联吡啶四氮唑单核钆配合物的甲基化影响
2017-09-06狄宝生罗燕生曾雪花何丽华陈景林廖金生刘遂军温和瑞
狄宝生 罗燕生 曾雪花 何丽华 陈景林 廖金生 刘遂军 温和瑞
(江西理工大学冶金与化学工程学院,赣州341000)
狄宝生 罗燕生 曾雪花 何丽华 陈景林*廖金生 刘遂军 温和瑞
(江西理工大学冶金与化学工程学院,赣州341000)
应用6-(氢-5-四氮唑基)-2,2′-联吡啶(tbpyH)和6-(氢-5-四氮唑基)-4,4′-二甲基-2,2′-联吡啶(tmbpyH)配体,合成得到2个新的单核钆配合物[Gd(tbpy)2(DMF)(H2O)2]NO3·2H2O(1)和[Gd(tmbpy)2(DMF)(NO3)]·DMF·THF(2)。X射线单晶衍射表明,每个钆离子均表现为1个畸变的三冠三角棱柱体,包含了2个四氮唑基N-H去质子化而产生的一价阴离子三齿螯合配体。此外,在2,2′-联吡啶环上引入2个甲基对钆金属中心的配位环境有显著影响,表现为2个单齿配位的水分子被1个螯合配位的硝酸根离子取代。
钆配合物;联吡啶四氮唑;甲基化;晶体结构
0 Introduction
Mono-anionic polydentate chelating ligand is a preferred candidate,which can saturate the coordination sphere of the Lnion and completely compensate the charge in the case where tris-ligand complexes are generated,an advantage for application in photoelectric devices.Bipyridyl tetrazole(Scheme 1)may be an alternative class of N-heterocyclic ligands which may be easily modified and generate strong bonding with transition metals,giving robust complexes,where the N-H deprotonation of the tetrazolyl ring gives rise to themono-anionictridentatechelatingligands[23-26]. Herein,we report the synthesis and crystal structures oftwonewmononuclearGdcomplexeswith deprotonated bipyridyl tetrazole tridentate chelating ligands,and the influence of the methylation of the 2,2′-bipyridylringonthestructuresofGdcomplexes.
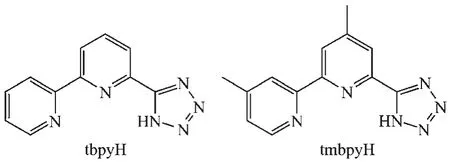
Scheme 1Structures of the tbpyH and tmbpyH ligands
1 Experimental
1.1 Materials and measurements
All the chemicals used for synthesis were of analytical grade and used without further purification unless otherwise stated.6-(1H-tetrazole-5-yl)-2,2′-bipyridyle(tbpyH)and 6-(1H-tetrazole-5-yl)-4,4′-dimethyl -2,2′-bipyridyle(tmbpyH)were synthesized according to literature methods[23-24].Elemental analyses of carbon, hydrogen and nitrogen were conducted on a Perkin-Elmer model 240C elemental analyzer.Infrared(IR) spectra were recorded on a Bruker Optics ALPHA FT-IRspectrometerusingKBrpellets.Crystal structures were determined on a Bruker D8 QUEST diffractometer.
1.2 Preparations of complexes 1 and 2
1.2.1 [Gd(tbpy)2(DMF)(H2O)2]NO3·2H2O(1)
An ethanol solution(10 mL)of Gd(NO3)3·6H2O (32.1 mg,0.071 mmol)and tbpyH(49.3 mg,0.220 mmol)wasrefluxedfor12h,givingawhite precipitate.The precipitate was isolated by filtration, washed with diethyl ether and dried under vacuum. Colorless crystals of 1 were afforded via slow diffusion of CH3CN into the DMF solution of 1 after 6 days. Yield:46%(based on Gd(NO3)3·6H2O).Anal.Calcd. for C25H29GdN14O8(%):C,37.03;H,3.60;N,24.18. Found(%):C,37.06;H,3.63;N,24.14.IR(KBr,cm-1): 3 412(m),3 083(w),1 663(vs,C=O),1 601(s),1 429(vs), 1 384(vs,NO3-),1 314(m),1 243(w),1 186(w),1 110(w), 1 060(w),1 009(m),829(w),790(m),758(m),688(m).
1.2.2 [Gd(tmbpy)2(DMF)(NO3)]·DMF·THF(2)
Complex 2 was synthesized following the procedure for 1,using Gd(NO3)3·6H2O(52.2 mg,0.116 mmol) and tmbpyH(90.4 mg,0.358 mmol).Colorless crystals of 2 were afforded by slow diffusion of THF into the DMF solution of 2 after 5 days.Yield:41%(based on Gd(NO3)3·6H2O).Anal.Calcd.for C36H44GdN15O6(%): C,45.99;H,4.72;N,22.35.Found(%):C,45.94;H, 4.75;N,22.32.IR(KBr,cm-1):3 431(m),3 066(w), 2 926(w),2 588(w),1 656(s,C=O),1 618(vs),1 564(m), 1 482(m),1 388(s,NO3-),1 239(vs),1 157(vs),1 010(m), 940(m),877(w),835(w),789(w),748(w),687(w),628 (m),553(m),505(s),432(w).
1.3 X-ray crystallography
The measurements of single crystals of 1 and 2 were performed on a Bruker D8 QUEST diffractometer using a graphite-monochromated Mo Kα radiation(λ= 0.071 073 nm).The program CrystalClear was used for the integration of the diffraction profile.Structures were solved by direct methods and refined by full-matrix least-squares technique on F2using the SHELXTL software package[27-28].The heavy atoms were located from E-map and other non-hydrogen atoms were found in subsequent difference Fourier syntheses.All nonhydrogen atoms were refined anisotropically,while hydrogen atoms were generated geometrically with isotropicthermalparameters.Thecrystallographic data and structure refinement details of 1 and 2 are listed in Table 1,and the selected bond lengths and angles for 1 and 2 are summarized in Table 2.
CCDC:1496645,1;1496646,2.
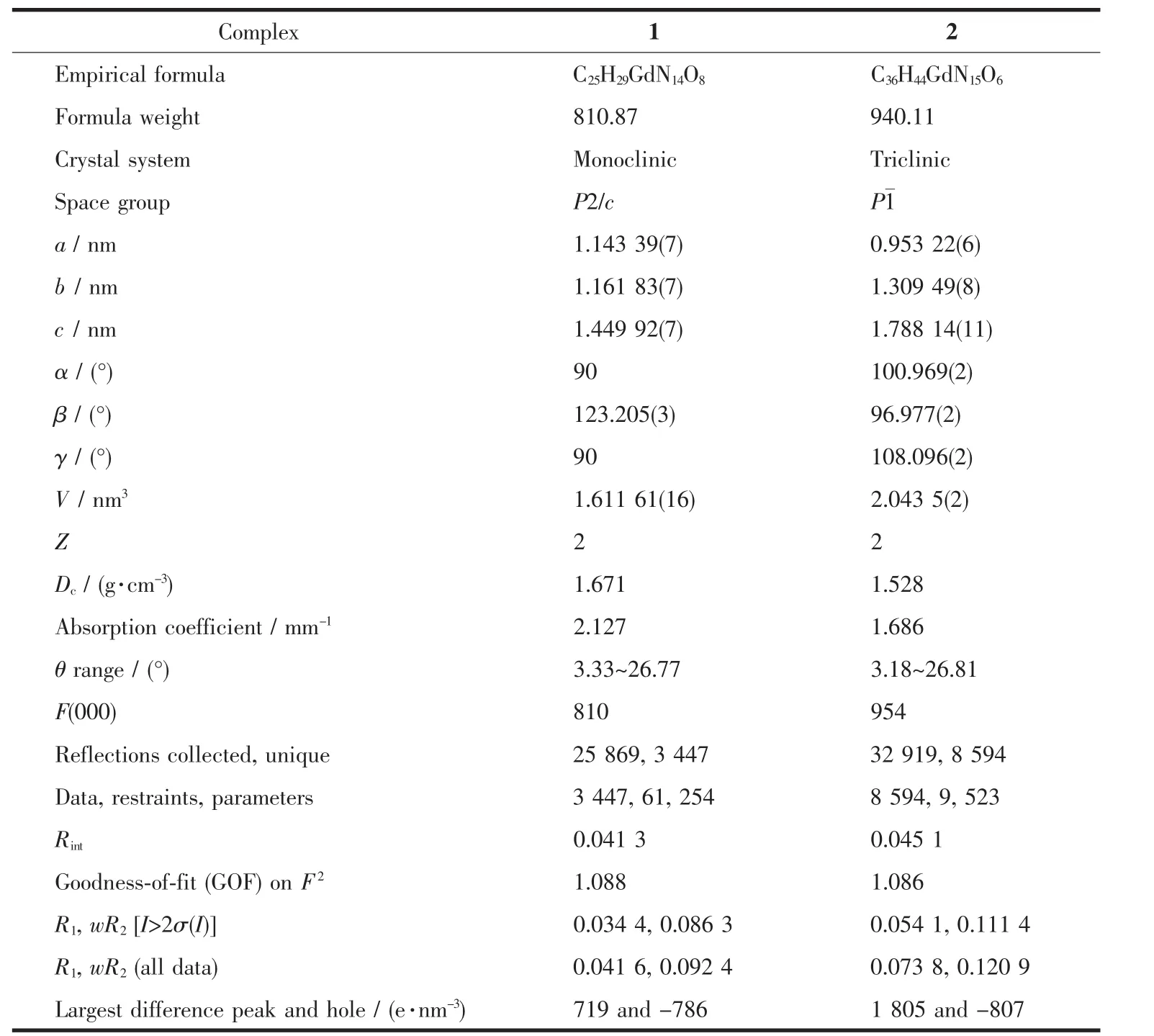
Table 1Crystal data and structure refinement for 1 and 2

Table 2Selected bond lengths(nm)and angles(°)for 1 and 2
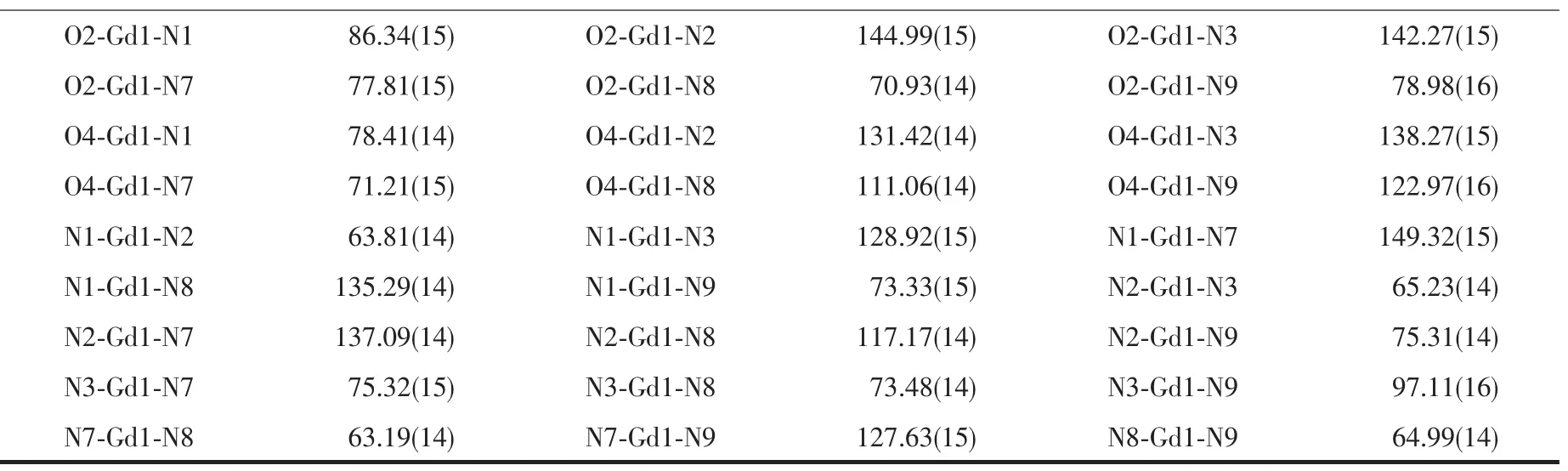
Continued Table 2
2 Results and discussion
2.1 Synthesis and characterization
2.1 Crystal structural description

Fig.1Molecular structure of the cation with 30%probability ellipsoids(a)and coordination geometry around the Gdion(b)of complex 1
The exact structures of 1 and 2 were established by single-crystal X-ray crystallography.Complex 1 crystallizes in the monoclinic system,space group P2/ c.The asymmetric unit of 1 contains one[Gd(tbpy) (DMF)(H2O)2]+cation,one NO3-anion and two H2O solvent molecules.As indicated in Fig.1a,the Gdion of 1 is nine-coordinated by six N atoms from two mono-anionic tbpy tridentate chelates and three O atoms from one DMF and two H2O molecules.The coordination polyhedron around the Gdcenter can be described as a distorted tricapped trigonal prism (Fig.1b),with two N atoms from the central pyridyl rings of two tmbpy chelates and one O atom from oneDMF molecule in capping positions(these three atoms [N,N and O]are in-plane with the Gdcation).The tbpy ligand ligates the Gdion in a mono-anionic tridentate chelating coordination fashion via the N-H cleavage of the tetrazolyl ring.The Gd1-N3 distance (0.249 8(3)nm)(tetrazolyl-N atom)is shorter than those between the Gdion and the pyridyl-N atoms(Gd1-N1 0.258 5(4)nm;Gd1-N2 0.259 9(3)nm],implying a stronger bonding of the Gdion to the tetrazolyl-N atomandasignificantinfluenceoftheN-H deprotonation of the tetrazolyl ring on the binding strength of the ligand.The Gd1-O1 distance(hydrate oxygen atom)is 0.242 1(3)nm,and the Gd1-O2 length (DMF oxygen atom)is 0.241 2(5)nm.
However,complex 2 crystallizes in the triclinic system,space group P1,in which tmbpy exhibits a mono-anionic tridentate chelating coordination manner similar to the tbpy ligand of 1.The asymmetric unit of 2 includes one[Gd(tmbpy)(DMF)(NO3)]molecule,one DMF and one THF solvent molecule.As shown in Fig.2a,the coordination number of the Gdion is also nine,similar to 1,of which seven positions are also occupied by six N atoms from two mono-anionic tmbpy tridentate chelating ligands and one O atom from one DMF solvent molecule,the remaining two positions are occupied by two O atoms from one chelating nitrate anion instead of two coordinated H2O molecules of 1.Analogous to 1,the coordination geometry around the Gdion of 2 is also a distorted tricapped trigonal prism(Fig.2b),with two N atom from the central pyridyl rings of two tmbpy chelates and one O atom from the nitrate anion in capping positions(these three atoms[N,N and O]are also inplane with the Gdion).The Gd-Ntetrazolyldistances (Gd1-N3 0.247 8(5)nm;Gd1-N9 0.248 6(5)nm)are shorter than the Gd-Npyridyllengths(Gd1-Npyridyl0.254 6(4)~0.255 7(4)nm),indicative of a stronger bonding of the Gdion to the tetrazolyl-N atom.It is also noted that the Gd-N lengths of 2 are slightly shorter than those of 1,implying an important influence of two electron-donatingmethylgroupsonthebipyridyl tetrazole chelate.As is further supported by the fact that the Gd1-O1 length(DMF oxygen atom)of 2 (0.235 0(4)nm)is somewhat shorter than the Gd1-O2 length(DMF oxygen atom)of 1(0.241 2(5)nm).The Gd-Onitratedistances are 0.250 0(4)and 0.252 0(4)nm.

Fig.2Molecular structure of the cation with 30%probability ellipsoids(a)and coordination geometry around the Gdion(b)of complex 2
3 Conclusions
We have synthesized and characterized two new mononuclearGdcomplexeswithdeprotonated bipyridyltetrazoletridentatechelatingligands, [Gd(tbpy)2(DMF)(H2O)2]NO3·2H2O(1)and[Gd(tmbpy)2(DMF)(NO3)]·DMF·THF(2),in which tbpyH and tmbpyH serve as the mono-anionic tridentate chelating ligands via deprotonation of the tetrazolyl-NH.It is demonstratedthatthemethylationofthe2,2′-bipyridyl ring has an important influence on the molecular structures of Gdcomplexes,exhibitingthat two mono-coordinated H2O molecules are replaced by one bidentate chelating nitrate.We believe that the results presented herein might provide new insight into the design and synthesis of new Lncomplexes.
Acknowledgements:We thank the financial supports from the National Natural Science Foundation of China(Grants No.21561013,21501077),theMajorProgramofJiangxi Provincial Natural Science Foundation of China(Grants No. 20143ACB21017,20161ACB21013),theNaturalScience FoundationofJiangxiProvinceofChina(GrantsNo. 20142BAB203001,20151BAB213003)and the Program for Qingjiang Excellent Young Talents of Jiangxi University of Science and Technology.
[1]Leonard J P,Nolan C B,Stomeo F,et al.Top.Curr.Chem., 2007,281:1-43
[2]Eliseeva S V,Bünzli J C G.Chem.Soc.Rev.,2010,39:189-227
[3]Binnemans K.Chem.Rev.,2009,109:4283-4374
[4]Gao F,Cui L,Liu W,et al.Inorg.Chem.,2013,52:11164-11172
[5]Gao F,Cui L,Song Y,et al.Inorg.Chem.,2014,53:562-567
[6]Li H X,Cheng M L,Ren Z G,et al.Inorg.Chem.,2006,45: 1885-1887
[7]Liu L L,Ren Z G,Zhu L W,et al.Cryst.Growth Des.,2011, 11:3479-3488
[8]BING Ying-Ying(邴颖颖),WU Zhen-Ting(吴振廷),HU Ming (胡明).Chinese J.Inorg.Chem.(无机化学学报),2015,31: 2059-2064
[9]Zebret S,Dupont N,Bernardinelli G,et al.Chem.Eur.J., 2009,15:3355-3358
[10]Zebret S,Dupont N,Besnard C,et al.Dalton Trans.,2012, 41:4817-4823
[11]Rybak J C,Meyer L V,Wagenhfer J,et al.Inorg.Chem., 2012,51:13204-13213
[12]Gusev A N,Shul′gin V F,Meshkova S B,et al.Inorg.Chim. Acta,2012,387:321-326
[13]Gusev A N,Hasegawa M,Nishchymenko G A,et al.Dalton Trans.,2013,42:6936-6943
[14]D′Alessio D,Muzzioli S,Skelton B W,et al.Dalton Trans., 2012,41:4736-4739
[15]Facchetti A,Abbotto A,Beverina L,et al.Chem.Commun., 2004,15:1770-1771
[16]Andrews P C,Junk P C,Massi M,et al.Chem.Commun., 2006,31:3317-3319
[17]Giraud M,Andreiadis E S,Fisyuk A S,et al.Inorg.Chem., 2008,47:3952-3954
[18]Bozoklu G,Marchal C,Pécaut J,et al.Dalton Trans.,2010, 39:9112-9122
[19]Andreiadis E S,Imbert D,Pécaut J,et al.Dalton Trans., 2012,41:1268-1277
[20]Lin J M,Guan Y F,Wang D Y,et al.Dalton Trans.,2008, 37:6165-6169
[21]Wu M F,Wang M S,Guo S P,et al.Crystal.Growth Des., 2011,11:372-381
[22]Liang L,Peng G,Ma L,et al.Crystal.Growth Des.,2012, 12:1151-1158
[23]Kratsch J,Beele B B,Koke C,et al.Inorg.Chem.,2014,53: 8949-8958
[24]Song Y H,Chiu Y C,Chi Y,et al.Organometallics,2008, 27:80-87
[25]Chen J L,Tan X Z,Chen X X,et al.Inorg.Chem.Commun., 2013,30:120-123
[26]Chen J L,Luo Y S,Gao G P,et al.Polyhedron,2016,117: 388-393
[27]Sheldrick G M.Acta Crystallogr.Sect.A,1990,46:467-473
[28]Sheldrick G M.Acta Crystallogr.Sect.A,2008,64:112-122
[29]Fukuda Y,Nakao A,Hayashi K.J.Chem.Soc.,Dalton Trans., 2002:527-533
[30]LIU Ying-Hong(刘迎红),REN Qing(任请),MA Jian-Ru(马建茹),et al.Chinese J.Inorg.Chem.(无机化学学报),2007, 23:1322-1328
Effect of the Methylation on Mononuclear GdBipyridyl Tetrazolate Complexes
DI Bao-ShengLUO Yan-ShengZENG Xue-HuaHE Li-Hua CHEN Jing-Lin*LIAO Jin-ShengLIU Sui-JunWEN He-Rui
(School of Metallurgy and Chemical Engineering,Jiangxi University of Science and Technology,Ganzhou,Jiangxi 341000,China)
Two new mononuclear Gdcomplexes,[Gd(tbpy)2(DMF)(H2O)2]NO3·2H2O(1)and[Gd(tmbpy)2(DMF) (NO3)]·DMF·THF(2),have been synthesized by using 6-(1H-tetrazole-5-yl)-2,2′-bipyridyle(tbpyH)and 6-(1H-tetrazole-5-yl)-4,4′-dimethyl-2,2′-bipyridyle(tmbpyH).As revealed by single-crystal X-ray diffraction,each Gdion has a distorted tricapped trigonal prism with two mono-anionic tridentate chelating ligands,originating from N-H deprotonation of the tetrazolyl ring,and the introduction of two methyl groups into the 2,2′-bypridyl ring has a significant effect on the coordination environment of the Gdcore,showing that two mono-coordinated H2O molecules are displaced by one chelating nitrate.CCDC:1496645,1;1496646,2.
Gdcomplex;bipyridyl tetrazole;methylation;crystal structure
O614.33+9
A
1001-4861(2017)02-0323-06
10.11862/CJIC.2017.031
2016-08-08。收修改稿日期:2016-12-07。
国家自然科学基金(No.21561013,21501077)、江西省青年科学基金重大项目(No.20143ACB21017,20161ACB21013)、江西省自然科学基金(No.20142BAB203001,20151BAB213003)和江西理工大学清江青年英才支持计划资助。
*通信联系人。E-mail:gzchenjinglin@126.com
