自体心包修复儿童主动脉瓣狭窄9例病例系列报告
2017-09-22单亚平张惠锋
单亚平 贾 兵 张惠锋 叶 明 沈 华
·论著·
自体心包修复儿童主动脉瓣狭窄9例病例系列报告
单亚平 贾 兵 张惠锋 叶 明 沈 华
目的 总结并分析以自体心包修复儿童先天性主动脉瓣狭窄的手术方法和早期预后。方法 纳入2013年7月至2015年6月在复旦大学附属儿科医院行自体心包主动脉瓣成形术的先天性主动脉瓣狭窄患儿,收集患儿的一般资料,围手术期情况,并发症,术前、术中、术后和随访时的超声心动图资料。结果 符合本文纳入标准的9例患儿进入本文分析,男6例,女3例,年龄4月龄至9岁。术前超声提示,三叶式和二叶式主动脉瓣分别为3例和6例;重度狭窄6例,中度狭窄1例,重度狭窄伴中度返流2例。9例均以自体心包再造或扩大主动脉瓣瓣叶。术后即刻与术前超声心动图比较:主动脉瓣最大跨瓣压差 [(31.6±9.4)mm Hgvs(73.0±22.2)mm Hg,P=0.000]和主动脉瓣平均跨瓣压差 [(15.8±18.3)mmHgvs(35.8±18.3)mmHg,P= 0.004]均下降。术后随访24~48(32±8)月,无死亡和再干预病例,未见严重不良事件,未见主动脉瓣重度狭窄或重度反流、升主动脉狭窄或扩张、主动脉瓣瓣环狭窄与扩张、瓣膜脱垂或瓣膜赘生物病例;3例患儿分别在随访18、24和12个月时出现瓣叶活动僵硬,瓣叶开放不完全。术后左室后壁厚径均呈下降趋势;末次随访时,8例患儿左室后壁厚径Z值(Z-LVPWd)均下降至正常水平(<2)。结论 以自体心包修复主动脉瓣可改善先天性主动脉瓣狭窄患儿的血流动力学,手术风险低,术后早期疗效可,避免或延缓儿童主动脉瓣置换术,减少施行ROSS手术机会。
主动脉瓣成形; 自体心包; 先天性主动脉瓣狭窄
儿童主动脉瓣病变的治疗目前仍面临巨大挑战。经皮主动脉球囊扩张术、瓣膜交界切开术和瓣膜置换术等,术后往往有残余狭窄、瓣膜返流和需终身抗凝等问题,严重影响了术后早期效果及患儿生存质量。Ross手术无需抗凝,自体肺动脉瓣具有一定的生长潜能,但手术风险高,且两个瓣膜的手术远期并发症较多[1]。主动脉瓣成形术围术期病死率低、并发症少,但手术难度大,缺乏理想的修补材料和手术方式。近年来,国外多个中心报道,以自体心包修复主动脉瓣的近期及远期预后尚可[2-4]。然而,在国内尚无以自体心包修复儿童主动脉瓣病变的报道。现回顾性分析复旦大学附属儿科医院(我院)心血管中心应用自体心包主动脉瓣成形术治疗先天性主动脉瓣狭窄患儿的手术经验及早期预后情况。
1 方法
1.1 先天性主动脉瓣狭窄患儿行自体心包主动脉瓣成形术的适应证和禁忌证 适应证:主动脉瓣平均跨瓣压差>50 mmHg;或症状体征明显,如呼吸急促、喂养困难、晕厥,体格检查见周围动脉搏动减弱、上下肢压差>20 mmHg,肢体皮肤苍白、偏冷,上下肢差异性发绀等。禁忌证:①可行主动脉瓣球囊扩张的主动脉瓣狭窄;②多次瓣叶成形效果不满意者;③瓣叶严重受损,无法修补,需瓣膜置换者;④主动脉瓣瓣环发育差者;⑤左心室发育不良者;⑥伴发其他系统严重疾病者。
1.2 病例纳入标准 在我院行自体心包主动脉瓣成形术的先天性主动脉瓣狭窄的连续患儿。
1.3 主动脉狭窄程度判断 主动脉瓣平均压差<25 mmHg为轻度狭窄,~50 mmHg为中度狭窄,>50 mmHg为重度狭窄。
1.4 自体心包主动脉瓣成形术 术前禁食6 h,全麻后取胸骨正中切口,打开心包,剪取心包(至少5 cm×4 cm),充分剔除筋膜组织,用0.6%戊二醛溶液处理10 min,以丝线牵引,使其充分展开以免皱缩,生理盐水清洗3次,每次6 min。主动脉和上、下腔静脉分别做荷包缝线,插管建立体外循环,阻断升主动脉后,先顺行灌注心肌保护液;伴主动脉瓣返流者,切开主动脉根部,经左、右冠状动脉开口分别灌注心肌保护液。探查主动脉瓣,明确主动脉瓣病变类型。参照文献 [5-8]的方法以自体心包修复主动脉瓣。伴有房间隔缺损或室间隔缺损者一并常规修补。手术结束后入心脏监护室。
术后予口服阿司匹林6个月。术后1周和1、3、6个月及其后每6个月随访超声心动图、X线胸片和心电图。
1.5 超声心动图及相关观察指标 仪器为荷兰Philip公司IE33、美国GE公司vivid7和vivid i,探头采用S8-3、X5-1、M4S和7S-RS,频率5.0~7.5MHz。观察指标[9]:①左心室射血分数(EF);②升主动脉内径Z值(Z-AAO):{ln (y) - [2.773+ 0.494×ln (x)]}/0.095,x指体表面积,y指实际测得的升主动脉内径;③主动脉瓣瓣环Z值(Z-ARD):{ln (y) - [2.693+ 0.473×ln (x)]}/0.0949,x指体表面积,y指实际测得的主动脉瓣瓣环内径;④左室后壁厚径Z值(Z-LVPWd): {ln (y) - [1.783+ 0.373×ln (x)]}/0.141,x指体表面积,y指实际测得的左室后壁厚径;⑤主动脉瓣最大、平均跨瓣压差;⑥主动脉瓣返流程度;⑦主动脉瓣瓣叶运动:观察瓣叶能否完全开放和闭合,有无脱垂,有无赘生物。②~④公式中x是指BSA,y是指实际测得升主动脉内径。
1.6 手术效果的观察指标 ①本文1.1适应证中的阳性症状和体征的变化,②主动脉瓣有无残余狭窄及狭窄程度,③主动脉瓣返流程度,④主动脉瓣叶及活动情况,⑤冠状动脉无损伤,⑥出院前无再干预。
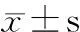
2 结果
2.1 一般情况 2013年7月至2015年6月符合本文纳入标准的9例患儿进入本文分析,男6例,女3例,年龄4月龄至9岁,一般情况见表1。术前超声心动图:9例EF 55~71(64.5±4.9)%;主动脉瓣最大跨瓣压差42~123(73.0±22.2)mmHg,主动脉瓣平均跨瓣压差22~83(35.8±18.3)mmHg;主动脉瓣重度狭窄6例,主动脉瓣中度狭窄1例,主动脉瓣重度狭窄伴主动脉瓣中度返流2例;Z-LVPWd 2.16~5.00(3.34±1.09),左心室均有不同程度肥厚;Z-AAO 0.34~1.89(0.99±0.60),均在正常值范围;Z-ARD -0.09~1.66 (0.53±0.57) ,均在正常值范围。
2.2 手术及围术期情况 9例体外循环时间(120.0±68.4)min,主动脉阻断时间(67.2±26.4)min。主动脉瓣成形方法见表1。
术后即刻超声心动图提示:主动脉最大、平均跨瓣压差与术前比较下降(表2),差异有统计学意义(P<0.05),无中、重度残余狭窄;瓣叶活动可,开放、闭合可,无瓣叶脱垂、撕裂等;例1、3、4、5和9主动脉瓣轻度返流,无中、重度主动脉瓣返流病例;EF 47%~65%,EF仅例9<50%;升主动脉内径与术前比较无明显狭窄及扩张(表2,P>0.05),主动脉瓣环内径与术前比较无明显狭窄及扩张(表2,P>0.05),左室后壁厚径与术前比较无明显缩小与增厚(表2,P>0.05);伴室间隔缺损或房间隔缺损者无残余分流。
术后无患儿发生明显残余狭窄或严重返流、心包填塞和感染性心内膜炎;例1~6、8发生高血压,给予相应处理后血压均降至正常水平;发生心律失常3例,例1和8为室上性心动过速,给予地高辛后心律恢复正常,例3为Ⅰ度房室传导阻滞,未予处理。
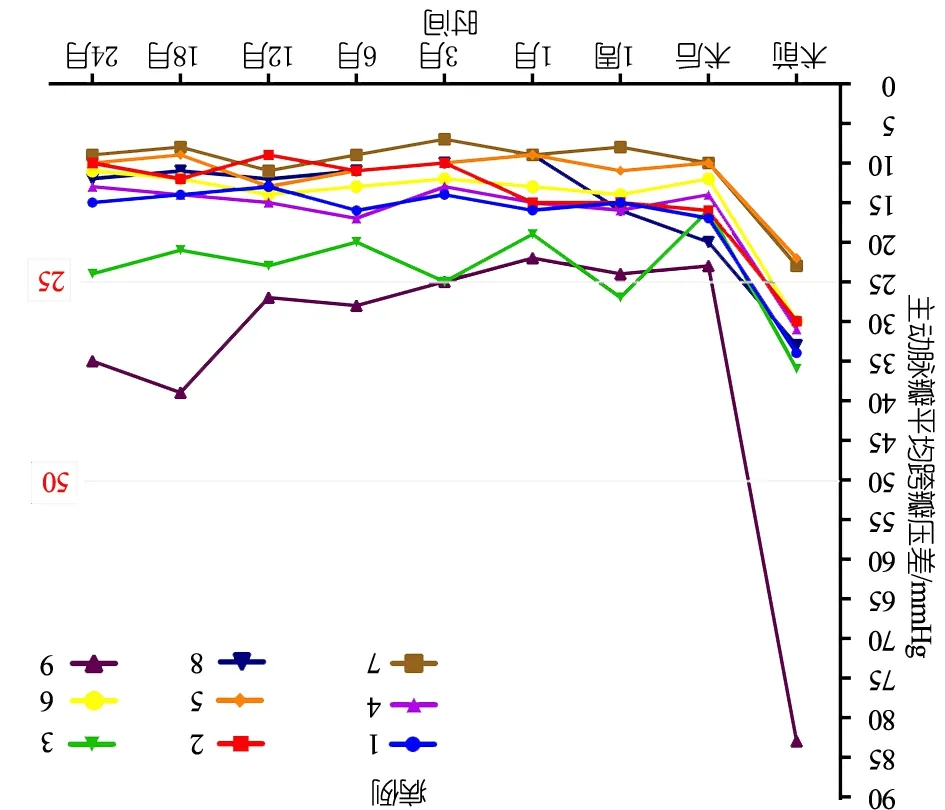
表2 手术前后超声心动图指标比较

指标术前术后即刻tPAVPG-max73.0±22.231.6±9.47.5180.000AVPG-mean35.8±18.315.8±18.34.0680.004Z-LVPWd3.34±1.093.28±1.102.1350.065EF/%64.4±4.965.2±7.9-5.5360.606Z-AAO1.56±0.541.51±0.471.4200.193Z-ARD0.26±0.710.27±0.72-1.6440.139
注 AVPG-max:主动脉瓣最大跨瓣压差(mmHg);AVPG-mean:主动脉瓣平均跨瓣压差(mmHg);Z-LVPWd:左室后壁厚径Z值;EF:左心室射血分数;Z-AAO:升主动脉内径Z值;Z-ARD:主动脉瓣瓣环Z值
9例术后平均监护时间(3.2±1.6)d,住院时间(14±3.7)d,无术中和出院前死亡病例,无出院二次手术病例。
2.3 随访情况 术后随访24~48(32±8)月,无出院死亡和再干预病例,无感染性心内膜炎等不良事件,患儿生活质量可。
末次随访超声心动图提示:9例EF 61%~80% [(71.1±6.5)%];主动脉瓣最大跨瓣压差19~63(34.4±14.5)mmHg,主动脉瓣平均跨瓣压差9~35(15.4±8.5)mmHg,除例9外主动脉瓣平均跨瓣压差均<25 mmHg。图1显示术后2年内9例患儿主动脉瓣平均跨瓣压差变化。图2显示,术后例1~6、8为中度返流,例7和9为轻度反流;Z-LVPWd 0.72~2.51(1.35±0.56),除例9>2,余8例均处于正常范围。术后2年,升Z-AAO 0.34~1.89(0.99±0.60),Z-ARD -0.09~1.66 (0.53±0.57),均在正常范围;无瓣膜脱垂或瓣膜赘生物病例,例3、7和9分别在随访18、24和12个月时瓣叶活动僵硬,瓣膜开放不完全。
3 讨论
儿童主动脉瓣病变主要由先天性瓣膜结构异常以及继发性变性所致,虽然瓣膜置换术是治疗瓣膜病变的金标准,但是瓣膜成形术对于儿童来说有独特优势,一方面,患儿瓣环有机会长至成人大小,避免了多次瓣膜置换;另一方面,可避免人工瓣膜植入造成的感染、瓣膜衰败、血栓和出血等并发症。国外的中长期随访表明,瓣膜成形术术后并发症少,中远期不良事件发生率低,再干预率低,可避免和延缓主动脉瓣换瓣时间,减少施行高难度ROSS术的必要[10]。在中国,缺乏同种瓣膜,且能实施高难度心脏手术的中心不多,瓣膜成形术的应用更为重要。
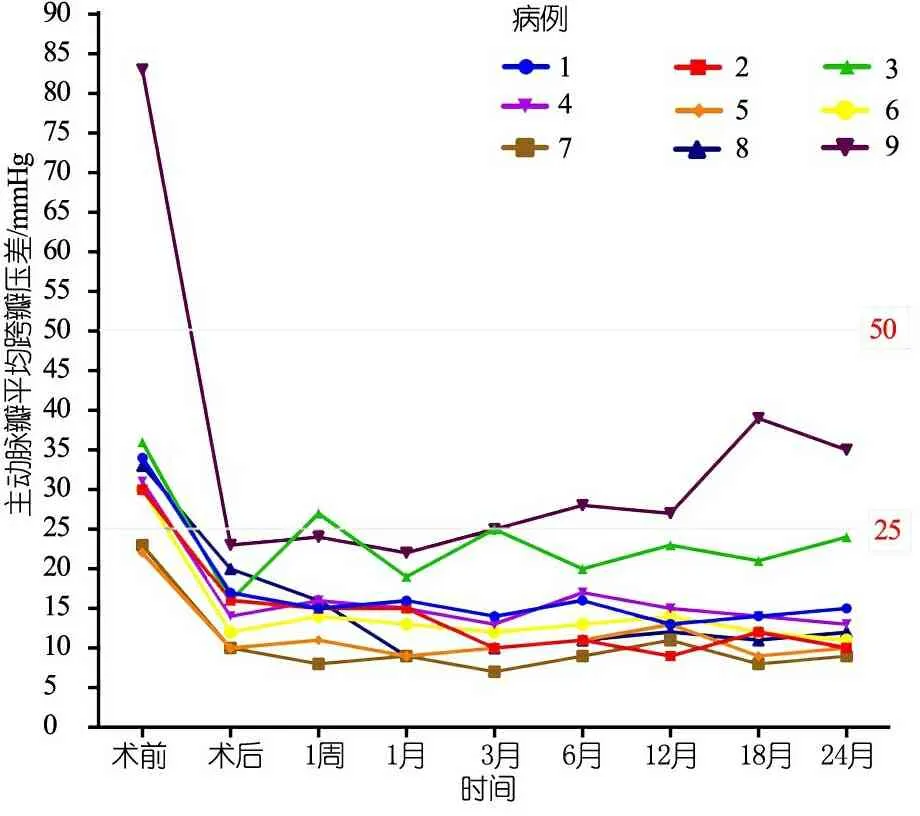
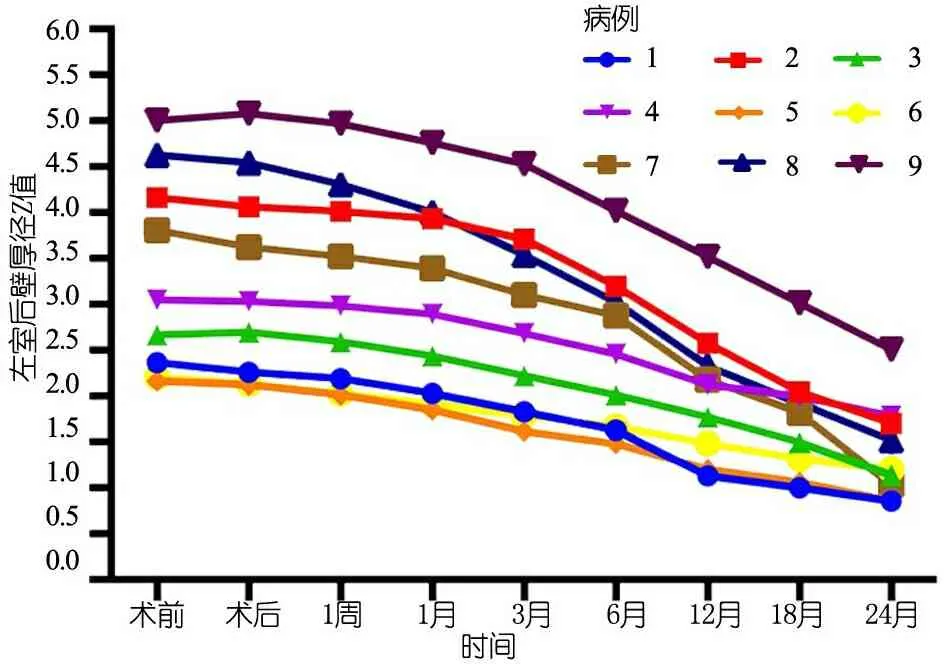
图1 术后2年内9例患儿主动脉瓣平均跨瓣压差变化
图2 术后2年内9例患儿左室后壁厚径Z值变化
传统的瓣膜成形术是指对患者自身主动脉瓣的修复,包括瓣膜交界切开、瓣环成形和瓣叶边缘强化等,主要用于主动脉瓣返流的治疗,这些方法在二叶式主动脉瓣等先天性主动脉瓣叶数量异常或3个瓣叶严重损害的主动脉瓣疾病中的使用受到限制。本研究采用日本Ozaki等发明的瓣膜成形方法,将自身瓣叶切除,用自体心包再造主动脉瓣。
该术式的优点:①适用于任何年龄的患者[11,12]和任何主动脉瓣病变,如先天性或后天性主动脉瓣狭窄,先天性或后天性主动脉瓣返流,主动脉瓣狭窄伴返流,主动脉瓣畸形[13,14];②将瓣叶直接缝合于瓣环,同时将主动脉瓣三叶化,避免了使用支架,获得较大主动脉瓣开放面积,从而获得最佳的血流动力学[15]。国外长达16年的随访远期衰败率低,再干预率低[16]。
瓣膜成形术研究的热点集中在修补材料上,应用较多的材料有牛心包和自体心包等,自体心包较牛心包有免疫原性低、厚度更薄和不易钙化等优势。Al Halees等[16]在对用牛心包和自体心包重建主动脉瓣长达16年的随访过程中发现,牛心包的钙化率明显高于自体心包。1997年,Haydar等[17]报告了44例行自体心包主动脉瓣成形术的病例,平均随访2.6年,无死亡,根据NYHA分级,术后心功能较术前好转,主动脉反流程度较术前下降,由心包瓣膜衰败导致的再手术率仅7%, 提示自体心包修复主动脉瓣手术风险低,早中期结果可;2006年,Alsoufi等[18]报告了22例自体心包延长主动脉瓣的病例,平均年龄11.4岁,随访5年,均存活,主动瓣关闭不全、主动脉瓣狭窄程度、左心室功能与术前相比有所改善;避免瓣膜置换的自由度为75%。2015年,日本Ozaki等[5]对416例主动脉瓣狭窄的患者用自体心包重建主动脉瓣,平均年龄(71.2±12.0)岁,平均随访(25.2±17.5)月,术前主动脉平均压差(79.0±33.6)mmHg,术后1周和5年分别为(21.2±10.7)mmHg和(14.3±5.0)mmHg,3年无需再干预率高达96.7%。自体心包是修复主动脉瓣病变的合适材料之一,修复瓣叶活动可,虽有残余的血流动力学缺陷,如轻中度的主动脉瓣狭窄和主动脉瓣反流,但是有研究表明,对于主动脉轻度狭窄(心导管提示主动脉瓣平均跨瓣<25 mm Hg )或主动脉瓣轻中度返流的患者,90%的患者10年内无需手术治疗[19,20]。
本文9例先天性主动脉瓣狭窄患儿行自体心包主动脉瓣成形术前后的主动脉瓣最大跨瓣压差和平均跨瓣压差下降(P<0.05),提示手术有效缓解了主动脉狭窄;术后仅1例(例9)中度主动脉瓣狭窄、2例(例7、9)中度主动脉瓣反流,升主动脉内径和主动脉瓣环内径无扩张或狭窄,提示以自体心包修复儿童主动脉瓣狭窄可明显改善患儿血流动力学。在术后以及随访过程中9例的Z-LVPWd明显下降,提示该术式虽有残余的血流动力学异常,但是从保护心脏几何形态和预防心室重构的角度来看,该术式是成功的;从另一个角度来看,Z-LVPWd下降提示了术后残余的血流动力学异常在长期可耐受的范围内。然而,本文病例中仍有3例(例3、7和9)心超随访提示瓣叶活动僵硬,可能与心包处理方法、瓣叶交界处缝合、心包与瓣叶组织结构不同等因素有关。
自体心包主动脉瓣成形术的关键因素:①瓣叶的数量(三叶式主动脉瓣血流动力学更好)和质量(修补瓣叶的材料)。②瓣叶交界处的处理、瓣叶的运动和残余反流程度[21]。③心包的处理:使用戊二醛处理自体心包可预防再造瓣叶的挛缩及衰败[5];心包的修剪和瓣叶的高度对术后血流动力学影响非常重要,可制作合适尺寸的模具来确定瓣叶的形状和大小。本研究中心近期采用标准化模具裁剪心包,发现术后患儿瓣膜运动更自然,血流动力学纠治更完善。④患儿的选择[22]:该术式可切除所有瓣叶,采用自体心包重建3个瓣叶,从某种程度上,比传统的瓣膜成形术适应证更广,瓣叶严重受损、主动脉瓣返流和瓣膜赘生物的患儿均可采用,但需排除伴其他瓣膜严重病变者、多次瓣膜成形不满意者及需要瓣膜置换的患儿;此外,心肌收缩力差、左心室发育不良、主动脉瓣瓣环发育差的患儿不宜采用。
综上所述,以自体心包修复主动脉瓣可改善先天性主动脉瓣狭窄患儿的血流动力学,手术风险低,术后早期疗效可。对于部分主动脉瓣狭窄的患儿可采用该术式可避免或延缓瓣膜置换、减少ROSS术的实施。需进一步随访明确该术式的长期疗效。
[1]Hraska V, Krajci M, Haun Ch, et al. Ross and Ross-Konno procedure in children and adolescents: mid-term results. Eur J Cardiothorac Surg,2004,25(5):742-747 .
[2]Zacek P, Holubec T, Vobornik M, et al. Quality of life after aortic valve repair is similar to Ross patients and superior to mechanical valve replacement: a cross-sectional study. BMC Cardiovasc Disord, 2016, 16(1):1-8 .
[3]Ozaki S, Kawase I, Yamashita H, et al. A total of 404 cases of aortic valve recon-struction with glutaraldehyde-treated autologous pericardium. J Thorac Cardiovasc Surg, 2014, 147(1):301-306 .
[4]Ozaki S, Kawase I, Yamashita H, et al. Aortic valve reconstruction using autolo-gous pericardium for patients aged less than 60 years. J Thorac Cardiovasc Surg, 2014, 148(3):934-938 .
[5]Ozaki S, Kawase I, Yamashita H, et al. Aortic Valve Reconstruction Using Autologous Pericardium for Aortic Stenosis. Circ J,2015,79(7):1504-1510 .
[6]Ozaki S, Kawase I, Yamashita H, et al. Reconstruction of bicuspid aortic valve with autologous pericardium—usefulness of tricuspidization.Circ J, 2014,78(5):1144-1151 .
[7]Kawase I, Ozaki S, Yamashita H, et al. Aortic valve reconstruction of unicuspid aortic valve by tricuspidization using autologous pericardium. Ann Thorac Surg, 2012, 94(4):1180-1184 .
[8]Hammer PE, del Nido PJ. Guidelines for sizing pericardium for aortic valve leaflet grafts. Ann Thorac Surg, 2013, 96(1):e25-27 .
[9]黄国英.小儿超声心动图学. 上海 : 上海科学技术出版社, 2015:93,135 .
[10]Tweddell JS, Pelech AN, Frommelt PC, et al. Complex aortic valve repair as a durable and effective alternative to valve replacement in children with aortic valve disease. J Thorac Cardiovasc Surg,2005,129(3):551-558 .
[11]Ozaki S, Kawase I, Yamashita H, et al. Aortic valve reconstruction using autologous pericardium for patients aged less than 60 years. Journal of Thoracic & Cardio-vascular Surgery, 2014, 148(3):934-938 .
[12]Ozaki S, Kawase I, Yamashita H, et al. Aortic valve reconstruction using autologous pericardium for ages over 80 years. Asian Cardiovascular & Thoracic Annals, 2014, 22(8):903-908 .
[13]Kawase I, Ozaki S, Yamashita H, , et al. Original aortic valve plasty with autologous pericardium for quadricuspid valve. Ann Thorac Surg, 2011,91(5): 1598-1599 .
[14]Kawase I, Ozaki S, Yamashita H, et al. Aortic valve reconstruction of unicuspid aortic valve by tri-cuspidization using autologous pericardium. Ann Thorac Surg 2012, 94(4): 1180-1184 .
[15]Yaku H. Aortic valve reconstruction with autologous glutaraldehyde-treated pericardium—a new paradigm for aortic valve surgery? Circ J, 2014, 78(5):1063-1065 .
[16]Al Halees Z, Al Shahid M, Al Sanei A, et al. Up to 16 years follow-up of aortic valve reconstruction with pericardium: a stentless readily available cheap valve? Eur J Cardiothorac Surg,2005, 28(2):200-205 .
[17]Haydar HS, He GW, Hovaguimian H, et al. Valve repair for aortic insufficiency: surgical classification and techniques. Eur J Cardiothorac Surg,1997,11(2):258-265 .
[18]Alsoufi B, Karamlou T, Bradley T, et al. Short and midterm results of aortic valve cusp extension in the treatment of children with congenital aortic valve disease. Ann Thorac Surg,2006,82(4):1292-1299, 1300 .
[19]Siddiqui J, Brizard CP, Konstantinov IE, et al. Outcomes after operations for bicuspid aortic valve disease in the pediatric population. Ann Thorac Surg,2013,96(6):2175-2183 .
[20]Gersony WM, Hayes CJ, Driscoll DJ, et al. Second natural history study of congenital heart defects. Quality of life of patients with aortic stenosis, pulmonary stenosis, or ventricular septal defect. Circulation,1993,87(2 Suppl):I52-I65 .
[21]Mitchell BM, Strasburger JF, Hubbard JE, et al. Serial exercise performance in children with surgically corrected congenital aortic stenosis. Pediatr Cardio, 24(4):319-324 .
[22]Mastrobuoni S, El Khoury G. Aortic valve repair and Ross operation in children: the importance of patient selection and surgical technique. Eur J Cardiothorac Surg,2016,49(3):892-893
Surgical repair of aortic stenosis by using autologous pericardium in pediatric population: a report of 9 cases
SHANYa-ping,JIABing,ZHANGHui-feng,YEMing,SHENHua
(CardiovascularCenter,Children'sHospitalofFudanUniversity,Shanghai201102,China)
JIA Bing,E-mail:jiabing2012@hotmail.com
ObjectiveTo summarize the surgical technique of repairing congenital aortic valve stenosis by using autologous pericardium and analyze its early outcomes in children.MethodsA total of children with congenital aortic stenosis who underwent autologous pericardial aortic valvuloplasty at the Children's Hospital of Fudan University from July 2013 to June 2015 were collected for clinical information, including general informations, perioperative conditions, complications , preoperative, intraoperative, postoperative color echocardiograph data, and follow-up information. Results9 children according with the inclusion criteria for this article were analyzed: There were 6 males and 3 females, aged from 4 months to 9 years old. Preoperative color echocardiograph showed tricuspid aortic valve (n=3), bicuspid aortic valve(n=6); severe aortic valve stenosis (n=6), moderate aortic valve stenosis(n=1) and severe aortic valve stenosis with moderate aortic valve re-gurgitation (n=2). The surgical procedures of 9 patients were all based on reconstruction aortic valve (reconstructed leaflets or expand leaflets) by autologous pericardium. Compared to the preoperative echocardiograph, immediately postoperative echocardiography results showed that aortic valve peak pressure gradient [(31.6±9.4)mmHgvs(73.0±22.2)mmHg,P=0.000]and aortic valve average pressure gradient [(15.8±18.3)mmHgvs(5.8±18.3)mmHg,P= 0.004]were decreased significantly. 24~48(32±8)months were followed up after operation, no death events or reoperation events or adverse event were recorded, and there was no patient with severe aortic stenosis or sever aortic regurgitation , ascending aortic stenosis or dilatation, aortic valvular ring stenosis or dilatation. And there were no patients with aortic valve prolapse or aortic valve vegetation.3 patients encountered with aortic valve stiffness and restriction of aortic valve open at the18, 24 and 12 months after operation respectively.The left ventricular posterior wall diameter was decreased in all patients ,and except for 1 case, the Z-LVPWd of rest 8 patients was at normal level upon the last follow-up. Conclusion Using autologous pericardium to repair aortic valve lesions can improve the hemodynamics of children with congenital aortic valve stenosis, the risk of this operation is low and the short-term results are excellent. Furthermore, this operation technique can avoid or put off the valve replacement operation and reduce the operation of Ross procedure.
Aortic valvuloplasty; Autologous pericardium; Congenital Aortic valve stenosis
2017-07-19
2017-08-08)
(本文编辑:张崇凡,孙晋枫)
复旦大学附属儿科医院心血管中心 上海,201102
贾兵,E-mail: jiabing2012@hotmail.com
10.3969/j.issn.1673-5501.2017.04.006
