖能力 NPCs生长曲线呈S形:1~2天细胞生长缓慢;3~7天细胞生长迅速,进入对数生长期;8天后细胞增殖缓慢,进入平台期。连续培养12天,第1、2、3代NPCs相同时间点吸光度值比较差异均无统计学意义(P均>0.05)。见表1。

表1 第1、2、3代NPCs连续培养1~12天的吸光度值
2.3 NPCs免疫组化鉴定结果 NPCs中Col-2、KRT-18、KRT-19、GLUT-1、HIF-1α、Sox-9、ACAN、CD24阳性表达率分别为87.7%、88.1%、89.1%、85.6%、91.2%、90.1%、89.8%、87.0%。Col-2、KRT-18、KRT-19、GLUT-1、ACAN、CD24主要表达于NPCs的细胞质,HIF-1α主要表达于NPCs的细胞核,Sox-9在NPCs的细胞质和细胞核均有表达。见插页Ⅰ图1。
3 讨论
目前NPCs分离培养应用最多的为酶消化法,酶消化法又可分为酶序贯消化法和单纯Ⅱ型胶原酶消化法。前者先采用胰蛋白酶消化20~30 min,再予Ⅱ型胶原酶消化数小时,能分离出较多的细胞,但酶的联合应用可加重细胞损伤,并增加污染概率。而单纯Ⅱ型胶原酶消化法相对简单实用,对细胞影响小,但各研究采用酶的浓度(0.025%~2%)及酶作用的时间不尽相同[9~11]。本研究选用Ⅱ型胶原酶的浓度为0.2%,消化时间为4 h,结果显示获取的NPCs贴壁较快,生长状态良好。
NPCs的培养方法可分为三维培养法和单层贴壁培养法[12,13]。三维培养法利用藻酸盐等材料构建三维空间,NPCs在三维空间内通过材料的孔隙通道获取外界营养,该方法有利于NPCs维持软骨样细胞表型[14],但细胞增殖缓慢、不易观察细胞形态等缺点限制了该方法的研究应用范围。单层贴壁法培养的细胞增殖速度快,可在短期内获得足量NPCs。本研究第1、2、3代的NPCs生长曲线呈S形, 3~7天细胞生长迅速迅速,进入对数生长期,此时细胞活力最佳,实验时应尽量选择此时对数生长期的细胞;而培养8天后NPCs生长曲线进入平台期,细胞停止增殖,此时需进行消化传代,否则细胞会发生形态改变,甚至死亡。但随着传代数的增加,细胞易出现去分化现象[15]。本研究结果表明,第4代后的NPCs细胞突起延长,呈长梭形,生长缓慢,出现老化现象,提示以NPCs为研究对象的相关研究应尽量选取第3代以内的细胞。
NPCs无特异性的细胞表面标识,其鉴定目前尚无统一标准[16,17]。首先,NPCs可表达软骨细胞表型,如Col-2、ACAN、Sox-9,在正常NPCs表达均呈阳性。有研究认为,NPCs中ACAN/Col-2比值远远大于软骨细胞,提示NPCs高表达ACAN[18]。其次,NPCs表达脊索细胞表型,如T基因、KRT-18、KRT-19、配对盒基因1等[19,20],这些基因高表达提示NPCs来源于胚胎发育过程中脊索细胞在椎间盘内的残留。再次,NPCs可高表达环境相关性基因,如HIF-1α、GLUT-1、碳酸酐酶12、血管内皮细胞生长因子等。纤维环、软骨终板、髓核均可表达HIF-1β,但仅有髓核表达HIF-1α[21]。另外,GLUT-1、碳酸酐酶12、血管内皮细胞生长因子受HIF-1α调控,三者在NPCs内高表达,但在纤维环及软骨终板内不表达或低表达[22]。最后,NPCs高表达CD24、CD44、CD90等表面标识物[23],其中CD24被认为是NPCs特异性的表面标识物[24,25]。本研究免疫组化染色结果显示,NPCs中Col-2、KRT-18、KRT-19、GLUT-1、HIF-1α、Sox-9、ACAN、CD24的阳性表达率均呈高表达(85.6%~91.2%),证明本研究分离、培养的细胞为NPCs。
综上所述,单纯0.2% Ⅱ型胶原酶消化法及单层贴壁法可分离、培养人NPCs;传代3代以内的NPCs细胞形态及增殖能力均无明显改变,可作为椎间盘退变相关研究的种子细胞。
[1] Takatalo J, Karppinen J, Niinimaki J, et al. Does lumbar disc degeneration on magnetic resonance imaging associate with low back symptom severity in young finnish adults[J]. Spine, 2011,36(25):2180-2189.
[2] Feng C, Liu H, Yang M, et al. Disc cell senescence in intervertebral disc degeneration: causes and molecular pathways[J]. Cell Cycle, 2016,15(13):1674-1684.
[3] Le Maitre CL, Binch AL, Thorpe AA, et al. Degeneration of the intervertebral disc with new approaches for treating low back pain[J]. J Neurosurg Sci, 2015,59(1):47-61.
[4] Rim DC. Quantitative pfirrmann disc degeneration grading system to overcome the limitation of Pfirrmann disc degeneration grade[J]. Korean J Spine, 2016,13(1):1-8.
[5] Horner HA, Urban JP. 2001 Volvo Award winner in basic science studies: effect of nutrient supply on the viability of cells from the nucleus pulposus of the intervertebral disc[J]. Spine, 2001,26(23):2543-2549.
[6] Zeckser J, Wolff M, Tucker J, et al. Multipotent mesenchymal stem cell treatment for discogenic low back pain and disc degeneration[J]. Stem Cells Int, 2016(2016):3908389-3908401.
[7] Vasiliadis ES, Pneumaticos SG, Evangelopoulos DS, et al. Biologic treatment of mild and moderate intervertebral disc degeneration[J]. Mol Med, 2014,20(1):400-409.
[8] Paesold G, Nerlich AG, Boos N. Biological treatment strategies for disc degeneration: potentials and shortcomings[J]. Eur Spine J, 2007,16(4):447-468.
[9] Wang F, Wu XT, Zhuang SY, et al. Ex vivo observation of human nucleus pulposus chondrocytes isolated from degenerated intervertebral discs[J]. Asian Spine J, 2011,5(2):73-81.
[10] Wang YY, Zhu QS, Wang YW, et al. Thymosin beta-4 recombinant adeno-associated virus enhances human nucleus pulposus cell proliferation and reduces cell apoptosis and senescence[J]. Chin Med J, 2015,128(11):1529-1535.
[11] Li ZY, Xiong SH, Hu M, et al. Knockdown epithelial membrane protein 1 suppresses human degenerative intervertebral disc-derived nucleus pulposus cell proliferation[J]. Cartilage, 2011,2(3):300-306.
[12] Gruber HE, Hanley EN Jr. Human disc cells in monolayer vs 3D culture: cell shape, division and matrix formation[J]. BMC Musculoskelet Disord, 2000(1):1-5.
[13] Cheng CC, Uchiyama Y, Hiyama A, et al. PI3K/AKT regulates aggrecan gene expression by modulating Sox9 expression and activity in nucleus pulposus cells of the intervertebral disc[J]. J Cell Physiol, 2009,221(3):668-676.
[14] Kluba T, Niemeyer T, Gaissmaier C, et al. Human anulus fibrosis and nucleus pulposus cells of the intervertebral disc: effect of degeneration and culture system on cell phenotype[J]. Spine, 2005,30(24):2743-2748.
[15] Mern DS, Thome C. Identification and characterization of human nucleus pulposus cell specific serotypes of adeno-associated virus for gene therapeutic approaches of intervertebral disc disorders[J]. BMC Muscul Dis, 2015(16):341-352.
[16] Risbud MV, Schoepflin ZR, Mwale F, et al. Defining the phenotype of young healthy nucleus pulposus cells: recommendations of the Spine Research Interest Group at the 2014 annual ORS meeting[J]. J Orthop Res, 2015,33(3):283-293.
[17] Rodrigues-Pinto R, Richardson SM, Hoyland JA. Identification of novel nucleus pulposus markers: interspecies variations and implications for cell-based therapiesfor intervertebral disc degeneration[J]. Bone Joint Res, 2013,2(8):169-178.
[18] Clouet J, Grimandi G, Pot-Vaucel M, et al. Identification of phenotypic discriminating markers for intervertebral disc cells and articular chondrocytes[J]. Rheumatology (Oxford), 2009,48(11):1447-1450.
[19] Minogue BM, Richardson SM, Zeef LA, et al. Characterization of the human nucleus pulposus cell phenotype and evaluation of novel marker gene expression to define adult stem cell differentiation[J]. Arthritis Rheum, 2010,62(12):3695-3705.
[20] Risbud MV, Schaer TP, Shapiro IM. Toward an understanding of the role of notochordal cells in the adult intervertebral disc: from discord to accord[J]. Dev Dyn, 2010,239(8):2141-2148.
[21] Rajpurohit R, Risbud MV, Ducheyne P, et al. Phenotypic characteristics of the nucleus pulposus: expression of hypoxia inducing factor-1, glucose transporter-1 and MMP-2[J]. Cell Tissue Res, 2002,308(3):401-407.
[22] Richardson SM, Knowles R, Tyler J, et al. Expression of glucose transporters GLUT-1, GLUT-3, GLUT-9 and HIF-1alpha in normal and degenerate human intervertebral disc[J]. Histochem Cell Biol, 2008,129(4):503-511.
[23] Risbud MV, Guttapalli A, Tsai TT, et al. Evidence for skeletal progenitor cells in the degenerate human intervertebral disc[J]. Spine, 2007,32(23):2537-2544.
[24] Navone SE, Marfia G, Canzi L, et al. Expression of neural and neurotrophic markers in nucleus pulposus cells isolated from degenerated intervertebral disc[J]. J Orthop Res, 2012,30(9):1470-1477.
[25] Sakai D, Nakamura Y, Nakai T, et al. Exhaustion of nucleus pulposus progenitor cells with ageing and degeneration of the intervertebral disc[J]. Nat Commun, 2012(3):1264-1273.
Establishment and significance of separation and culture of human nucleus pulposus cells
LIDapeng1,WUYan,HUANGYonghui,JIANGLu,SUNJifu,YUEJiawei
(1AffiliatedHospitalofJiangsuUniversity,Zhenjiang212001,China)
国家自然科学基金资助项目(81601931);江苏省自然科学基金资助项目(BK20150475)。
李大鹏(1981-),男,副主任医师,研究方向为椎间盘退变的理论与治疗。E-mail: lidapeng706@163.com
黄永辉(1966-),男,副教授,研究方向为椎间盘退变的理论与治疗。E-mail: huangyh8855@163.com
10.3969/j.issn.1002-266X.2017.32.001
R681.5
A
1002-266X(2017)32-0001-04
2016-11-12)
