热应激对不同品种(系)青年肉牛生产性能、营养物质表观消化率及血液生化指标的影响
2017-09-16蒲启建王之盛彭全辉景小平邹华围
蒲启建 王之盛 彭全辉 张 灿 景小平 胡 瑞 邹华围
(四川农业大学动物营养研究所,牛低碳养殖与安全生产重点实验室,雅安625014)
热应激对不同品种(系)青年肉牛生产性能、营养物质表观消化率及血液生化指标的影响
蒲启建 王之盛*彭全辉 张 灿 景小平 胡 瑞 邹华围
(四川农业大学动物营养研究所,牛低碳养殖与安全生产重点实验室,雅安625014)
本试验旨在研究热应激对不同品种(系)青年肉牛生产性能、营养物质表观消化率及血液生化指标的影响,探究不同品种(系)肉牛的耐热性差异。选取体重[(185.89±14.02) kg]相近、健康的青年西杂牛(西门塔尔牛×宣汉黄牛)、地方黄牛(宣汉黄牛)和犏牛(娟姗牛×麦洼牦牛)各6头为研究对象,试验期间不同品种(系)的青年肉牛饲喂相同饲粮。试验时间为2015年4—9月,预试期7 d,正试期180 d。结果表明:1)试验牛舍5—8月温湿度指数(THI)高于72,属于热应激期,且显著高于4月(热应激发生前)和9月(热应激发生后)(P<0.05);热应激环境导致各试验牛呼吸频率和直肠温度显著升高(P<0.05)。试验期间犏牛呼吸频率和直肠温度显著高于西杂牛和地方黄牛(P<0.05)。2)热应激期内西杂牛、地方黄牛和犏牛单位体重干物质采食量均有不同程度降低,以6月为最低,均较4月显著降低(P<0.05),且降低幅度为犏牛>西杂牛>地方黄牛。西杂牛、地方黄牛和犏牛的平均日增重(ADG)均在6月最低,分别较4月降低了27.62%(P<0.05)、10.81%(P>0.05)和46.15%(P<0.05);西杂牛的ADG在4—7月显著高于犏牛(P<0.05)。西杂牛和犏牛的料重比(F/G)以6月为最高,分别较4月升高了63.01%(P<0.05)和89.03%(P<0.05);地方黄牛的F/G在9月最高,较4月升高了53.12%(P<0.05)。3)热应激期内西杂牛、地方黄牛和犏牛的粗蛋白质(CP)表观消化率均有不同程度降低,且7月均较4月显著降低(P<0.05);西杂牛7月的粗脂肪(EE)表观消化率较4月显著降低(P<0.05),而热应激对地方黄牛和犏牛的EE表观消化率无显著影响(P>0.05);热应激期内犏牛中性洗涤纤维(NDF)(6月)和酸性洗涤纤维(ADF)的表观消化率(5—8月)较热应激发生前显著降低(P<0.05);热应激期内西杂牛和犏牛钙(Ca)(西杂牛:6—8月;犏牛:5—8月)和磷(P)的表观消化率(西杂牛:6—7月;犏牛:5—8月)较热应激发生前显著降低(P<0.05)。试验期间,不同品种(系)牛的CP表观消化率无显著差异(P>0.05),西杂牛和地方黄牛的EE表观消化率显著高于犏牛(P<0.05),地方黄牛的ADF表观消化率显著高于西杂牛和犏牛(P<0.05),西杂牛和地方黄牛的P表观消化率显著高于犏牛(P<0.05)。此外,5—9月地方黄牛的Ca表观消化率显著高于犏牛(P<0.05)。4)西杂牛、地方黄牛和犏牛血清中葡萄糖(GLU)浓度均以6月最低,较4月分别降低了16.82%(P<0.05)、12.82%(P>0.05)和15.90%(P<0.05);热应激期间各试验牛血清中非酯化脂肪酸(NEFA)浓度均以7月最低,显著低于4月(P<0.05);试验期间西杂牛和地方黄牛血清中尿素氮(UN)浓度随年龄的增大呈先升高后降低变化,而犏牛血清中UN浓度则持续升高;6—9月各试验牛血清肌酐(CRE)浓度均显著高于4、5月(P<0.05),且7—9月犏牛血清CRE浓度显著高于地方黄牛(P<0.05)。综上所述,热应激导致不同品种(系)青年肉牛生产性能降低、饲粮营养物质消化率降低,地方黄牛的耐热应激能力强于西杂牛和犏牛,犏牛对热应激最敏感。
热应激;肉牛;生产性能;营养物质表观消化率;血液生化指标
随着我国人们生活水平的提高,消费者对牛肉的消费需求逐年增加,然而国内牛肉却因牛源不足连续减产。《中华人民共和国国民经济和社会发展第十三个五年规划纲要》提出要分区域推进现代草业和草食畜牧业发展。然而,当前牧区牲畜超载过牧越来越严重,草畜矛盾越来越尖锐。我国南方地区因其气候优势,草场的单位面积产量高、开发潜力较大,肉牛养殖有着可观的发展前景。“牧繁农育”的提出,为缓解牧区草畜矛盾、提高农区肉牛产量提供了解决办法。但是,在我国南方高温高湿环境下牛极易出现热应激反应[1],严重降低其生产性能[2-3],给养殖业造成巨大损失[4]。国内外常将环境温度和湿度相结合即温湿度指数(temperature humidity index,THI)来评价外界环境,且大量研究认为当THI大于72时,奶牛即处于热应激状态[5-7]。
牛的耐热性与品种(系)、生产性能有密切关系[8-9]。西杂牛是为改善我国地方黄牛生产性能而与西门塔尔牛杂交的改良种;黄牛是我国固有牛种,其养殖数量在我国牛类中居首,其适应能力强,耐粗饲;犏牛则是青藏高原地区为改善牦牛生产性能而与其他普通牛种杂交的品种,能适应高海拔、低气压和冷季长的生态环境。上述3种肉牛在我国农区和牧区肉牛养殖中有着举足轻重的地位。目前关于反刍动物热应激的研究主要集中于奶牛,关于肉牛热应激的相关研究还相对较少,因此探究西杂牛、地方黄牛和犏牛的耐热能力对南方高温高湿地区肉牛养殖具有重要意义。鉴于此,本试验选用西杂牛(西门塔尔牛×宣汉黄牛)、地方黄牛(宣汉黄牛)和犏牛(娟姗牛×麦洼牦牛)3种肉牛,在南方地区(四川省雅安市)经4—9月连续6个月饲养,通过生产性能、营养物质表观消化率、血液生化指标等考察热应激对不同品种(系)肉牛的影响,为我国南方地区肉牛养殖品种的选择提供参考。
1 材料与方法
1.1试验动物与试验设计
本试验于2015年4—9月在四川农业大学动物营养研究所试验场(平均海拔598 m)进行。选取体重[(185.89±14.02) kg]相近、健康的青年西杂牛(西门塔尔牛×宣汉黄牛)、地方黄牛(宣汉黄牛)、犏牛(麦洼牦牛×娟姗牛)去势公牛各6头,分为3个组,即西杂牛组、地方黄牛组和犏牛组,每组6个重复,每个重复1头牛。试验期间不同品种(系)的青年肉牛饲喂相同饲粮。西杂牛和地方黄牛购于四川省达州市宣汉县(平均海拔780 m),犏牛购于四川省阿坝藏族羌族自治州红原县(平均海拔3 500 m)。
1.2饲养管理与饲粮
所有牛只均舍饲饲养,预试期7 d,正试期180 d。饲养开始前,对试验牛进行驱虫处理。每天09:00和15:00定时饲喂2次,以预试期测定的采食量为基础,自由采食和饮水,所有余料在第2天晨饲前记录。
根据中国《肉牛饲养标准》(NY/T 815—2004)中200 kg体重、日增重800 g肉牛营养推荐值,以玉米、豆粕、小麦麸、菜籽粕等为精料,以稻草、白酒糟为粗料设计配方。饲粮组成及营养水平见表1。
1.3样品采集和指标测定
1.3.1 样品采集
试验期内,在牛舍前、中、后部距地面1.5 m高处各悬挂1支干湿温度计,于每天08:00、11:00、14:00和17:00记录牛舍的干球温度(Td)和湿球温度(Tw),利用下列公式计算THI。
THI=0.72×(Td+Tw)+40.6[10]。
参考Johnson等[11]的方法每10 d分别于08:00和14:00测量各组肉牛直肠温度并记录呼吸频率。
试验期间每天记录各试验牛精料、粗料实际饲喂量,并计算干物质采食量(DMI);根据DMI和体重计算单位体重DMI。

表1 饲粮组成及营养水平(风干基础)
1)预混料为每千克饲粮提供The premix provided the following per kg of the diet:VA 3 300 IU,VD 880 IU,VE 60 IU,Cu (as copper sulfate) 10 mg,Fe (as ferrous sulfate) 50 mg,Mn (as manganese sulfate) 20 mg,Zn (as zinc sulfate) 30 mg,I (as potassium iodide) 0.50 mg,Se (as sodium selenite) 0.10 mg,Co (as cobalt chloride) 0.1 mg。
2)综合净能根据我国《肉牛饲养标准》(NY/T 815—2004)计算得出,其余为实测值。NEmfwas calculated according to the ChineseFeedingStandardofBeefCattle(NY/T 815—2004), while the other nutrient levels were measured values.
分别在正式试验第0天(正式试验开始前)、第30天、第60天、第90天、第120天、第150天、第180天晨饲前空腹称重,计算各试验牛的平均日增重(ADG)并计算料重比(F/G);并由颈静脉采集试验牛血液15 mL,静置30 min后4 000 r/min离心15 min制备血清,-20 ℃保存待测血液生化指标。
试验期内每30 d采集1次饲料样,-20 ℃保存。分别在正式试验第0天、第30天、第60天、第90天、第120天、第150天连续7 d 08:00、14:00、20:00采集各试验牛鲜粪样100 g左右,于-20 ℃保存,待7 d粪样收集完全后混匀,按粪样重量的5%加入浓度为10%的稀硫酸固氮,-20 ℃保存待测[12]。
1.3.2 指标测定
参照张丽英[13]的方法测定饲粮及粪样中干物质(DM)、粗蛋白质(CP)、粗纤维(CF)、中性洗涤纤维(NDF)、酸性洗涤纤维(ADF)、钙(Ca)和磷(P)的含量。采用内源指示剂[酸不溶灰分(AIA)]法测定试验牛的营养物质表观消化率[12]。
某营养物质表观消化率(%)=100-100×[(F2/F1)×(A1/A2)]
式中:A1为饲粮中AIA含量(%);A2为粪中AIA含量(%);F1为饲粮中该营养物质含量(%);F2为粪中该营养物质含量(%)。
采用全自动生化分析仪(AUTOLAB PM-4000,意大利)通过比色法测定血清中葡萄糖(GLU)、甘油三酯(TG)、尿素氮(UN)、肌酐(CRE)的浓度;采用酶联免疫吸附测定(ELISA)法测定血清中非酯化脂肪酸(NEFA)的浓度,试剂盒购于南京建成生物工程研究所,具体操作方法参考说明书进行。
1.4数据分析
试验数据经Excel 2016初步分析后,用SPSS 19.0统计软件进行单因素方差分析(one-way ANOVA)程序方差分析,有显著差异(P<0.05)时,以Duncan氏法进行多重比较,结果以平均值±标准差表示。
2 结果与分析
2.1牛舍THI与肉牛直肠温度、呼吸频率
试验期间牛舍THI指数变化见表2和图1。试验期间牛舍内温度逐渐升高,THI也逐渐升高,5—8月牛舍THI显著高于4月和9月(P<0.05)。根据THI将整个试验阶段分为热应激发生前(4月,THI<72)、热应激期(5—8月,THI≥72)和热应激结束后(9月,THI<72)。
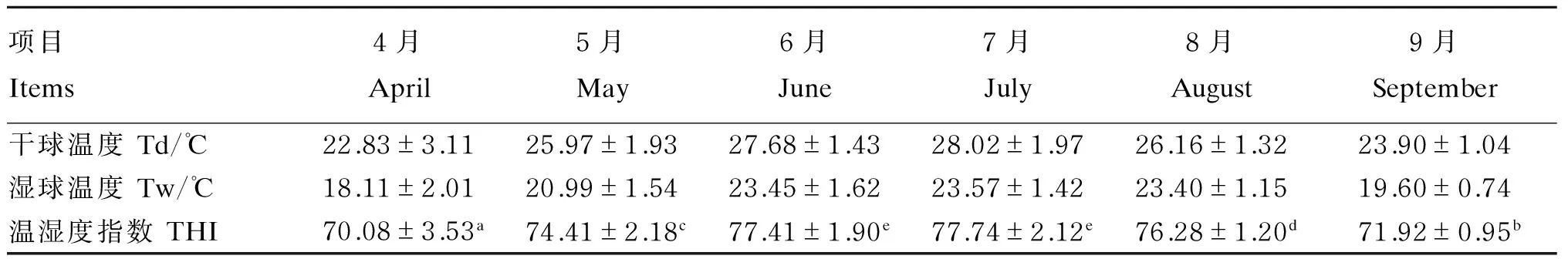
表2 试验期间牛舍THI变化
同行数据肩标无字母或相同小写字母表示差异不显著(P>0.05),不同小写字母表示差异显著(P<0.05)。
In the same row, values with the same or no small letter superscripts mean no significant difference (P>0.05), while with different small letter superscripts mean significant difference (P<0.05)。
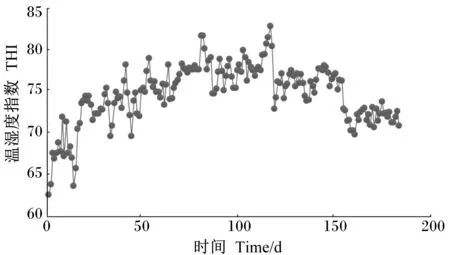
图1 试验期间牛舍THI
热应激对肉牛呼吸频率和直肠温度的影响见表3。
各试验牛只试验期间呼吸频率随THI的升高而升高,湿热应激期内各试验牛呼吸频率较湿热应激发生前显著升高(P<0.05)。试验期间犏牛呼吸频率显著高于西杂牛和地方黄牛(P<0.05),而西杂牛呼吸频率显著高于地方黄牛(P<0.05)。
各试验牛直肠温度均是在热应激期中的6月开始显著升高(P<0.05)。西杂牛、地方黄牛和犏牛均以7月直肠温度最高,分别较热应激发生前升高了1.15%(P<0.05)、0.84%(P<0.05)和1.98%(P<0.05),而热应激结束后直肠温度均较7月显著降低(P<0.05)。试验期间犏牛直肠温度显著高于西杂牛和地方黄牛(P<0.05)。
不同月份THI和各试验牛只直肠温度、呼吸频率的变化表明西杂牛、地方黄牛和犏牛在5—8月处于热应激状态。
2.2热应激对肉牛生产性能的影响
热应激对肉牛生产性能的影响见表4。
试验期间随着试验牛年龄的增大,西杂牛、地方黄牛和犏牛DMI均有不同程度升高,9月相对于4月分别升高了65.01%(P<0.05)、37.83%(P<0.05)和33.89%(P<0.05);9月时西杂牛DMI显著高于地方黄牛和犏牛(P<0.05)。热应激期内西杂牛、地方黄牛和犏牛单位体重DMI均有不同程度降低,且以6月为最低,较热应激发生前分别降低了11.17%(P<0.05)、8.83%(P<0.05)和16.15%(P<0.05);犏牛单位体重DMI的降低较西杂牛和地方黄牛发生早,与热应激发生前相比,西杂牛和地方黄牛单位体重DMI在6月开始显著降低(P<0.05),而犏牛在5月即开始显著降低(P<0.05)。
各试验牛ADG在6月最低,其中西杂牛6月的ADG较热应激发生前降低了27.62%(P<0.05),地方黄牛6月的ADG较热应激发生前降低了10.81%(P>0.05),犏牛6月的ADG较热应激发生前降低了46.15%(P<0.05);在整个试验期内,西杂牛的ADG均高于地方黄牛和犏牛,且在4—7月与犏牛的差异达到显著水平(P<0.05)。
热应激期内西杂牛和犏牛的F/G均有不同程度升高,且以6月为最高,分别较热应激发生前升高了63.01%(P<0.05)和89.03%(P<0.05);试验期间地方黄牛F/G随年龄的增大逐渐升高,9月的F/G较热应激发生前升高了53.12%(P<0.05);在整个试验期内,犏牛F/G均高于西杂牛和地方黄牛,且5—7月与西杂牛和地方黄牛的差异达到显著水平(P<0.05)。
2.3热应激对肉牛营养物质表观消化率的影响
由表5可知,热应激期内西杂牛、地方黄牛和犏牛的CP表观消化率均有不同程度降低,且以7月最低,分别较热应激发生前降低了10.30%(P<0.05)、10.17%(P<0.05)和9.77%(P<0.05);西杂牛7月的EE表观消化率较热应激发生前显著降低(P<0.05),而热应激对地方黄牛和犏牛的EE表观消化率无显著影响(P>0.05);热应激期内犏牛NDF和ADF的表观消化率均有不同程度降低,其NDF的表观消化率在6月显著低于热应激发生前(P<0.05),ADF的表观消化率在整个热应激期内均显著低于热应激发生前(P<0.05);热应激期内西杂牛和犏牛Ca的表观消化率均有不同程度降低,其中西杂牛Ca的表观消化率在6—8月显著低于热应激发生前(P<0.05),犏牛Ca的表观消化率在整个热应激期内均显著低于热应激发生前(P<0.05);热应激期内西杂牛和犏牛P的表观消化率均有不同程度降低,其中西杂牛P的表观消化率在6—7月显著低于热应激发生前(P<0.05),犏牛P的表观消化率在整个热应激期内均显著低于热应激发生前(P<0.05)。试验期间不同品种(系)牛的CP表观消化率无显著差异(P>0.05),西杂牛和地方黄牛的EE表观消化率显著高于犏牛(P<0.05),地方黄牛的ADF表观消化率均显著高于西杂牛和犏牛(P<0.05),西杂牛和地方黄牛的P表观消化率显著高于犏牛(P<0.05);5—9月地方黄牛的Ca表观消化率显著高于犏牛(P<0.05)。
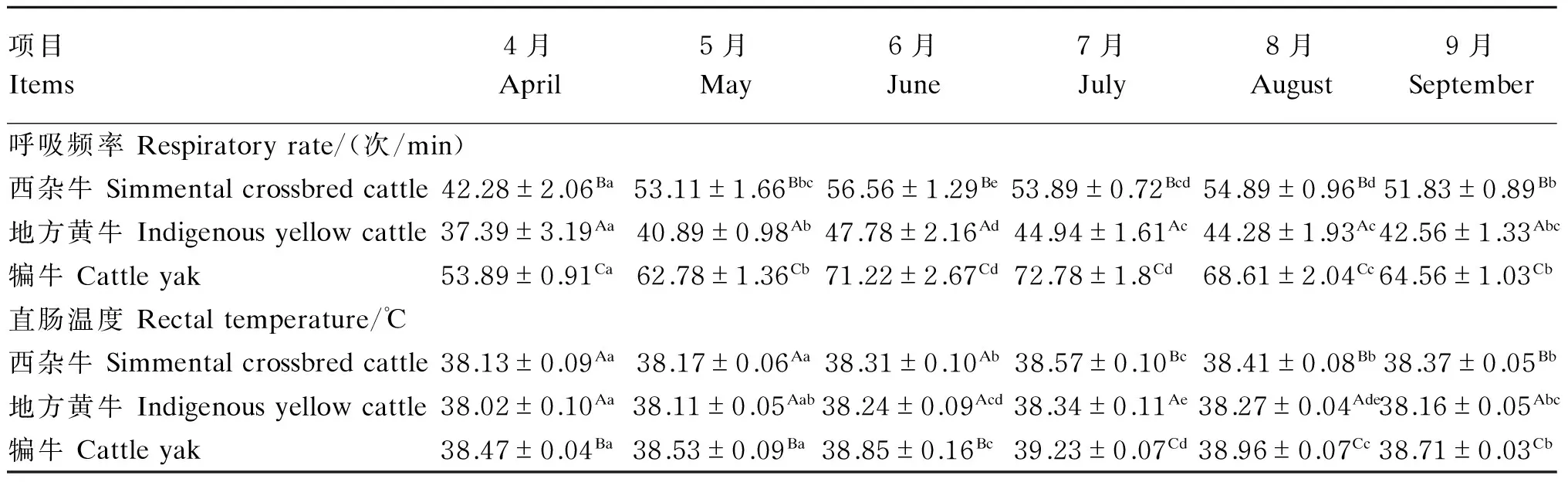
表3 热应激对肉牛呼吸频率和直肠温度的影响
同行数据肩标无字母或相同小写字母表示差异不显著(P>0.05),不同小写字母表示差异显著(P<0.05)。同列数据肩标无字母或相同大写字母表示差异不显著(P>0.05),不同大写字母表示差异显著(P<0.05)。下表同。
In the same row, values with the same or no small letter superscripts mean no significant difference(P>0.05), while with different small letter superscripts mean significant difference (P<0.05). In the same column ,values with the same or no capital letter superscripts mean no significant difference (P>0.05), while with different capital letter superscripts mean significant difference (P<0.05). The same as below.
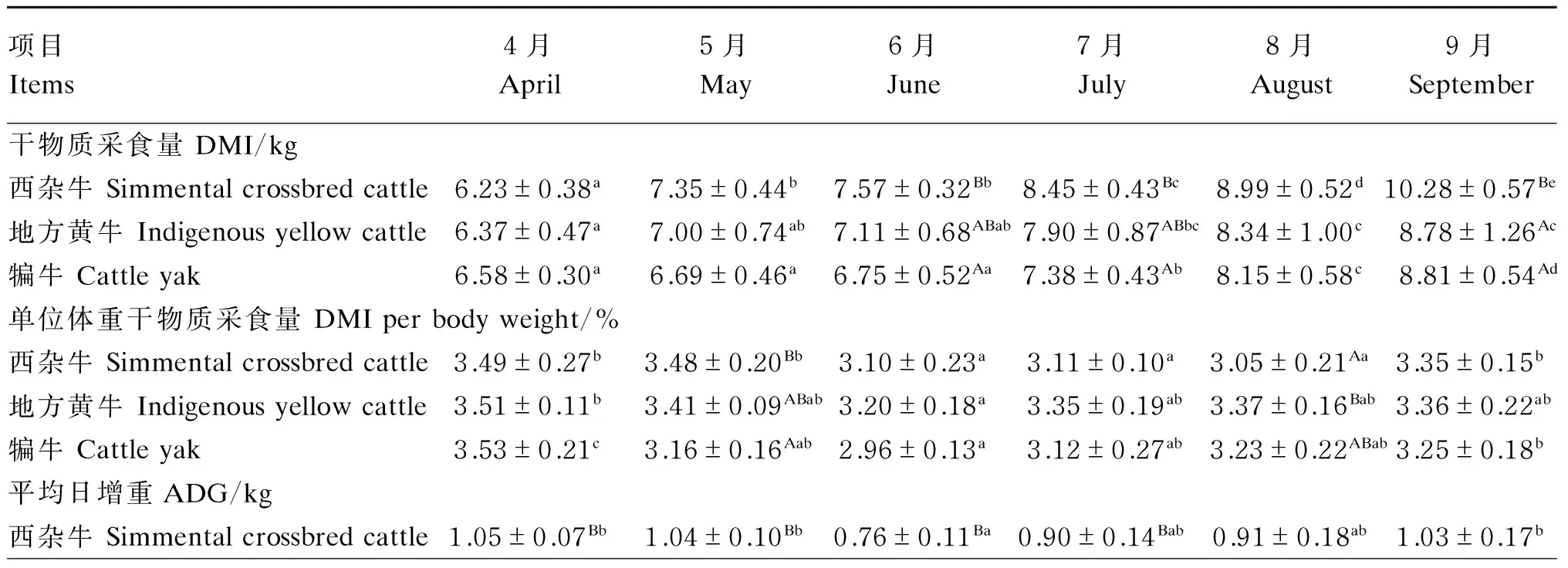
表4 热应激对肉牛生产性能的影响

续表4项目Items4月April5月May6月June7月July8月August9月September地方黄牛Indigenousyellowcattle0.74±0.19A0.81±0.28B0.66±0.23B0.74±0.28AB0.71±0.240.71±0.29犏牛Cattleyak0.78±0.07Ab0.53±0.13Aa0.42±0.09Aa0.53±0.09Aa0.66±0.17ab0.62±0.14ab料重比F/G西杂牛Simmentalcrossbredcattle6.11±0.68Aa7.05±0.81Aab9.96±1.43Ac9.45±1.56Abc9.87±2.43c9.81±0.21c地方黄牛Indigenousyellowcattle7.70±0.35Ba8.92±2.88Aab10.83±2.64Aab11.04±1.57Aab11.69±2.27b11.79±2.31b犏牛Cattleyak8.57±0.96Ba12.57±1.08Bab16.20±3.18Bb14.38±2.07Bab12.71±2.16ab13.24±3.68ab
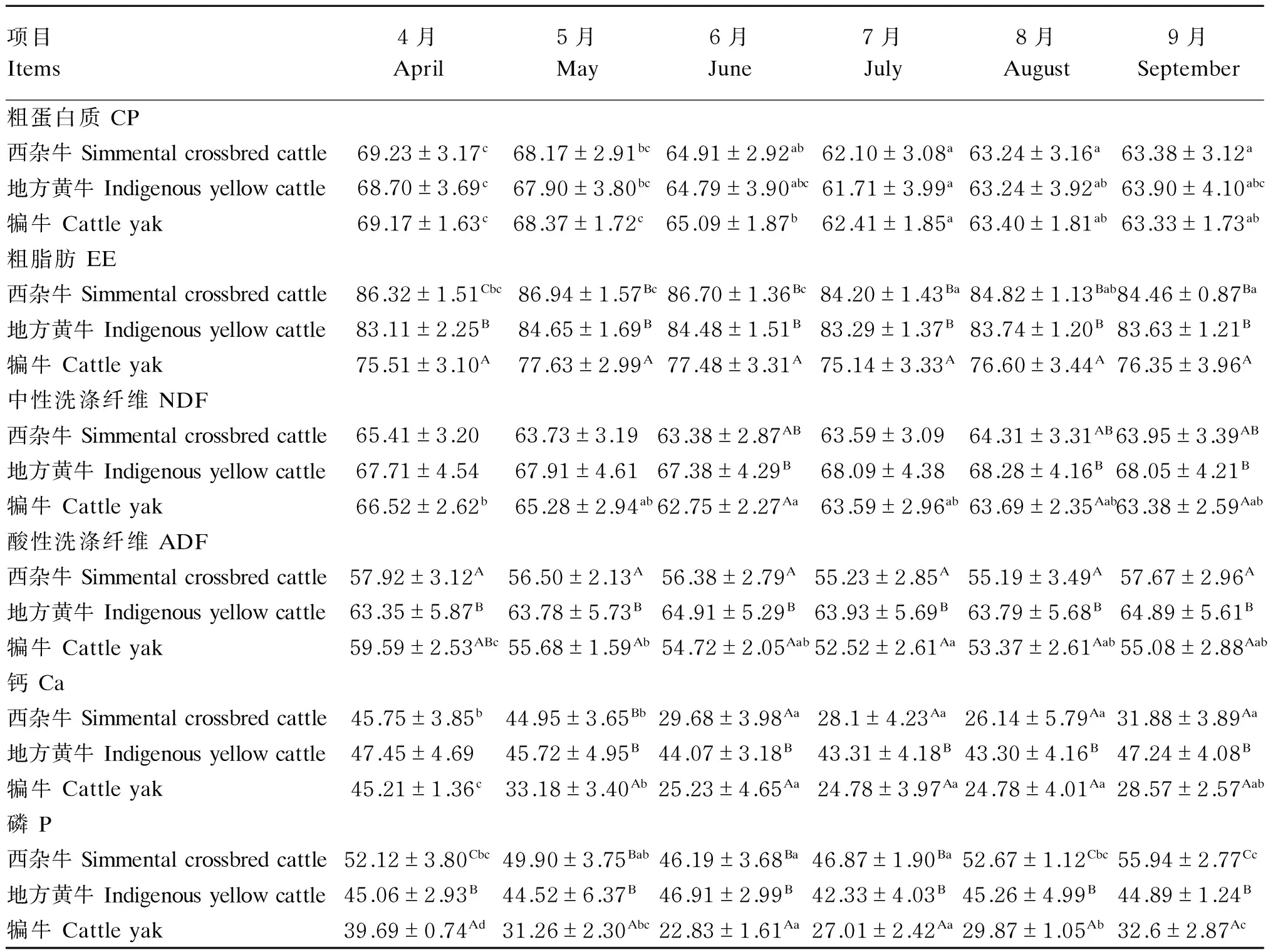
表5 热应激对肉牛营养物质表观消化率的影响
2.4热应激对肉牛血液生化指标的影响
由表6可知,热应激期内西杂牛、地方黄牛和犏牛血清中GLU浓度均先降低后升高,且均以6月最低,较热应激发生前分别降低了16.82%(P<0.05)、12.82%(P>0.05)和15.90%(P<0.05)。试验期间各试验牛血清中TG浓度随年龄的增大逐渐升高,热应激结束后血清中TG浓度显著高于热应激发生前(P<0.05),且热应激结束后西杂牛血清中TG浓度显著高于地方黄牛和犏牛(P<0.05)。此外,热应激期间各试验牛血清中NEFA浓度均有不同程度降低,且以7月最低,显著低于热应激发生前(P<0.05);热应激结束后西杂牛和犏牛血清中NEFA浓度较8月显著升高(P<0.05),而地方黄牛NEFA浓度仍维持在较低水平。试验期间西杂牛和地方黄牛血清中UN浓度随年龄的增大呈先升高后降低变化,而犏牛血清中UN浓度则持续升高。西杂牛7、8月血清中UN浓度较热应激发生前显著升高(P<0.05);地方黄牛6、7月血清中UN浓度显著高于热应激发生前和热应激结束后(P<0.05);犏牛热应激结束后血清中UN浓度较热应激发生前升高了93.75%(P<0.05)。6—9月各试验牛血清CRE浓度均显著高于4、5月(P<0.05),且7—9月犏牛血清CRE浓度显著高于地方黄牛(P<0.05)。
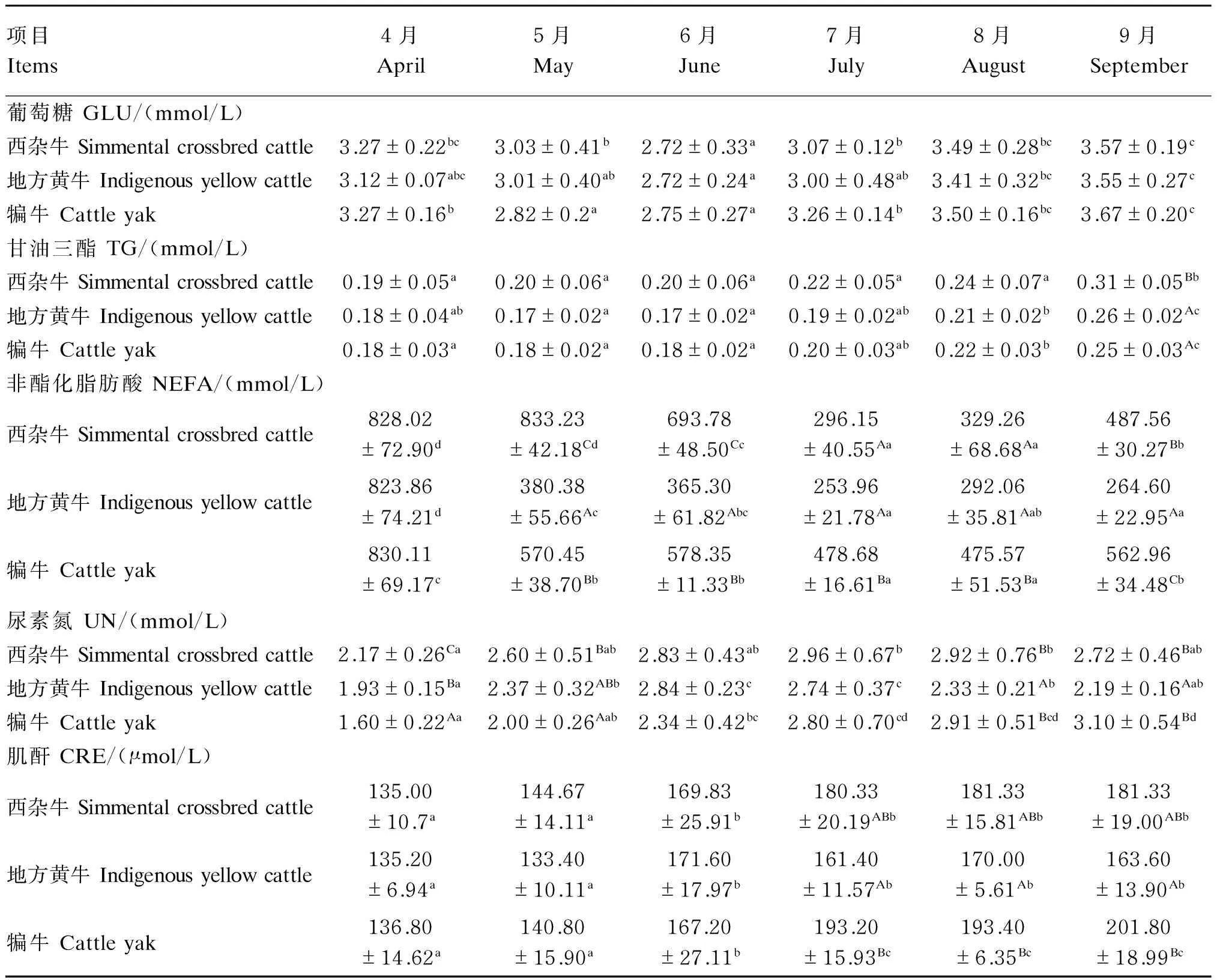
表6 热应激对肉牛血液生化指标的影响
3 讨 论
3.1热应激对肉牛直肠温度和呼吸频率的影响
我国南方地区夏季高温高湿,肉牛极易处于热应激状态,对其生产性能、健康状况造成不利影响[1,14]。本试验于四川省降水最多的地域雅安市进行,夏季闷热潮湿。本研究发现5—8月牛舍THI均高于72,肉牛处于热应激环境下,而4和9月牛舍THI低于72,肉牛处于非热应激环境下。
在适宜环境温度下,牛呼吸频率为20~40次/min,体温为38.5 ℃左右。热应激状态下肉牛通过增加呼吸频率来增加蒸发散热[15],本研究中热应激状态下各试验牛呼吸频率较直肠温度先升高,说明当呼吸代偿仍不能有效缓解环境温度对机体的影响时肉牛体温才会升高。试验期间5月时各试验牛呼吸频率即显著升高,说明5月肉牛即进入热应激状态。试验期内犏牛呼吸频率和直肠温度均显著高于西杂牛和地方黄牛,说明犏牛热应激反应较西杂牛和地方黄牛严重且可能4月已处于热应激状态,推测是因为犏牛属高原牛种且带有牦牛血统,被毛较西杂牛和地方黄牛厚,阻碍了体表的辐射散热;此外,犏牛源自我国气候偏冷、长冬无夏西北高原地区,因而南方地区高温高湿对犏牛造成了较严重的热应激。7、8月时地方黄牛直肠温度显著低于西杂牛和犏牛,说明地方黄牛体温受环境影响较小,抗热应激能力强于西杂牛和犏牛。
3.2热应激对肉牛生产性能和营养物质表观消化率的影响
在热应激状态下,动物通过降低采食量降低机体热增耗以维持机体热平衡[16],这将导致动物生产性能降低甚至是负增长。而本研究中,试验期间各试验牛DMI并未降低,且9月西杂牛、地方黄牛和犏牛DMI相对于4月分别增加了65.01%、37.83%和33.89%,推测是本试验牛只为处于生长发育快速时期的青年牛,因热应激减少的采食量小于肉牛因生长发育需要而增加的采食量[17]。当采用单位体重DMI来评价热应激对肉牛采食量的影响时,可发现西杂牛、地方黄牛和犏牛单位体重DMI均是6月最低,较热应激发生前分别降低了11.17%、8.83%和16.15%,由此可知热应激对地方黄牛采食量的影响要小于西杂牛和犏牛,对犏牛采食量影响最大。
西杂牛和犏牛ADG在热应激状态下出现不同程度降低,与前人研究结果[18]一致。西杂牛、地方黄牛和犏牛6月ADG最低且较热应激发生前分别降低了27.62%、10.81%和46.15%,说明热应激对地方黄牛ADG影响小于西杂牛和犏牛,对犏牛ADG影响最大。结合THI变化和单位体重DMI可发现,各试验牛6月受热应激影响较大,采食量降低导致ADG降低。试验期间西杂牛ADG均高于地方黄牛和犏牛,说明西杂牛生产性能优于地方黄牛和犏牛。
热应激状态下肉牛大量血液被分配到体表帮助散热而使得消化道血流量减少,导致营养物质表观消化率降低[19-20]。本试验中,夏季西杂牛CP、EE、Ca和P的表观消化率受热应激影响而降低;但地方黄牛仅CP的表观消化率受热应激影响而降低;而犏牛CP、EE、NDF、ADF、Ca和P的表观消化率均有所降低,说明同样环境条件下热应激对地方黄牛营养物质表观消化率影响较西杂牛和犏牛小。各试验牛CP的表观消化率均因热应激而降低,推测是因为动物机体摄入蛋白质后的热增耗较其他营养物质高[21],CP表观消化率降低可降低肉牛内源性产热。此外,试验期间地方黄牛ADF的表观消化率高于西杂牛和犏牛,说明地方黄牛更耐粗饲。
3.3热应激对肉牛血液生化指标的影响
血清代谢物是反映机体代谢变化的敏感指标。Scharf等[22]研究发现,肉牛血清中GLU浓度在急性热应激时会升高,而在慢性热应激时则降低。本试验中,试验牛的热应激状态是在自然条件下随本地区季节、气候变化而变化的,属于慢性热应激。6月各试验牛血清中GLU浓度最低,说明其通过利用GLU供能加快外周循环以增强机体散热[23];随着热应激时间的持续,肉牛血清中GLU浓度逐渐升高,推测是因为糖异生作用加强以维持血糖浓度稳定,增强机体对热应激的抵抗力[24]。与西杂牛、地方黄牛相比,犏牛血清中GLU浓度下降出现的较早,5月血清中GLU浓度即显著降低,说明犏牛对热应激更加敏感。
大量研究发现,热应激有增加机体脂肪沉积的趋势[25-27]。本研究中,肉牛在热应激状态下血清中TG浓度升高而NEFA浓度降低,说明热应激使肉牛脂肪合成代谢加强,这与前人研究发现的热应激抑制泌乳早期奶牛血清中NEFA浓度升高的结果[23,28]相似。这可能是血清中NEFA浓度降低可减少细胞线粒体基质的氧化分解反应,减少内源性产热和活性氧化物的产生,减缓热应激带来的副作用[29-31]。地方黄牛和犏牛血清中NEFA浓度降低较西杂牛发生早且地方黄牛热应激期内血清中NEFA浓度显著低于犏牛,说明犏牛和地方黄牛对热应激较西杂牛敏感且地方黄牛内源性产热低于犏牛。
热应激时动物DMI的下降会使得糖原的储存量减少,血糖浓度需要糖异生作用来维持[32]。在这种情况下,氨基酸代谢的增加会使血清中UN浓度增加。而CRE是由动物机体肌肉蛋白质水解产生的,其浓度主要与动物机体肌肉总量有关[33]。本试验中,西杂牛、地方黄牛和犏牛血清中UN浓度在热应激期内显著升高,推测可能是热应激状态下各肉牛单位体重DMI降低使得机体糖原储存量减少,导致血糖浓度需要通过氨基酸糖异生作用来维持[32]。热应激发生前犏牛血清中UN浓度显著低于西杂牛和地方黄牛,说明犏牛氮利用效率高于西杂牛和地方黄牛[34]。而热应激结束后西杂牛、地方黄牛和犏牛血清中UN浓度较热应激发生前分别升高了25.35%、13.47%和93.75%,说明热应激对犏牛氨基酸代谢影响较大。试验期间各试验牛血清中CRE浓度随着热应激时间的持续而逐渐升高,可能是由于各试验牛处于生长发育迅速的青年时期,其肌肉总量逐渐增加。但7、8、9月犏牛血清中CRE浓度高于西杂牛和地方黄牛,但其试验期间增重较西杂牛和地方黄牛少,说明热应激使得犏牛肌肉蛋白质水解增加,氨基酸代谢加强,使得血清中UN浓度持续升高,这与其ADG的降低密切相关[35]。
4 结 论
热应激状态下西杂牛、地方黄牛和犏牛的呼吸频率和直肠温度升高,生产性能和营养物质表观消化率降低。地方黄牛的耐热应激能力强于西杂牛和犏牛,犏牛对热应激最敏感,且热应激降低了犏牛的氮利用效率。
[1] 王祖新,王之盛,王立志,等.不同季节温湿度指数对奶牛生产性能和生理生化指标的影响[J].中国畜牧杂志,2009,45(23):60-63.
[2] LEFCOURT A M,ADAMS W R.Radiotelemetry measurement of body temperatures of feedlot steers during summer[J].Journal of Animal Science,1996,74(11):2633-2640.
[3] MADER T L,DAVIS M S,BROWN-BRANDL T.Environmental factors influencing heat stress in feedlot cattle[J].Journal of Animal Science,2006,84(3):712-719.
[4] ST-PIERRE N R,COBANOV B,SCHNITKEY G.Economic losses from heat stress by us livestock industries 1[J].Journal of Dairy Science,2003,86(Suppl.1):E52-E77.
[5] YOUSEF M K,JOHNSON H D.Calorigenesis of dairy cattle as influenced by thyroxine and environmental temperature[J].Journal of Animal Science,1966,25(1):150-156.
[6] BOHMANOVA J,MISZTAL I,COLE J B.Temperature-humidity indices as indicators of milk production losses due to heat stress[J].Journal of Dairy Science,2007,90(4):1947-1956.
[7] ARMSTRONG D V.Heat stress interaction with shade and cooling[J].Journal of Dairy Science,1994,77(7):2044-2050.
[8] SILANIKOVE N.Effects of heat stress on the welfare of extensively managed domestic ruminants[J].Livestock Science,2000,67(1/2):1-18.
[9] COLLIER R J,COLLIER J L,RHOADS R P,et al.Invited review:genes involved in the bovine heat stress response[J].Journal of Dairy Science,2008,91(2):445-454.
[10] LEE D G K.Climatic stress indices for domestic animals[J].International Journal of Biometeorology,1965,9(1):29-35.
[11] JOHNSON J S,SCHARF B,WEABER R L,et al.Patterns of heat response and adaptation on summer pasture:a comparison of heat-sensitive (Angus) and-tolerant (Romosinuano) cattle[J].Journal of Thermal Biology,2012,37(4):344-350.
[12] VAN KEULEN J,YOUNG B A.Evaluation of acid-insoluble ash as a natural marker in ruminant digestibility studies[J].Journal of Animal Science,1977,44(2):282-287.
[13] 张丽英.饲料分析及饲料质量检测技术[M].2版.中国农业大学出版社,2003.
[14] COLLIER R J,BEEDE D K,THATCHER W W,et al.Influences of environment and its modification on dairy animal health and production[J].Journal of Dairy Science,1982,65(11):2213-2227.
[15] EIGENBERG R A,BROWN-BRANDL T M,NIENABER J A,et al.Dynamic response indicators of heat stress in shaded and non-shaded feedlot cattle,part 2:predictive relationships[J].Biosystems Engineering,2005,91(1):111-118.
[16] DERNO M,JENTSCH W,SCHWEIGEL M,et al.Measurements of heat production for estimation of maintenance energy requirements of Hereford steers[J].Journal of Animal Science,2005,83(11):2590-2597.
[17] BURROW H M,PRAYAGA K C.Correlated responses in productive and adaptive traits and temperament following selection for growth and heat resistance in tropical beef cattle[J].Livestock Production Science,2004,86(1/2/3):143-161.
[18] O’BRIEN M D,RHOADS R P,SANDERS S R,et al.Metabolic adaptations to heat stress in growing cattle[J].Domestic Animal Endocrinology,2010,38(2):86-94.
[19] KADZERE C T,MURPHY M R,SILANIKOVE N,et al.Heat stress in lactating dairy cows:a review[J].Livestock Production Science,2002,77(1):59-91.
[20] BERNABUCCI U,LACETERA N,DANIELI P P,et al.Influence of different periods of exposure to hot environment on rumen function and diet digestibility in sheep[J].International Journal of Biometeorology,2009,53(5):387-395.
[21] TASAKI I,KUSHIMA M.Heat production when single nutrients are given to fasted cockerels[M]//MOUNT L E.Energy metabolism.Amsterdam:Elsevier,1980:253-256.
[22] SCHARF B,CARROLL J A,RILEY D G,et al.Evaluation of physiological and blood serum differences in heat-tolerant (Romosinuano) and heat-susceptible (Angus)Bostauruscattle during controlled heat challenge[J].Journal of Animal Science,2010,88(7):2321-2336.
[23] WHEELOCK J B,RHOADS R P,VANBAALE M J,et al.Effects of heat stress on energetic metabolism in lactating Holstein cows[J].Journal of Dairy Science,2010,93(2):644-655.
[24] 刘铀,林红英,罗东君,等.热应激对肉鸡血液生化指标及内分泌机能的影响[J].湛江海洋大学学报,1999,19(1):61-64.
[25] 王启军.高温环境对不同生长阶段北京油鸡脂肪沉积及脂质代谢的影响[D].硕士学位论文.杨凌:西北农林科技大学,2006.
[26] 李洁蕾,杨培歌,冯跃进,等.L-肉碱对热应激大鼠机体脂质代谢的影响[J].动物营养学报,2015,27(9):2849-2855.
[27] KOUBA M,HERMIER D,LE DIVIDICH J.Influence of a high ambient temperature on lipid metabolism in the growing pig[J].Journal of Animal Science,2001,79(1):81-87.
[28] RHOADS M L,RHOADS R P,VANBAALE M J,et al.Effects of heat stress and plane of nutrition on lactating Holstein cows:Ⅰ.Production,metabolism,and aspects of circulating somatotropin[J].Journal of Dairy Science,2009,92(5):1986-1997.
[29] BERNABUCCI U,LACETERA N,BAUMGARD L H,et al.Metabolic and hormonal acclimation to heat stress in domesticated ruminants[J].Microchemical Journal,2010,4(7):1167-1183.
[30] BAUMGARD L H,RHOADS R P.Effects of heat stress on postabsorptive metabolism and energetics[J].Annual Review of Animal Biosciences,2013,1(2):311-337.
[31] ZHANG F J,WENG X G,WANG J F,et al.Effects of temperature-humidity index and chromium supplementation on antioxidant capacity,heat shock protein 72,and cytokine responses of lactating cows[J].Journal of Animal Science,2014,92(7):3026-3034.
[32] BAIRD G D,HEITZMAN R J,HIBBITT K G.Effects of starvation on intermediary metabolism in the lactating cow.A comparison with metabolic changes occurring during bovine ketosis[J].Biochemical Journal,1972,128(5):1311-1318.
[33] ASAI H,HAYASHI N,TAKAI N,et al.Estimation of daily urinary potassium excretion using urinary creatinine as an index substance in prepartum dairy cows[J].Animal Science Journal,2005,76(1):51-54.
[34] WANG H,LONG R,ZHOU W,et al.A comparative study on urinary purine derivative excretion of yak (Bosgrunniens),indigenous cattle (Bostaurus),and crossbred (Bostaurus×Bosgrunniens) in theQing-haiTibetan plateau,China[J].Journal of Animal Science,2009,87(7):2355-2362.
[35] RHOADS R P,OBRIEN M D,GREER K,et al.Consequences of heat stress on the profile of skeletal muscle gene expression in beef cattle[J].The FASEB Journal,2008,22(Suppl.1):1165.1
*Corresponding author, professor, E-mail: zswangsicau@126.com
(责任编辑 菅景颖)
Effects of Heat Stress on Performance, Nutrient Apparent Digestibility and Blood Biochemical Indices of Different Breeds of Young Beef Cattle
PU Qijian WANG Zhisheng*PENG Quanhui ZHANG Can JING Xiaoping HU Rui ZOU Huawei
(Animal Nutrition Institute of Sichuan Agricultural University, Key Laboratory of Low Carbon Culture and Safety Production in Cattle in Sichuan, Ya’an 625014, China)
This experiment was conducted to investigate the effects of heat stress on performance, nutrient apparent digestibility and blood biochemical indices of different breeds of young beef cattle, in order to explore the heat resistance difference of different breeds of beef cattle. Six individuals of Simmental crossbred cattle (Simmental cattle×Xuanhanyellow cattle), indigenous yellow cattle (Xuanhanyellow cattle) and cattle yak (Maiwayak×Jersey cattle) were chosen based on similar body weight [(185.89±14.02) kg]. Animals were fed with the same diet and feeding experiment lasted 187 days, including 7-days adaption period and 180-days test period. The experiment from April 2015 to September 2015. The results showed as follows: 1) the temperature humidity index (THI) of barn was above 72 from May to August and this stage belonged to heat stress (HS) period, and the THI of HS period was higher than April (pre HS) and September (after HS) (P<0.05). During the HS period, the respiratory frequency and rectal temperature were significantly increased (P<0.05) for all experimental animals. The respiratory frequency and rectal temperature of cattle yak was significantly higher than those of Simmental crossbred cattle and indigenous yellow cattle in the period of experiment (P<0.05). 2) The dry matter intake per body weight of Simmental crossbred cattle, indigenous yellow cattle and cattle yak was decreased in HS period, and the lowest value in June and significantly lower than April (P<0.05), the degree of decreased was cattle yak>Simmental crossbred cattle>indigenous yellow cattle. The lowest values of average daily gains of Simmental crossbred cattle, indigenous yellow cattle and cattle yak were all in June, and decreased by 27.62% (P<0.05), 10.81% (P>0.05) and 46.15% (P<0.05) as contrasted April, respectively. The average daily gain of Simmental crossbred cattle was significantly higher than that of cattle yak from April to July (P<0.05). The highest values of feed/gain of Simmental crossbred cattle and cattle yak in June, and increased by 63.01% (P<0.05) and 89.03% (P<0.05)as contrasted April, respectively; the highest value of feed/gain of indigenous yellow cattle in September, and increased by 53.12% as contrasted to April (P<0.05). 3) The apparent digestibility of crude protein (CP) of Simmental crossbred cattle, indigenous yellow cattle and cattle yak was different degrees of reduction in HS period and significantly decreased in July compared with April (P<0.05). The apparent digestibility of ether extract (EE) of Simmental crossbred cattle was significantly decreased in July compared with April (P<0.05), but the HS had no significant effects on the apparent digestibility of EE for indigenous yellow cattle and cattle yak (P>0.05). The apparent digestibility of neutral detergent fiber (NDF) (June) and acid detergent fiber (ADF) (from May to August) of cattle yak was significantly decreased in HS period compared with pre HS (P<0.05). The apparent digestibility of calcium (Ca) of Simmental crossbred cattle (from June to August) and cattle yak (from May to August) was significantly decreased in HS period compared with pre HS (P<0.05), and the apparent digestibility of phosphorus (P) of Simmental crossbred cattle (from June to July) and cattle yak (from May to August) was significantly decreased in HS period compared with pre HS, too (P<0.05). During the experiment period, the apparent digestibility of CP had no significant difference among the three breeds of young beef cattle (P>0.05), the apparent digestibility of EE of Simmental crossbred cattle and indigenous yellow cattle was significantly higher than that of cattle yak (P<0.05), the apparent digestibility of ADF of indigenous yellow cattle was significantly higher than that of Simmental crossbred cattle and cattle yak (P<0.05), and the apparent digestibility of P of Simmental crossbred cattle and indigenous yellow cattle was significantly higher than that of cattle yak (P<0.05). Moreover, the apparent digestibility of Ca of indigenous yellow cattle was significantly higher than that of cattle yak from May to September (P<0.05). 4) Serum glucose (GLU) concentration of Simmental crossbred cattle, indigenous yellow cattle and cattle yak had the lowest value in June and decreased by 16.82% (P<0.05), 12.82% (P>0.05) and 15.90% (P<0.05) compared with April, respectively. For all the experimental cattle, the non-esterfied fatty acid (NEFA) concentration had the lowest value in July and significantly lower than that in April (P<0.05). Serum urea nitrogen (UN) concentration of Simmental crossbred cattle and indigenous yellow cattle was increased at first and then decreased in the experiment period with the age increasing, while the serum UN concentration of cattle yak was continually increased. Serum creatinine (CRE) concentration of all experimental animals in June to September was significantly higher than that in April and May (P<0.05), and the serum CRE concentration of cattle yak was significantly higher than that of indigenous yellow cattle from July to September (P<0.05). In conclusion, the performance and apparent digestibility of nutrients of different breeds of young beef cattle are decreased under heat stress condition. Indigenous yellow cattle are more tolerance of heat stress than Simmental crossbred cattle and cattle yak, and cattle yak are more sensitive to heat stress.[ChineseJournalofAnimalNutrition,2017,29(9):3120-3131]
heat stress; beef cattle; performance; nutrient apparent digestibility; blood biochemical indices
2017-02-20
国家肉牛牦牛产业技术体系资金资助(CARS-38)
蒲启建(1990—),男,四川成都人,硕士研究生,从事反刍动物营养与饲料科学研究。E-mail: penqiji@live.com
*通信作者:王之盛,教授,博士生导师,E-mail: zswangsicau@126.com
10.3969/j.issn.1006-267x.2017.09.013
S816
:A
:1006-267X(2017)09-3120-12
