改性PVDF膜抗污染性进展探究
2017-09-12贺慧妮代鹤峰
房 平,贺慧妮,代鹤峰
改性PVDF膜抗污染性进展探究
房 平,贺慧妮,代鹤峰
(西安工程大学, 陕西 西安 710048)
聚偏氟乙烯以其优异的力学性质和化学性质在膜分离技术中被广泛应用,其表面的极强疏水性,导致在水处理过程中极易受到污染且恢复率很低。所以膜的改善亲水性具有十分重要的意义。根据不同的改性粒子分类,结合主要的改性方法,将国内外近几年对PVDF改性效果较好的纳米粒子和两亲性共聚物进行综述和性能对比,着重从渗透性和抗污性比较了各种粒子的改性效果。最后对PVDF膜亲水性改善进行总结和展望。
聚偏氟乙烯;抗污染性;纳米粒子;两亲性共聚物
在膜技术材料常用的聚合物中,由于聚偏二氟乙烯(PVDF)膜优异的化学性,良好的机械强度和耐老化性,已广泛应用于许多领域[1,2]。但PVDF膜的疏水性使疏水性污染物会吸附到膜表面和表面孔隙中,这都导致严重的污染问题,甚至缩短膜的使用寿命[3-7],所以增加PVDF膜的亲水性具有非常重要的实际意义[8]。近几年的关于PVDF膜改性方法有很多[9]。一般使用共混和接枝的方法,效果较好的改性膜通常是几种改性方式联合使用。膜的结构特点,比如孔的形态、孔隙率、表面化学结构与组成等决定了改性膜的亲水性、渗透性和抗污染性等性能[10]。亲水性主要由接触角表征,接触角越小,亲水性越好,渗透性越高;抗污染性能主要由蛋白质静态吸附、通量恢复率(FRR)和不可逆污染物的量联合表征。本文主要是基于这几个指标通过某些粒子对PVDF改性膜的性能进行对比总结。
1 共混法与其他方法联合改性
1.1 氧化石墨烯(GO)对PVDF膜的相关改性
氧化石墨烯(GO)因含有大量的活性含氧基团如-COOH等,而具备优良的亲水性和其高的水分散性[11],这恰好与PVDF材料和其他易团聚粒子形成互补。
Jang等[12]和Zhao等[13]采用不同的方法制备出PVDF / GO混合膜,在水通量和抗污染性方面都有不错的效果。Wu等[14]将GO添加到凝胶浴里。与纯膜相比,改性膜的接触角降低,孔隙率略有增加,改性膜的纯水通量为467.75 L·m-2·h-1,对BSA的截留率增加了38.99%,其中不可逆污染占19.5%,其FRR达85.7%。而纯膜不可逆污染占56.8%
单一的GO改性有一定的局限性,而且受到材料本身缺陷的限制,加入其它粒子或者其他方式能在一定程度上提高性能。Safarpour等[15]将rGO/TiO2纳米复合材料和PVDF铸膜液共混。与PVDF纯膜相比,改性膜的接触角略有降低,纯水通量提高23.1%~69.2%,对BSA的截留率保持在90%以上,且纯膜的FRR只有30%,而改性膜的FRR为67%~92%。当rGO与TiO2采用合适比时,亲水性、纯水通量和抗污能力表现出最好的协同作用。Xu[16]等在铸膜液中添加GO/TiO2纳米复合材料后,水通量提高到487.8 L·m-2·h-1,对 BSA的截留率,从77.1%提高到92.5%。作者发现经水力与UV照射对膜进行清洗后,膜有更好的抗污染性和自清洁能力。Zhao[17]等人讨论了PVDF / GO复合膜在MBR中应用的实际效益。纯膜与复合膜对污水处理二者效果相当,重要的是纯膜的清洗周期是复合膜的3倍。且复合膜不可逆污染阻力仅占0.78%,远远小于纯膜的3.95%。表1具体总结和对比了GO对PVDF膜不同的改性结果。
1.2 TiO2对PVDF膜的相关改性
纳米颗粒TiO2由于其极高的亲水性和稳定性而引起广泛关注[18-22],但是TiO2高表面能和强范德华力,使TiO2纳米颗粒易于聚集,降低了膜的性能[18,21]。所以使TiO2纳米颗粒更好的分散于膜基质中以提高膜性能变的十分重要。
Moghadam等[19]将TiO2纳米颗粒分散在PVDF基质中,复合膜的水通量为100 L·m-2·h-1·bar-1,是纯膜的2倍,对BSA和EPS的截留率都提高30%左右,改性膜的FRR高达71.2%。Teow等[20]将PVDF通过Fenton反应羟基化后,以含TiO2的水溶液为凝胶浴,制备PVDF-OH/TiO2改性膜,与纯PVDF膜相比,改性膜的孔隙率和平均孔径略有减少,且纯膜和改性膜的截留率相当,但改性膜的FRR为87.32%,高于纯膜的值。Zeng等[21]将TiO2-HNTs纳米复合材料与PVDF铸膜液共混。相比纯膜,改性膜的水通量提高了264.8%,与此同时对BSA的截留略有下降。但改性膜的静态蛋白质降低了159%。作者进行过滤清洗三次循环后,改性膜的FRR任可达76.9%。将Qin 等[22]将TiO2和PVA层都结合到PVDF膜表面。膜的水通量提高了10.3%,对BSA的截留率从44.7%提高到86.5%。作者在二次过滤后,通量恢复率从纯膜的20%提高到改性膜的86.4%,且改性膜的不可逆污染物含量(11.5%)远低于纯膜的值(80%)。表1具体总结和对比了TiO2对PVDF膜不同改性的结果。
1.3 SiO2对PVDF膜的相关改性
SiO2同TiO2一样是无机纳米材料,具有大的比表面积、易于制备和永久亲水性的优点,纳米结构表面可以有效减少相同的纳米级污染物对膜的锚定位点数和物理锚定效应[23]。
Wang[24]等人将中空二氧化硅微球(HSiO2)与铸膜液共混,利用HSiO2特殊的中腔结构,构建阶梯式渗透通道,从而降低PVDF膜中水分子的传质阻力,且改性膜的FRR也提高了74.1%。Qin[25]等人用三乙酸甘油酯改性的SiO2纳米粒子与PVDF粉末共混。相比原膜,改性膜的机械性能提高,虽然改性膜的孔隙率只增加了27.4%,但水通量却提高了276%。对BSA截留测试显示,改性膜的 FRR为93.8%,远高于原膜的11.7%,且改性膜的不可逆污染物仅有6.3%。Li等[26]首次用非溶剂相转化法合成PVDF/SiO2@GO纳米混合膜。混合膜的表面趋于光滑,接触角明显下降,对BSA蛋白的静态吸收能力明显降低。 Pan[27]等制备Ag/SiO2-PVDF膜,相比原膜,改性膜的水通量明显提高,对BSA的截留率略有降低,接触角下降了57.8%。经俩次循环过滤后, FRR由原膜的36.2%提高到改性膜的50.2%,且可逆污染量由63.8%下降到54.2%。并且改性膜有一定的抗菌性。Zhao[28]等将SA和SiO2-NH2纳米颗粒组成的组装层以改性PVDF膜的表面,叠加六层组装层后,表面粗糙度基本与纯膜一致,但表面接触角降至6°,纯水通量从95 L·m-2·h-1提高到153 L·m-2·h-1,对BSA的截留率提高了50%,蛋白质静态吸附的量几乎为0。膜经二次循环测试,改性膜的 FRR高达99%。表1具体总结和对比了SiO2对PVDF膜不同改性的结果。
1.4 两亲聚合物对PVDF膜的相关改性
与均聚物相比,将两亲共聚物与主体膜材料共混制备的PVDF膜发生脱离现象的概率更小。在非溶剂诱导相分离(NIPS)期间,两亲共聚物的亲水链分离集中在膜表面上以使界面能最小化,疏水链段与PVDF链的缠结可将亲水链段紧密固定在膜表面上[2,29]。
Zhao[30]等将EW-326加入铸膜液中,制造仿蚊眼结构且具有规则纳米级凸起的新型聚PVDF超滤膜。仿生膜的粗糙度明显增加,接触角增加到133°对BSA的截留率有所降低却也保持在80%以上。将BSA与SA的混合液作为污染液时,仿生膜依然有80%的FRR值,高于原膜的值(56%)。Ma[31]等人制备了PEGMA-MMA两亲性共聚物,在基本相同的纯水渗透性条件下(约300 L·m-2·h-1·atm-1),越高的O/C比,膜的亲水性越差。作者将PEG侧链的长度、PEGMA / MMA、共聚物与PVDF的比率单体比例对膜抗污染性能的影响分别做了讨论,发现前俩者对于改善防污性能更有效。 Sun等[32]利用相同的原理,得出类似的结论,相比未改性膜,高链段比的VA / TFE共聚物制备出的共混膜,接触角无明显变化。作者发现BSA分子对共混膜的附着量很低,当采用合适的混合比时,共混膜在60 min内保持其初始水通量的70%,并且在反冲之后,水通量几乎完全恢复。Wang[33]等将SiO2-g-PDMS纳米颗粒与PVDF粉末共混,制备出的共混膜接触角下降到15°,水通量增加了76.9%,对BSA的截留略有减低,但仍保持80%以上,且通量恢复率达90%以上。Liu[34]等合成PMMA-b-PEG-b-PMMA两亲嵌段式共聚物。相比原膜,改性膜水通量提高了137.5%,对BSA的截留率保持在80%以上。且原膜的FRR仅58%,而改性膜高达90%以上,膜表面和孔道内形成亲水层是使改性膜的抗污染性显著提高的主要原因。表1具体总结和对比了两亲性物质对PVDF膜不同改性的结果。
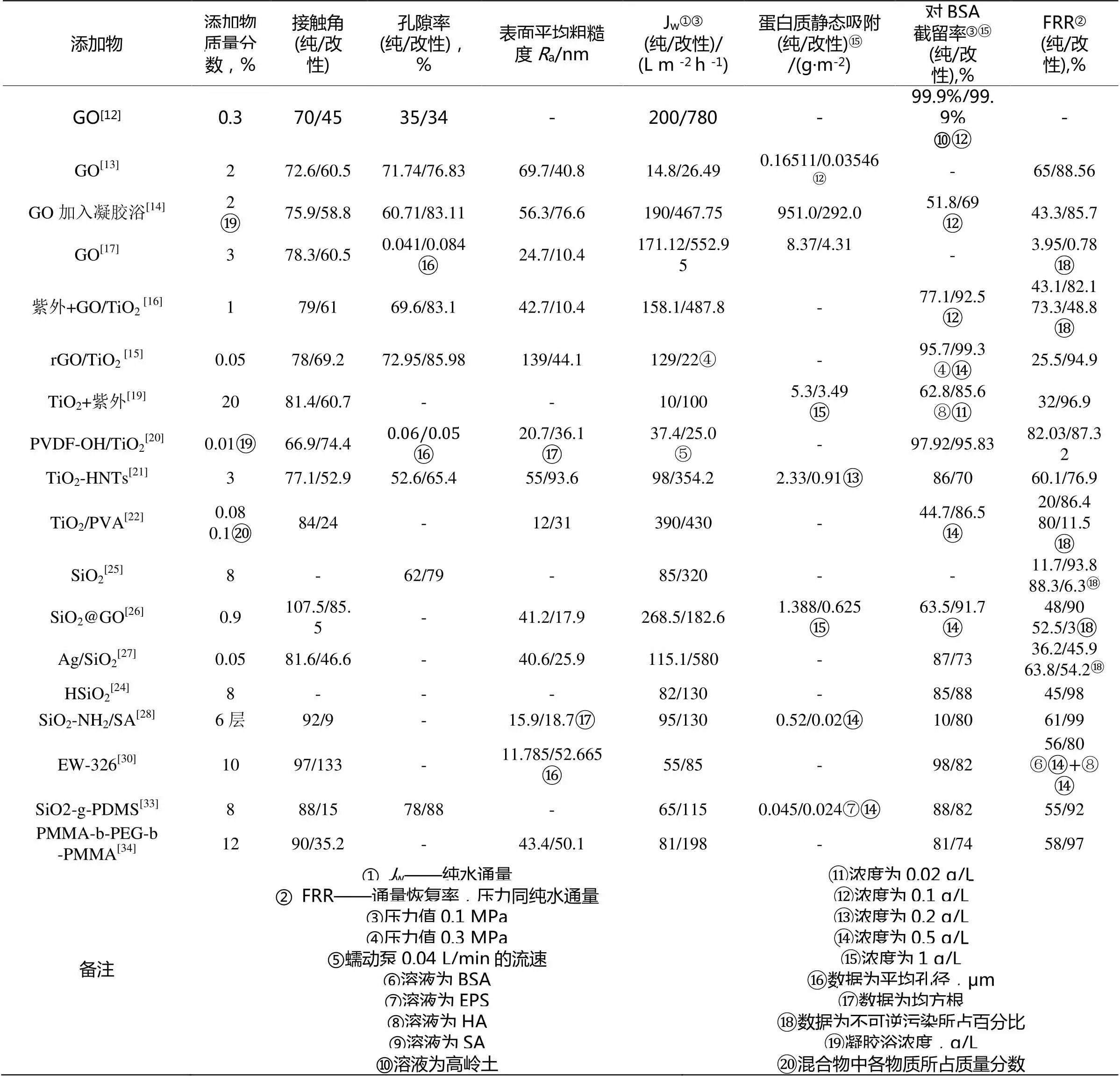
表1 改性PVDF膜性能对比
2 其他物质和方法对PVDF膜的防污改性
接枝法、等离子体改性和两性离子改性法等由于目的性强,效果明显被被学者采用,但是同时操作过程复杂且要求较高,所以广泛程度不高。
Lü等[35]将PU与PVDF共混制备改性膜,相比纯膜,接触角下降为40°,纯水通量是原膜的5.46倍(148.1 L·m-2·h-1)。纯膜和改性膜对BSA的截留率都高达90%以上,但改性膜对蛋白质的吸附量明显下降,通量恢复率也从58.7%提高到93%左右。Shen等[36]采用接枝共聚的方法,制备基于羧基甜菜碱的两性离子PVDF膜 ,改性PVDF膜接触角略降低,但对BSA的截留率高达96.3%,其中不可逆污染物仅占1.8%。经过俩次循环过滤后,通量恢复率FRR可达98.12%。Okuji等[37]采用离子辐射法对PVDF膜进行表面改性,蛋白质吸附量显著降低。Venault[38]等通过介质阻止放电(GDBD)将两性离子TMA / SA接枝在PVDF膜表面,测试表明当膜表面带有适量的负电荷时,膜的接触角下降到60°。当TMA:SA=1:1时,改性膜相对于纯膜的BSA吸附量为32%,且未检测出大肠杆菌的生长。在经三个循环过滤测试后,改性膜不可逆污染物仅11%,远低于原膜的值(42%)。其它学者也在两性离子方面做了相关研究[39-45],都取得了不错的效果。
3 结束语
基于PVDF的优异性,未来会成为水处理过程中分离膜的主要材料之一,但是其表面极高的表面能导致PVDF膜材料表面有极强的疏水性,这成为它发展的瓶颈,对其进行亲水性改性变的十分重要。现总结如下:
(1)本文综述了GO、TiO2、SiO2纳米粒子和两亲性物质对PVDF膜的相关改性。
(2)纳米粒子增强了膜的机械性能,但有分散不均的缺陷。
(3)两亲性物质中亲水段向膜表面聚集,形成亲水性的表面,疏水段稳定的与PVDF分子共存交联,并固定亲水段于膜的表面。相比通过纳米粒子来改善PVDF膜,两亲性物质与PVDF相容性好,且能稳定的存在于膜基质中,在很长一段时间里保持性质不变。
(4)在改性方法中,共混法改性操作简单,适合工业化,但是要想PVDF膜既拥有稳定的化学性质,又在水通量和截留率方面达到折衷效应,进而达到一个理想的状态,必须与接枝共聚等方法相结合。
[1]Kang G D, Cao Y M. Application and modification of poly(vinylidene fluoride) (PVDF) membranes – A review[J]. Journal of Membrane Science, 2014, 463(1):145-165.
[2] Liu F, Hashim N A, Liu Y, et al. Progress in the production and modification of PVDF membranes[J]. Journal of Membrane Science,2011, 375(1-2): 1-27.
[3] Mansouri J, Harrisson S, Chen V. Strategies for controlling biofouling in membrane filtration systems: challenges and opportunities[J]. Journal of Materials Chemistry, 2010, 20(22):4567-4586.
[4]Rana D, Matsuura T. Surface Modifications for Antifouling Membranes[J]. Chemical Reviews, 2010, 110(4):2448-71.
[5] Yuliwati E, Ismail A F. Effect of additives concentration on the surface properties and performance of PVDF ultrafiltration membranes for refinery produced wastewater treatment[J]. Desalination, 2011, 273(1): 226-234.
[6] Zhou R, Ren P F, Yang H C, et al. Fabrication of antifouling membrane surface by poly (sulfobetaine methacrylate)/polydopamine co-deposition[J]. Journal of Membrane Science, 2014, 466: 18-25.
[7]Nishigochi S, Ishigami T, Maruyama T, et al. Improvement of antifouling properties of polyvinylidene fluoride hollow fiber membranes by simple dip coating of phosphorylcholine copolymer via hydrophobic interactions[J]. Industrial & Engineering Chemistry Research, 2014, 53(6): 2491-2497.
[8] Cui Z, Drioli E, Lee Y M. Recent progress in fluoropolymers for membranes[J]. Progress in Polymer Science, 2014, 39(1):164-198.
[9] Liu F, Hashim N A, Liu Y, et al. Progress in the production and modification of PVDF membranes[J]. Fuel & Energy Abstracts, 2011, 375(1):1-27.
[10]Ji J, Liu F, Hashim N A, et al. Poly(vinylidene fluoride) (PVDF) membranes for fluid separation[J]. Reactive & Functional Polymers, 2015, 86:134-153.
[11]Kanrar S, Debnath S, De P, et al. Preparation, characterization and evaluation of fluoride adsorption efficiency from water of iron-aluminium oxide-graphene oxide composite material[J]. Chemical Engineering Journal, 2016, 306: 269-279.
[12]Jang W, Yun J, Jeon K, et al. PVdF/graphene oxide hybrid membranes via electrospinning for water treatment applications[J]. RSC Advances, 2015, 5(58): 46711-46717.
[13]Zhao C, Xu X, Chen J, et al. Effect of graphene oxide concentration on the morphologies and antifouling properties of PVDF ultrafiltration membranes[J]. Journal of Environmental Chemical Engineering, 2013, 1(3): 349-354.
[14]Wu T, Zhou B, Zhu T, et al. Facile and low-cost approach towards a PVDF ultrafiltration membrane with enhanced hydrophilicity and antifouling performance via graphene oxide/water-bath coagulation[J]. RSC Advances, 2015, 5(11): 7880-7889.
[15]Safarpour M, Khataee A, Vatanpour V. Effect of reduced graphene oxide/TiO2nanocomposite with different molar ratios on the performance of PVDF ultrafiltration membranes[J]. Separation and Purification Technology, 2015, 140: 32-42.
[16] Xu Z, Wu T, Shi J, et al. Photocatalytic antifouling PVDF ultrafiltration membranes based on synergy of graphene oxide and TiO2for water treatment[J]. Journal of Membrane Science, 2016, 520: 281-293.
[17]Zhao C, Xu X, Chen J, et al. Highly effective antifouling performance of PVDF/graphene oxide composite membrane in membrane bioreactor (MBR) system[J]. Desalination, 2014, 340: 59-66.
[18]Razmjou A, Mansouri J, Chen V. The effects of mechanical and chemical modification of TiO2nanoparticles on the surface chemistry, structure and fouling performance of PES ultrafiltration membranes[J]. Journal of Membrane Science, 2011, 378(1): 73-84.
[19]Moghadam M T, Lesage G, Mohammadi T, et al. Improved antifouling properties of TiO2/PVDF nanocomposite membranes in UV‐coupled ultrafiltration[J]. Journal of Applied Polymer Science, 2015, 132(21).
[20]Teow Y H, Latif A A, Lim J K, et al. Hydroxyl functionalized PVDF–TiO2 ultrafiltration membrane and its antifouling properties[J]. Journal of Applied Polymer Science, 2015, 132(21).
[21]Zeng G, He Y, Yu Z, et al. Preparation and characterization of a novel PVDF ultrafiltration membrane by blending with TiO 2-HNTs nanocomposites[J]. Applied Surface Science, 2016, 371: 624-632.
[22]Qin A, Li X, Zhao X, et al. Engineering a highly hydrophilic PVDF membrane via binding TiO2nanoparticles and a PVA layer onto a membrane surface[J]. ACS applied materials & interfaces, 2015, 7(16): 8427-8436.
[23]Pendergast M T M, Hoek E M V. A review of water treatment membrane nanotechnologies[J]. Energy & Environmental Science, 2011, 4(6): 1946-1971.
[24]Wang H, Zhao X, He C. Innovative permeation and antifouling properties of PVDF ultrafiltration membrane with stepped hollow SiO2microspheres in membrane matrix[J]. Materials Letters, 2016, 182: 376-379.
[25]Qin A, Wu X, Ma B, et al. Enhancing the antifouling property of poly (vinylidene fluoride)/SiO2hybrid membrane through TIPS method[J]. Journal of Materials Science, 2014, 49(22): 7797-7808.
[26]Li Z K, Lang W Z, Miao W, et al. Preparation and properties of PVDF/SiO2@ GO nanohybrid membranes via thermally induced phase separation method[J]. Journal of Membrane Science, 2016, 511: 151-161.
[27]Pan Y, Yu Z, Shi H, et al. A novel antifouling and antibacterial surface‐functionalized PVDF ultrafiltration membrane via binding Ag/SiO2nanocomposites[J]. Journal of Chemical Technology and Biotechnology, 2016.
[28]Zhao X, Xuan H, Chen Y, et al. Preparation and characterization of superior antifouling PVDF membrane with extremely ordered and hydrophilic surface layer[J]. Journal of Membrane Science, 2015, 494: 48-56.
[29]Wang D M, Lai J Y. Recent advances in preparation and morphology control of polymeric membranes formed by nonsolvent induced phase separation[J]. Current Opinion in Chemical Engineering, 2013, 2(2): 229-237.
[30]Zhao X, Liu C. One-step fabricated bionic PVDF ultrafiltration membranes exhibiting innovative antifouling ability to the cake fouling[J]. Journal of Membrane Science, 2016, 515: 29-35.
[31] Ma W, Rajabzadeh S, Shaikh A R, et al. Effect of type of poly (ethylene glycol)(PEG) based amphiphilic copolymer on antifouling properties of copolymer/poly (vinylidene fluoride)(PVDF) blend membranes[J]. Journal of Membrane Science, 2016, 514: 429-439.
[32]Sun Y, Rajabzadeh S, Ma W, et al. Preparation of PVDF/poly (tetrafluoroethylene‐co‐vinyl alcohol) blend membranes with antifouling propensities via nonsolvent induced phase separation method[J]. Journal of Applied Polymer Science, 2016, 133(32).
[33]Wang H, Zhao X, He C. Enhanced antifouling performance of hybrid PVDF ultrafiltration membrane with the dual-mode SiO2-g-PDMS nanoparticles[J]. Separation & Purification Technology, 2016, 166:1-8.
[34] Liu D, Li D, Du D, et al. Antifouling PVDF membrane with hydrophilic surface of terry pile-like structure[J]. Journal of Membrane Science, 2015, 493:243-251.
[35]Lü X, Wang X, Guo L, et al. Preparation of PU modified PVDF antifouling membrane and its hydrophilic performance[J]. Journal of Membrane Science, 2016, 520: 933-940.
[36]Shen X, Gao Y, He Y, et al. Preparation and anti-fouling property of carboxybetaine-based zwitterionic PVDF membrane[J]. Separation Science and Technology, 2016, 51(7): 1189-1198.
[37]Okuji S, Kitazawa H, Takeda Y. Time of flight-secondary ion mass spectrometry analysis of protein adsorption on a polyvinylidene difluoride surface modified by ion irradiation[J]. Colloids and Surfaces B: Biointerfaces, 2016, 148: 249-254.
[38]Venault A, Wei T C, Shih H L, et al. Antifouling pseudo-zwitterionic poly (vinylidene fluoride) membranes with efficient mixed-charge surface grafting via glow dielectric barrier discharge plasma-induced copolymerization[J]. Journal of Membrane Science, 2016.
[39]Zhao X, Su Y, Liu Y, et al. Multiple antifouling capacities of hybrid membranes derived from multifunctional titania nanoparticles[J]. Journal of Membrane Science, 2015, 495: 226-234.
[40]Carretier S, Chen L A, Venault A, et al. Design of PVDF/PEGMA-b-PS-b-PEGMA membranes by VIPS for improved biofouling mitigation[J]. Journal of Membrane Science, 2016, 510: 355-369.
[41]Venault A, Huang W Y, Hsiao S W, et al. Zwitterionic Modifications for Enhancing the Antifouling Properties of Poly (vinylidene fluoride) Membranes[J]. Langmuir, 2016, 32(16): 4113-4124.
[42]Ma W, Rajabzadeh S, Matsuyama H. Preparation of antifouling poly (vinylidene fluoride) membranes via different coating methods using a zwitterionic copolymer[J]. Applied Surface Science, 2015, 357: 1388-1395.
[43]Zhao X, He C. Efficient Preparation of Super Antifouling PVDF Ultrafiltration Membrane with One Step Fabricated Zwitterionic Surface[J]. ACS applied materials & interfaces, 2015, 7(32): 17947-17953.
[44]u K, Shen P, Li J, et al. Preparation of enduringly antifouling PVDF membrane with compatible zwitterionic copolymer via thermally induced phase separation[J]. Journal of Applied Polymer Science, 2015, 132(7).
[45]Shen X, Gao Y, He Y, et al. Preparation and anti-fouling property of carboxybetaine-based zwitterionic PVDF membrane[J]. Separation Science and Technology, 2016, 51(7): 1189-1198.
Research Progress in Anti-fouling Property of Modified PVDF Membrane
,,
(Xi'an Polytechnic University, Shaanxi Xi’an710048,China)
PVDF membrane with excellent mechanical properties and chemical properties is widely used in membrane separation technology. Because of high hydrophobicity of the surface, PVDF membrane is easily polluted in the water treatment process and the recovery rate is very low. So improving the hydrophilicity of the membrane is of great significance. In this paper, according to different modification particles and main modification methods, properties of nano-particles and amphiphilic copolymers with good modification effect on PVDF in recent years were summarized and compared, and modification effect of different particles was analyzed from the aspects of the permeability and anti-fouling property. Finally, the improvement of hydrophilicity of PVDF membrane was summarized and forecasted.
Polyvinylidene fluoride;Anti-fouling;Nano-particles;Amphiphilic copolymers
TQ 325
A
1671-0460(2017)08-1636-05
2016-12-27
房平(1975-),男,吉林省吉林市人,副教授,博士,2011年毕业于西安建筑科技大学环境工程,水处理膜材料与技术。E-mail:469493@qq.com。
