合成温度对全梯度正极材料LiNi0.7Co0.15Mn0.15O2的结构和电化学性能影响
2017-09-12谭潮溥胡国荣杜柯骆宏钧黄殿华段建国曹雁冰
谭潮溥胡国荣杜柯*,骆宏钧黄殿华段建国曹雁冰
合成温度对全梯度正极材料LiNi0.7Co0.15Mn0.15O2的结构和电化学性能影响
谭潮溥1胡国荣1杜柯*,1骆宏钧2黄殿华2段建国1曹雁冰1
(1中南大学冶金与环境学院,长沙410083)
(2广州锂宝新材料有限公司,广州510800)
通过控制结晶法和浓度梯度进料的方式制备了Ni、Co和Mn三元素组分含量呈全梯度分布的类球形Ni0.7Co0.15Mn0.15(OH)2前驱体,与LiOH·H2O均匀混合并焙烧后获得LiNi0.7Co0.15Mn0.15O2正极材料,系统研究了不同焙烧温度对材料Ni、Co和Mn三元素扩散情况、晶体结构及电化学性能的影响规律。通过能谱仪(EDXS)分析不同焙烧温度下材料颗粒中Ni、Co、Mn三元素的扩散程度。研究结果表明,在800℃下焙烧得到的正极材料梯度分布特征明显且电化学性能最佳,首次放电比容量为186.1 mAh·g-1(2.8~4.3 V,0.2C),2C大倍率充放电条件下循环200次后容量保持率为90.1%。这种材料兼具高比容量及良好的循环稳定性,可以用作下一代高能量密度锂离子电池正极材料。
锂离子电池;正极材料;全梯度;元素扩散
0 Introduction
Lithium-ion battery(LIB)has been widely used in portable electronic devices,electric vehicles and efficient energy storage systems owing to its high energy density and outstanding cycle life[1].With further demand for the cycle life and safety of the power batteries,developing the novel cathode material with higher energy density and better rate performance is essential[2].The layered Ni-rich LiNixCoyMn1-x-yO2(x>0.6)compounds with higher energy density,low toxicity and relatively low cost have attracted more attention as cathode materials for electric vehicles[3-4]. However,its poor cyclic performance,rate performance and thermal stability caused by cation mixing between Li+and Ni2+and the reactions with electrolytes restrict its further application[5-6].
To solve the issues such as short cycle life and poor thermal stability,extensive studies have been carried out by doping other metals such as Ti,Al or Mg into the LiNixCoyMn1-x-yO2framework[7-9],which help to stabilize the layered structure.Another approach to stabilize the transition-metal oxide framework is to coat the materials with a layer of stable compounds such as Al2O3,SiO2,AlF3,and ZrO2,resulting in materials with modified characteristics such as superior cyclic performance[10-13].Inaddition,the cathode materials such as Li(Ni1/3Co1/3Mn1/3)O2and Li(Ni0.5Mn0.5) O2have been coated on the material′s surface, improving the cycle performance and thermal stability of the material without significantly reducing the capacity,thus being of significant research value[14-15]. A series of researches on diverse core-shell,gradient core-shell and the full gradient materials were also developed[16-18].For example,Sun et al.developed a concentration-gradient Li(Ni0.72Co0.18Mn0.10)O2with a high discharge capacity of 193 mAh·g-1(3.0~4.3 V,0.2C rate,temperature 55℃),After 50 cycles,the capacity was maintained at 95.3%[19].Du et al.synthesized spherical Li(Ni0.8Co0.1Mn0.1)O2with a full concentration gradient(FCG)of Ni and Mn in a particle,in which the Mn concentration decreased linearly whereas the Ni concentration increased linearly from the outer layer to the center of each particle,which achieved an initial capacity of 185.2 mAh·g-1at 1C(3.0~4.3 V, 0.2C rate,temperature 25℃)and retains 93.2%after 100 cycles[20].The surface of the FCG material is abundant in stable Mn4+while is poor in the active Ni4+being tend to trigger phase transition.It is possible to reduce the corrosion of the electrolytic solution on the surface of the electrode material during the longterm circulation and to suppress the increase in the interfacialresistanceofthematerial.Meanwhile, according to the relevant research,it′s worth noting that the Ni element will diffuse from the inside of the particle to the surface during the high temperature sintering process,while the Co and Mn elements will diffuse from the surface of the particle to the center[19,21].
In this paper,Ni0.7Co0.15Mn0.15(OH)2precursor with the FCG structure was prepared by a co-precipitated method.Then,a uniform mixture of the as-prepared precursor and LiOH·H2O was sintered at different temperature to synthesize the FCG-LiNi0.7Co0.15Mn0.15O2cathodematerials.Thechangeofconcentration gradient distribution of Ni,Co,and Mn in the products was carefully investigated.The structural properties, morphologies,and electrochemical properties of these materials were also studied in detail.
1 Experimental
1.1 Materials synthesis
FCG-Ni0.7Co0.15Mn0.15(OH)2precursor was synthesized in NaOH-NH3-H2O system.According to the component design of the precursor,NiSO4·6H2O, CoSO4·7H2O,and MnSO4·H2O,with the cation′s molar ratio of nNi∶nCo∶nMn=0.87∶0.15∶0.15,were firstly dissolved in deionized water to prepare solution A of 2.0 mol·L-1.NiSO46H2O,CoSO4·7H2O,and MnSO4· H2O,with the cation′s molar ratio of nNi∶nCo∶nMn=1∶1∶1, were dissolved in deionized water to prepare solution B of 2 mol·L-1.During the co-precipitation reaction, the solution B was continuously pumped into solution A at a flow rate of 5.0 mL·min-1,while the mixed solution of A and B was maintaining stirred.Then,the mixed solution was pumped into a continuous stirred tank reactor(50 L)filled with nitrogen at a flow rateof 10.0 mL·min-1.While a NaOH precipitate solution (4.0 mol·L-1)and NH4OH solution(4.0 mol·L-1)as a chelating agent were simultaneously pumped into the reactor.The pH of the solution in the reactor was set to 11.5 and real-time monitored using an online pH analyzer.The temperature and stirring speed of the mixture in the reactor were kept at 55℃and 600 rpm,respectively.With the continuous progress of the reaction,theconcentrationofNiinsolutionA continuously decreased,while the Co and Mn contents gradually increased,forming full gradient distribution of Ni,Co,and Mn from the center to the surface of the precursor particles growing in the reactor.The volume ratio of the solution A and B was 1∶1 and two solutions were simultaneously consumed off after 40 h.Then,the obtained precipitate was filtered,washed several times and dried at 120℃for 24 h.After being sieved using a 400 mesh,the as-designed FCGNi0.7Co0.15Mn0.15(OH)2precursor was obtained.
The obtained spherical precursor was mixed with LiOH·H2O(a molar ratio of nLi∶(nNi+nCo+nMn)=1.05∶1) uniformly using an inclined mixer and then calcined at 500℃for 6 h in air.Then,the product was calcined at different temperature for 12 h in oxygen atmosphere to obtain FCG-LiNi0.7Co0.15Mn0.15O2.
1.2 Physical and chemical characterization
The chemical composition of the materials was evaluated by the inductively coupled plasma test (ICP).The structure of the samples was identified by Powder X-ray diffraction(XRD,D/max-r A type,40 kV, 300 mA,Rigaku)using Cu Kα radiation(λ=0.154 05 nm).The XRD data were collected between 2θ range of 10°~80°and a scanning rate of 2°·min-1.The microstructure and morphology of compounds were analyzed by scanning electron microscopy(SEM FEI Quanta 750,USA)operated with an accelerating voltage of 20 kV.The chemical composition of the samples was characterized by energy disperse X-ray spectrometer(EDX-720,Japan,theacceleration voltage was 20 kV).The cross-section samples of the material particles were prepared by cutting a small amount of the material powder into the middle of the two slides,and then the SEM and EDXS analysis were conducted.
1.3 Electrochemical measurement
The material was first made into an electrode and then assembled into a CR2025 type coin cell in a glove box full of dry argon(Mikrouna,O2and H2O~0.5 ppm respectively)for the electrochemical performance test.A certain amount of cathode material,polyvinylidene fluoride(PVDF)binder and acetylene black were mixed at a mass ratio of 8∶1∶1 with N-methylpyrrolidone as a solvent in an agate mortar to form a slurry. The slurry was uniformly spread onto an aluminum foil(thickness:14 μm)and then transferred to a vacuum oven at 110℃.After 12 h heating,the dried foil was punched to obtain a round electrode plate with a diameter of 11 mm,and the membrane density of electrode plate was 3.5 mg·cm-2.LiPF6organic solution(1 mol·L-1)using mixed solvents of ethylmethyl carbonic acid ester,ethylene carbonate,and dimethyl carbonate at a volume ratio of 1∶1∶1 was used as the electrolyte.A round lithium plate with a diameter and thickness of 15.4 and 1.0 mm,respectively,was used as the negative electrode and Celgard 2400 as the separator.The fabricated coin cells were subjected to electrochemicalperformancetestonanLAND CT2001A system with potential range of 2.8~4.3 V,a current density of 1C=180 mA·g-1,and a stabilized temperature of 25℃.Electrochemical impedance spectroscopy(EIS)of the battery with fully discharged state was performed using a Model 2273A electrochemical workstation(Perkin Elmer,USA),under the frequency range 10 mHz~100 kHz and a amplitude for the AC signal of 5 mV.The battery rest for 2 h after assembling was subjected to EIS experiment. TheelectrochemicalimpedancesoftwareZview (Version 2.1,Scribner Associates,Inc.)was performed to analyze the Impedance data.
2 Results and discussion
Fig.1 shows the TG-DTA curves of the mixture of FCG-Ni0.7Co0.15Mn0.15(OH)2precursor and LiOH·H2O. As shown in the Fig.1,three endothermic peaks corresponding to three weight loss stages are observed within the temperature range of 50~600℃.Amongthem,the first obvious weight loss stage occurs from the room temperature to 100℃results from the evaporation of water absorbed on the surface and the crystal water in the hydroxide;the second endothermic peak appearing at 279℃,can be attributed to the decomposition of M(OH)2to the oxide of transition metal(MOx);the third endothermic peak appears at 458℃,caused by the combination of melting of LiOH·H2O and MOxto form Li-M-O compounds. Then,no obvious phase change is observed when the temperature is higher than 500℃.However,a small amount of Li2O volatilized from the mixture in case of overheatwilldeterioratethematerial′selectrochemical performance.Thus,an excessive amount of LiOH·H2O is added in the process of sintering to compensate the loss of Li2O by volatilization.In view of the above analysis,the synthesis of the target material FCG-LiNi0.7Co0.15Mn0.15O2is divided into two steps:the first step is the 6 h calcination of the precursor mixed with LiOH·H2O at 500℃;Following is the calcination process temperature range of 750~900℃to synthesize the final products.
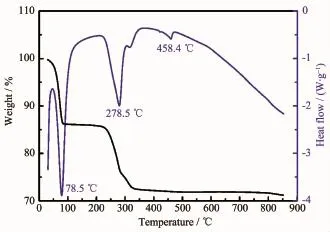
Fig.1 TG/DSC curves of mixture of FCG-Ni0.7Co0.15Mn0.15(OH)2and LiOH·H2O
The XRD pattern of FCG-Ni0.7Co0.15Mn0.15(OH)2precursor is shown in Fig.2a.The typical diffraction peaks are indexed well with that of Ni(OH)2with a space group of R3m[22-23],The cell parameters of the layered FCG-Ni0.7Co0.15Mn0.15(OH)2are a=0.309 9 nm and c=0.459 0 nm,indicating that Ni2+is partially substituted by Co2+and Mn2+ions in the Ni(OH)2structure.Fig.2b shows the XRD patterns of FCGLiNi0.7Co0.15Mn0.15O2cathode materials prepared at 750, 800,850 and 900℃,indicating no extra reflection and the lattice type is close to that of LiNiO2with a typical α-NaFeO2-type layered structure,and belonging to the space group of R3m.In the XRD patterns of the layered nickel-rich cathode material,the characteristic peaks(006)/(102)and(108)/(110)are often used to characterize the ordering of the layered structure[24].As shown in Fig.2b,the(100)/(102)and (108)/(110)peaks split clearer with the calcination temperature increasing,showing that the materials have better hexagonal structure at higher temperature. The intensity ratio of(003)and(104)peaks I003/I104is usually used to reflect the cation distribution in the lattice of the layered oxide.The larger I003/I104and c/a indicate a lower degree of cation mixing[14,25].The lattice parameters a and c are listed in Table 1.As thecalcinationtemperatureincreases,aandc increases,and at 800℃,the values of c/a and the intensities of(003)and(004)are both higher thanthose at other calcination temperatures.The data in the table indicate that the FCG-LiNi0.7Co0.15Mn0.15O2cathode material sintered at 800℃has a more orderly layered structure,higher crystallinity,and lower mixing extent of cation in all the samples obtained,all these characteristics correspond to a well-ordered layered structure and good electrochemical activity.

Fig.2 XRD patterns for FCG-Ni0.7Co0.15Mn0.15(OH)2(a)and FCG-LiNi0.7Co0.15Mn0.15O2(b)samples prepared at 750,800,850 and 900℃
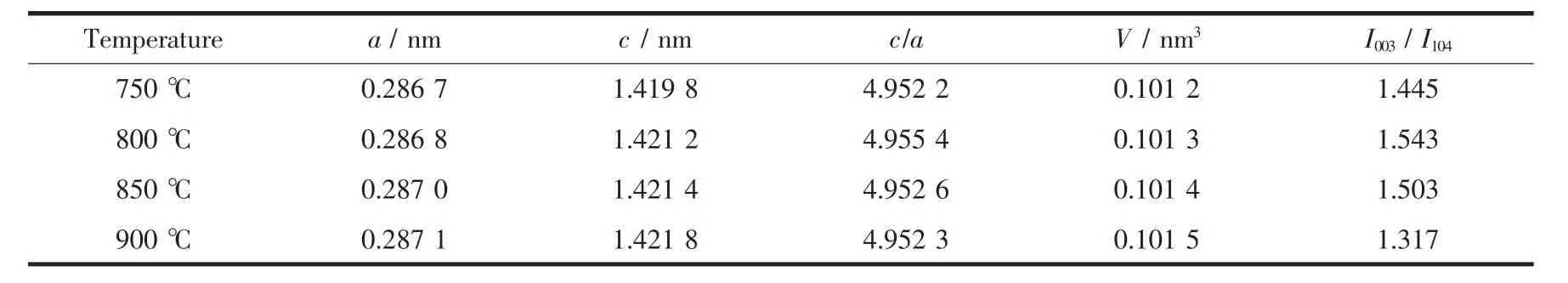
Table1 Lattice parameters of FCG-LiNi0.7Co0.15Mn0.15O2samples calcined at various temperatures
The SEM images of the FCG-Ni0.7Co0.15Mn0.15(OH)2precursor having aggregated spherical-like morphology with tiny spindle-like primary particles are shown in Fig.3a and b.The particle distribution is relatively concentrated with an average size of almost 12 μm in diameter.With a good morphology and particle size distribution,the tap density of the precursor reached to 2.16 g·cm-3.As shown in Fig.3c,the cross-section of the synthesized FCG-Ni0.7Co0.15Mn0.15(OH)2precursor exhibits an obvious concentration-gradient distribution of Ni,Co and Mn along the direction from the center to the surface of the particle.EDXS line scanning shows that the Ni content(a molar ratio of nNi/(nNi+nCo+nMn),similarly hereinafter)decreases continuously with almost linearly change from 0.87 to 0.36,while the Co and Mn contents increase gradually from nearly 0.15 to 0.32,respectively.The distance from the center to the outer shell is close to 6 μm.
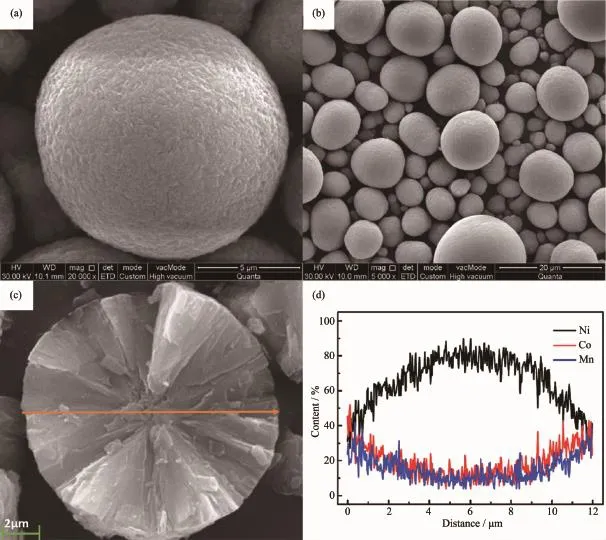
Fig.3 SEM images of FCG-Ni0.7Co0.15Mn0.15(OH)2samples at various temperature:×20 000(a),×5 000(b); cross-section(c)and EDXS line scanning(d)of the atomic ratio of transition metals across the diameter of a particle
TheSEMimagesofFCG-LiNi0.7Co0.15Mn0.15O2cathode materials calcined at various temperatures are shown by Fig.4.It is obvious that all of the material powders maintain the spherical morphology of the FCG-Ni0.7Co0.15Mn0.15(OH)2precursor after high-temperature calcination.The spherical particle is actually aggregated by the larger polyhedral primary particles changed from spindle-like primary particles of the precursor.As the calcination temperature increases, the size of primary particles increases(Fig.4).Generally, a fine grain size is conducive to the insertion and extractionoflithium-ionsduringcharge-discharge process,resultinginthepromotionofthe electrochemical performance[26].The XRD and SEM results indicate a well-ordered layered structure and good spherical morphology of the material calcined at 800℃.
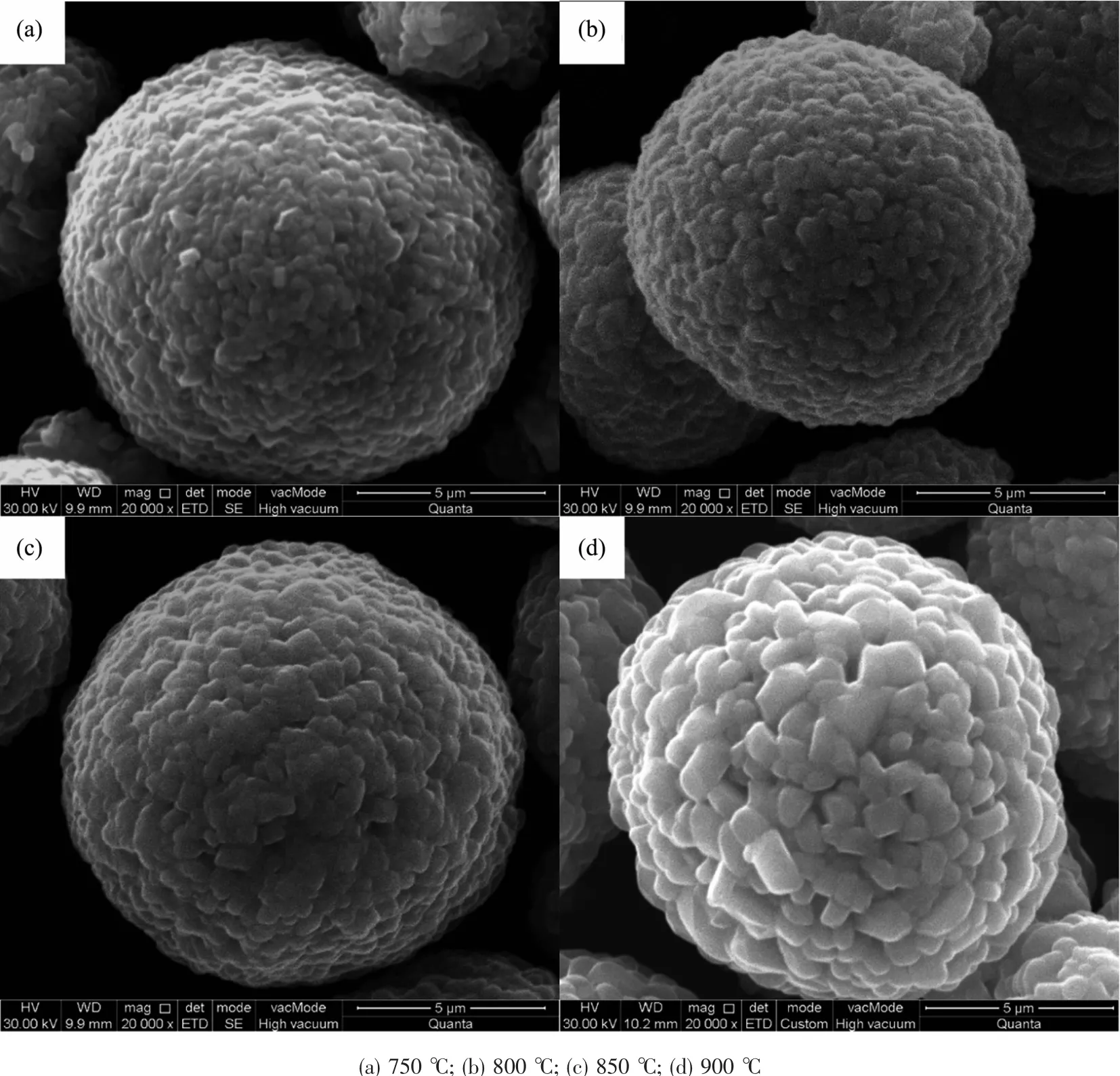
Fig.4 SEM images of FCG-LiNi0.7Co0.15Mn0.15O2powders calcined at various temperatures
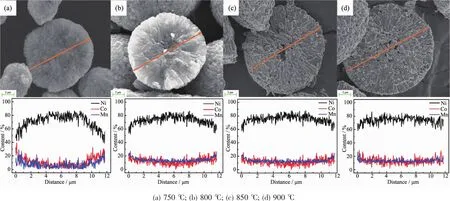
Fig.5 SEM images of the cross-section and EDXS line scanning results of the FCG-LiNi0.7Co0.15Mn0.15O2calcined at various temperatures
Fig.5 shows the EDXS line scanning results of the cross-section of FCG-LiNi0.7Co0.15Mn0.15O2cathode materials particles prepared by FCG-Ni0.7Co0.15Mn0.15(OH)2precursor at different calcination temperatures within the range of 750~900℃.The concentration distribution of Ni,Co,and Mn varies across the diameter of the particles′cross-section.As shown in the Fig.5a,the distribution of these three elements has the most obvious feature of concentration gradient, with the Ni content decreasing continuously with nearly linearly change from 0.83 to 0.42,while the Co and Mn contents increasing gradually from nearly 0.15 to 0.29,respectively.However,The XRD and SEM results indicate a poor layered structure and abnormal morphology of the material calcined at 750℃.It can be obviously observed from Fig.5b that with the calcination temperature reaching to 800℃,the Ni,Co and Mn variation of the cross-section of the lithiated oxide still retains the distribution characteristics in accordance with the FCG-Ni0.7Co0.15Mn0.15(OH)2precursor indicated in Fig.3c,and the Ni content decreases to 0.52 while the Co and Mn contents increase to 0.24 in the outer layer of the particle. With calcination temperature further increasing,the distribution of the three elements in the particles shows a trend of homogenization.As the calcination temperature increases to 850 and 900℃,the distribution inside materials′particles become significantly homogeneous,as shown in Fig.5c and d,at 900℃especially,the Ni,Co and Mn variation within the cross-section of the sample exhibit a homogeneous distribution from the center to the surface of the particle.
The electrochemical performance of the materials calcined at different temperatures is shown in Fig.6. The cells were subjected to the first charge and discharge test at a rate of 0.2C in the voltage range of 2.8~4.3 V(Fig.6a),while the cyclic performance is tested at the charge and discharge rate of 2C at the same potential range.The mass of the active material in the electrode plate is 3.2 to 3.3 mg.Fig.6a illustrates the initial charge-discharge performance for samples synthesized at different calcination temperatures,indicating the initial discharge capacity of the materials as 174.8,186.1,180.2,and 176.5 mAh·g-1accompanied by the initial charge-discharge efficiency of 88.3%,90.1%,86.4%and 82.7%at 750,800,850, and 900℃,respectively.As the XRD analysis shows above,the material sintered at 800℃has a more orderly layered structure and the highest crystallinity in all the samples obtained,and the higher initial charge-discharge efficiency benefits from the lower mixing extent of cation[27].Fig.6b shows the different cyclic performance of all of the samples as-synthesized. Within the voltage range of 2.8~4.3 V,the samples calcined at 750,800,850,and 900℃have the retention rates of 85.4%,90.1%,86.4%,and 80.8% after 200 cycles of charge-discharge,respectively. Above all,the higher manganese content and lower nickel content within the particles of the material calcined 800℃increases the stability of the material and reduces the side reaction of the material with the electrolyte,thusinhibitingtheincreaseofthe interfaceimpedanceandenhancingthecapacity retention during cycling[28].Combined with the XRD analysisandEDXSscanningresultsabove,thematerial with the most orderly layered structure and an obvious gradient distribution of the three elements at 800℃shows the optimal discharge capacity and cyclic performance.

Fig.6 Charge/Discharge curves at 0.2C(a)and Cyclic performance at 2C(b)in 2.8~4.3 V of the FCG-LiNi0.7Co0.15Mn0.15O2synthesized at various temperatures
The kinetic process of lithium ions intercalation/ deintercalation into the electrodes can be investigated by the electrochemical impedance spectroscopy(EIS)[29]. Fig.7a and b show the Nyquist plots of as-assembled and 200 cycles later for electrodes made from FCGLiNi0.7Co0.15Mn0.15O2calcined at 750,800,850,and 900℃,and it was achieved at room temperature(25℃)at the charged state(4.3 V),and the EIS parameters result from the test are presented in Table 2.The Nyquistplotsofas-assembledand200cycled batteries are shown in Fig.7c and d,which indicates the equivalent circuit model used to analyze the impedancespectra.Inthemodels,theohmic resistance of the electrode,separator,electrolyte,etc. in the cell is represented by Re.The capacitance and resistanceoflithiumionsmigrationthroughthe surface film which are reflected by the semicircle in the high-frequency region is represented by R1and CPE1;R2and CPE2 indicate the charge transfer resistance at the electrolyte/electrode interface which are related to the second semicircle existing in the medium and lower frequency region[30-31];Zwrepresents Warburg impedance which is associated with the slant inthelow-frequencyregion,resultedfromthediffusion of lithium ions in the electrode.Here,the pure capacitors are substituted by the constant-phase elements(CPE1 and CPE2).

Fig.7 Nyquist plots of FCG-LiNi0.7Co0.15Mn0.15O2synthesized at various temperatures after 0(a)and 200 cycles(b)and the equivalent circuit models(c,d)

Table2 Charge-transfer resistance(R2)for the FCG-LiNi0.7Co0.15Mn0.15O2samples synthesized at various temperatures after different cycles
Chen et al.reported that the impedance of cathode,especially charge-transfer resistance R2is the primary cause for the impedance of lithium-ion cells[32]. Thus,through comparing the second semicircle after cycles of cells which is related to R2,the difference between the surface of various materials can be obtained.As shown in Table 2,the value of the cells madeofFCG-LiNi0.7Co0.15Mn0.15O2beforecycleis 32.87,27.33,47.38,and 61.83 Ω for the samples calcined at the temperature of 750,800,850,and 900℃,respectively.Along with the cycling of the cells, the value increases to 196.33,137.19,261.64,and 446.26 Ω accordingly.Therefore,with a well-ordered structureandatypicalconcentrationgradient distribution of Ni,Co and Mn,the R2of the FCGLiNi0.7Co0.15Mn0.15O2synthesized at 800℃increases slowest from 27.33 Ω before cycle to 137.19 Ω after 200 cycles.Owing to the stable outer layer which has decreased Ni content and increased Mn content along with a weaker Li/Ni mixing,the FCG-LiNi0.7Co0.15Mn0.15O2performs the stable charge-transfer resistance.Thus, as to the lowst charge-transfer resistance of the electrode /electrolyteinterface,theFCG-LiNi0.7Co0.15Mn0.15O2synthesized at 800℃has an improved electrochemical performance compared with the other samples.
3 Conclusions
FCG-Ni0.7Co0.15Mn0.15(OH)2precursor was successfully synthesized by the special feed method in a NaOH-NH3-H2O system.The EDXS characterization results indicate that the semi-sphere of particle has an obvious concentration gradient distribution along the direction from the center to the surface.Different from the Ni-rich condition at the particle′s center,the Co and Mn enrich the outer layer of the particle with decreasingNielement.Duringtheprocessof sintering,the homogenized diffusion of the three elements in the interior of the particle is inevitable. Such diffusion process is more serious and tends to homogenize with increasing calcination temperature. Although the lower sintering temperature can maintain a more obvious concentration gradient distribution,it results in poorer layered structure and electrochemical performance.The experimental results show that all the three Ni,Co,and Mn elements still have a relatively obvious concentration gradient distribution at a sintering temperature of 800℃and a surface nickel content of the 52%,respectively.The increased stable Mn4+content and the reduced Ni4+amount in theparticlesurfacecandosignificantfavorto enhance the electrochemical stability of the cathode material.Meanwhile,it has a significant influence on restraining the increase of charge-transfer resistance duringcyclingandthuselevatesthecyclic performance.At a rate of 0.2C,the specific discharge capacity is 186.1 mAh·g-1and the retaining capacity percentage is 90.1%even after 200 cycles at a charge-discharge rate of 2C,representing superior electrochemical performance.
[1]AnderssonAM,Abraham D P,Haasch R,etal.J.Electrochem. Soc.,2002,149:A1358-A1369
[2]Goodenough J B,Kim Y.Chem.Mater.,2010,22:587-603
[3]Armand M,Tarascon J M.Nature,2008,451:652-657
[4]Liu W,Oh P,Liu X,et al.Angew.Chem.Int.Ed.,2015,54: 4440-4458
[5]Fu C,Li G,Luo D,et al.ACS Appl.Mater.Interfaces,2014, 18:15822-15831
[6]ZHANG Yu(张钰),SU Zhi(粟智),PAN Hui(潘会).Chinese J.Inorg.Chem.(无机化学学报),2015,31(9):1827-1830
[7]Mo Y,Li D,Chen Y,et al.RSC Adv.,2016,6:75293-75298
[8]LI Xiao-Wei(李晓炜),LIN Ying-Bin(林应斌),LIN Ying(林莹),et al.Chinese J.Inorg.Chem.(无机化学学报),2011,27 (4):643-648
[9]Liao P Y,Duh J G,Sheu H S.J.Power Sources,2008,183: 766-770
[10]Huang Y,Chen J,Cheng F,et al.J.Power Sources,2010, 195:8267-8274
[11]Liang L,Hu G,Jiang F,et al.J.Alloys Compd.,2016,657: 570-581
[12]WANG Xu-Yang(王旭阳),YE Xue-Hai(叶学海),ZHI Xiao-Ke(郅晓科),et al.Chinese J.Inorg.Chem.(无机化学学报), 2013,29(4):774-778
[13]Kong J Z,Wang S S,Tai G A,et al.J.Alloys Compd., 2016,657:593-600
[14]Cho S W,Kim G O,Ju J H,et al.Mater.Res.Bull.,2012, 47:2830-2833
[15]Lee K S,Myung S T,Sun Y K.J.Power Sources,2010,195: 6043-6048
[16]Myung S T,Noh H J,Yoon S J,et al.J.Phys.Chem.Lett., 2014,5:671-679
[17]Du K,Hua C,Tan C,et al.J.Power Sources,2014,263:203 -208
[18]Lee E J,Noh H J,Yoon C S,et al.J.Power Sources,2015, 273:663-669
[19]Sun Y K,Kim D H,Yoon C S,et al.Adv.Funct.Mater., 2010,20:485-491
[20]Hua C,Du K,Tan C,et al.J.Alloys Compd.,2014,614: 264-270
[21]Huang Z,Gao J,He X,et al.J.Power Sources,2012,202: 284-290
[22]Kim M H,Yang K S.J.Power Sources,2006,159:1328-1333
[23]HU Guo-Rong(胡国荣),LIU Qiang(刘强),DU Ke(杜柯), et al.Chinese J.Inorg.Chem.(无机化学学报),2012,28(6): 1171-1176
[24]Xiang M,Tao W,Wu J,et al.Ionics,2016,22:1003-1009
[25]LIU Xin-Yan(刘欣艳),ZHAO Yu-Juan(赵煜娟),LI Yan(李燕),et al.Chinese J.Inorg.Chem.(无机化学学报),2006,22 (6):1007-1012
[26]Wu K,Wang F,Gao L,et al.Electrochim.Acta,2012,75: 393-398
[27]Kang S H,Abraham D P,Yoon W S,et al.Electrochim. Acta,2009,54:684-689
[28]Abraham D P,Twesten R D,Balasubramanian M,et al.J. Electrochem.Soc.,2003,150:A1450-A1456
[29]Shaju K M,Subba Rao G V,Chowdari B V R.J.Electrochem. Soc.,2003,150:A1-A13
[30]Levi M D,Gamolsky K,Aurbach D,et al.Electrochim. Acta,2000,45:1781-1789
[31]Liu H,Zhang Z,Gong Z,et al.Solid State Ionics,2004,166: 317-325
[32]Chen C H,Liu J,Amine K.J.Power Sources,2001,96:321-328
Effect of Synthesis Temperature on the Structural and Electrochemical Properties of a Full Concentration Gradient LiNi0.7Co0.15Mn0.15O2Cathode Material
TAN Chao-Pu1HU Guo-Rong1DU Ke*,1LUO Hong-Jun2HUANG Dian-Hua2DUAN Jian-Guo1CAO Yan-Bing1
(1School of Metallurgy and Environment,Central South University,Changsha 410083,China)
(2Guangzhou Libode New Materials Co.,Ltd.,Guangzhou 510800,China)
Spherical Ni0.7Co0.15Mn0.15(OH)2precursor with a full concentration gradient(FCG)of Ni,Co and Mn elements was obtained via co-precipitation method.The precursor was evenly mixed with LiOH·H2O and then sintered at 750~900℃for 12 h in oxygen to synthesize FCG-LiNi0.7Co0.15Mn0.15O2cathode material with the Ni rich in core and Mn rich in the outer layer.The diffusion of Ni,Co,and Mn under different calcination temperatures led to various elements homogeneity,and was analyzed by energy-dispersive X-ray spectroscopy (EDXS).Then,the electrochemical properties of samples were investigated by the charge-discharge test and electrochemical impedance spectroscopy(EIS)test.The results indicate that the cathode material sintered at 800℃has an obvious concentration-gradient distribution with a shell of LiNi0.52Co0.24Mn0.24O2and exhibits the optimal electrochemical performance.Under the voltage range 2.8~4.3 V,it deliveres an initial discharge of 186.1 mAh· g-1at a charge-discharge rate of 0.2C,and shows an excellent capacity retention of 90.1%after 200 cycles at a high rate of 2C.
Lithium-ion battery;cathode material;full concentration-gradient;element diffusion
O646
A
1001-4861(2017)09-1537-10
10.11862/CJIC.2017.203
2017-06-19。收修改稿日期:2017-07-26。国家自然科学基金(No.51602352)资助项目。
*通信联系人。E-mail:csutcp@163.com
