Identification and characterization of Acidithiobacillusferrooxidans with high activity and resistance isolated from ancient mine area
2017-09-04FENGGuangzhiLIAOLimingLIUZiyingSHIYu
FENG Guang-zhi, LIAO Li-ming, LIU Zi-ying, SHI Yu
(Coll. of Food & Biotech., Wuhan Inst. of Design & Sci., Wuhan 430205)
Identification and characterization ofAcidithiobacillusferrooxidanswith high activity and resistance isolated from ancient mine area
FENG Guang-zhi, LIAO Li-ming, LIU Zi-ying, SHI Yu*
(Coll.ofFood&Biotech.,WuhanInst.ofDesign&Sci.,Wuhan430205)
A highly active ferrous iron oxidationAcidithiobacillusferrooxidansstrain SY, was isolated from an ancient copper mining area in Daye, Hubei Province. Analysis of 16S rDNA sequence showed that the strain has high similarity to the sequence ofA.ferrooxidans(DQ 062116.1). Physiological and biochemical determinations showed that the strain was a chemical energy autotrophically with the optimal growth pH at 2.0 and optimal growth temperature at 30 ℃. The MTC of the strain to resist Cu2+, Cd2+, Ni2+, and Zn2+were respectively at 300, 350, 700, and 800 (mmol/L), demonstrated it has high resistance against multiple heavy metal ions. Bioleaching data of SY showed its bioleaching rate on native ore was as high as 84.28%, higher than bioleaching rate on the ore from other mining areas, showing it has very high superiority on bioleaching native ore. The strain SY has great potential in the application of native minerals bioleaching.
Acidithiobacillusferrooxidans; physiological properties; biooxidation; heavy metal resistance; bioleaching
As natural resources are progressively being depleted, development of new technologies in recovering precious metals from low-grade ores is becoming important. Biohydrometallurgy means the oxidation of sulphide minerals by microorganisms to extract metal[1-2]. Microbe-based processes have clear economic advantages and are usually more environmentally friendly than physical-chemical processes[3-4]. Presently, bioleaching is the leading mineral-processing techniques about metals being recovered from low-content minerals. The commonly used microbial species in bioleaching processes might involveA.ferrooxidans,Leptospirillumferrooxidans,A.thiooxidans, andA.caldus, etc.A.ferrooxidanscould oxidize ferrous ions, elemental sulfur, thiosulfates and sulfides, which is considered to be the major and the widest researched leaching bacterium up to date[5-8].
The iron-oxidizing bacteriumA.ferrooxidansis considered one of the most important microorganisms in the bioleaching of sulfide ores. This might be due to its ability to utilize both Fe2+and sulfur moieties in sulfide ores for growth. It is known that metal ions are solubilized from metal sulfide directly byA.ferrooxidanscells (a direct bioleaching mechanism) or indirectly by ferric ion produced byA.ferrooxidanscells (a indirect bioleaching mechanism). Ferrous ions are oxidized to be ferric ions firstly in the energy metabolism process ofA.ferrooxidans, and then ferric ions play a more direct effect in the oxidation of sulfide minerals to make it break down, so that metal ions from the mineral are released[6-7,9-10]. Hence, it appears reasonable to conclude that theA.ferrooxidansstrain which has higher iron-oxidizing activity is preferable for more efficient bioleaching.
The metal tolerance of bacterial species has been considered one of the most important parameters in determining the efficiency of the bioleaching process[11-13]. The influence of different concentrations of base metal ions, such as Cu2+, Zn2+and Fe3+, when present either alone or in different possible binary and ternary combinations in 9K medium, on the ferrous ion oxidation ability ofA.ferrooxidanswas studied. The significance and relevance of multi-metal ion tolerance inA.ferrooxidanshas been highlighted with respect to bioleaching of sulphide mineral concentrates[11]. The ancient mine in Daye,Hubei Province, with a history of metallurgy more than 3 000 years has a lot of multi-metal ore. Mine tailings, wastes produced and low-grade ores are stacked in the ancient mine area after many years development. Sulfide-bearing mineralization, when exposed to weathering, could become a source of acid mine drainage (AMD) and induce an increase in heavy metal mobility, creating a potentially serious hazard for the surrounding environment. Acid water from ancient mine contains high levels of dissolved metals where produces the AMD. It is possible to consider this zone as an important source of microorganisms of relevant importance on multi-metal ion tolerance.
A number of studies have shown that indigenous microorganisms isolated from the locality of mineral deposit is more effectively on metal dissolution[14-18]. In their natural ecological niche, the acidophilic bacteria are constantly exposed to varying concentration of solutes and seasonal temperature changes. Thus, the indigenous bacteria present the advantage over a collection strain that they are adapted to the climatic conditions and the mineralogical and chemical composition of the ores to be treated. This particular advantage could lead to an increase in the metal extraction values during bioleaching process of native minerals.
In this study, multi-metal resistance strain was isolated and identified from the ancient mine area in Daye and the ability to oxidize ferrous iron at different pH, temperature, inoculation amount was described. This work presents a attempt to provide bacterial strains with high activity and multi-metal ion tolerance isolated from ancient mine area in Daye to research the effect of the indigenous strain in the bioleaching of ores.
1 Materials and methods
1.1 Water sample and isolation
Water samples for microorganism isolation were taken from AMD of Daye ancient mine area in Hubei province. The pH of the water was 4.5. Samples were stored at 4 ℃. The 9K medium was used to enrichA.ferrooxidans. The composition of the basal medium used was as follows[19]: Solution A: (NH4)2SO43 g/L,K2HPO40.5 g/L,MgSO40.5 g/L,KCl 0.1 g/L,Ca(NO3)20.01 g/L; solution B:FeSO4·7H2O 44.7 g/L. Solutions A and B were mixed at 7∶3 after being sterilized separately. The initial pH of the medium was adjusted to 2.0. Solid plates were prepared by adding 1.5% agar powder to the liquid 9K medium above, but the initial pH was adjusted to 2.5. 10 mL of the original 4 AMD samples were transferred into 90 mL of autoclaved 9K medium for enrichment. The flasks were then placed on constant-temperature shakers at 200 r/min and 30 ℃ until the color of the media became reddish-brown. Then, the cultures were subjected to two another cultivation cycles. After 3 generations of enrichment, serial dilutions from 10-1to 10-6were made and 100 μL aliquots plated on solid agar medium. After 7-9 d, many colonies formed on the surface of the agar plates. Colonies with different morphology and colors were picked from the 10-4and 10-6dilutions and transferred again to the liquid medium. In order to isolate pure bacterial strains, the cultures were subjected to two additional cultivation cycles, each including incubation in liquid followed by solid medium. An optical microscope was used to monitor the purity of the strains.
1.2 Preparation of DNA
8 mL liquid cultures of strain SY in the mid-exponential growth phase were filtrated and centrifugation at 2 000 r/min for 5 min to remove the jarosite in the liquid. The bacteria were harvested by centrifugation at 10 000 r/min for 10 supplementary minutes. The sedimentary cells were washed twice with de-ionized water. The DNA was extracted by phenol-chloroform method.
1.3 Amplification and sequencing of 16S rDNA gene
The 16S rDNA gene fragments were amplified by PCR using the forward primer 27f (5′-AGAGTTTGATCCTGGCTCAG-3′) and reverse primer 1492r (5′-GGCTACCTTGTTACGACT-3′). The PCR program was 94 ℃ for 5 min, followed by 30 cycles of 94 ℃ for 30 s, 54 ℃ for 50 s, and 72 ℃ for 90 s, and finally 72 ℃ for 8 min. A PCR product of approximately 1.5 kb corresponding to position 27 to 1492 was obtained (Escherichiacolinumbering). The PCR products were ligated to the PTA-2 vector and transformed into Escherichia coli strain DH5α, then plated on LB medium containing ampicillin, X-Gal and IPTG. White colonies maybe include the positive recon. For the ensurement of the correct insert, a PCR with M13+/- universal primers on a vector were proceed. The positive colonie was picked out and cultured, then sent to Shanghai Sangon Biological Engineering Technology And Service Co., Ltd for sequencing.
1.4 Phylogenetic analysis
The 16S rDNA gene sequence of strain SY was compared and analyzed with the known sequences published in GenBank using the BLAST search tool of the National Centre for Biotechnology Information database. Phylogenetic trees of the isolates and some known iron and sulfur-oxidizing acidophilic microorganisms obtained from the GenBank database were constructed by using the MEGA software.
1.5 Physiological characteristics
The optimal growth temperature, pH value and inoculation amount were determined in flask batch tests. Strains were cultivated in 250 mL flasks containing 100 mL 9K basal salt medium with ferrous ions as the sole energy on a shaker at 200 r/min at 30 ℃. The inoculum size comprised 5% (v/v) of total culture and the initial pH was 2.0. When a parameter that influenced the growth of theA.ferrooxidanswas changed, the other parameters were kept at optimum. The content of Fe2+in the medium was determined as indicator for growth ofA.ferrooxidansstrain. The ferrous oxidation ratio (the content of Fe2+) was determined by complexometric titration using potassium dichromate.
1.6 Heavy metal tolerance
Tolerance to copper, cadmium, nickel, zinc ions was assayed by growth experiment in tubes containing 10 mL growth media. The initial pH was adjusted to 2.0. Different concentration of metal salts were added to the media.The various metal ions tested in this study were Cu2+, Cd2+, Ni2+ and Zn2+ , using CuSO4·5H2O, CdSO4·8/3H2O, NiSO4·6H2O and ZnSO4·7H2O respectively. All tubes were inoculated with 20% (v/v) and cultivated in a rotary shaker at 200 r/min at 30 ℃. The ferrous ion concentrations in the 9K medium were determined each day for a long period using the potassium dichromate titration method.
1.7 Bioleaching of sulfide ores
The pure isolate strain SY was used to leach mixed sulphide-oxide ore, which was also collected from the ancient mine area in Hubei province and the other two were from Yunnan and Anhui provinces respectively. Experiments were carried out in 100 mL culture in 250 mL shake flasks containing ores sample (5%,w/v) in iron-free media with a pH of 2.0. The flasks were autoclaved (30 min, 120 ℃). The experiments were conducted at 30 ℃ and under 200 r/min of shaking condition. After 20 days of bioleaching, 1 mL samples were removed and diluted for determining the soluble metal ion concentrations of copper by atomic absorption spectrophotometer (Hitachi Z-8000). The lost water in the medium was supplemented with sterilized deionized water.
2 Results
2.1 Isolation of strain
In the initial enrichment experiment, four samples turned the medium reddish-brown, indicating the production of jarosite. Based on these observations, it is clear that these samples contain iron-oxidizing bacteria. From the enrchment cultures with ferrous iron medium, eight strains of iron-oxidizing bacteria were isolated. To obtain theA.ferrooxidansstrain active in sulfide ores bioleaching, ferrous iron oxidation activity was measured as the second screening for the four strains which turn the medium reddish-brown more quickly among the eight isolates. Strain SY was the most active on ferrous iron oxidation among theA.ferrooxidansstrains tested, it completely oxidized ferrous irons at the 60 h while strain BY at about 40% (Fig.1). Hence, strain SY was selected for further study.

Fig.1 The oxidation activity of four bacteria
2.2 Identification of strain SY
16S rDNA fragments of strain SY was amplified, cloned and sequenced. The results show that DNA sequences of strain SY is about 1 500 bp in size. The phylogenetic tree shown in Fig.2 summarized the phylogenetic relationship among the typical sulfur and iron oxidizing bacteria species. It revealed that the strain SY was closely related toA.ferrooxidans(DQ 062116.1) with 100.0% similarity. Therefore it is accepted that strain SY isA.ferrooxidans.
2.3 pH, temperature and inoculation amount Optima
All the isolates could grow in the media with an initial pH value varying from 2.0 to 5.0, but not at pH 1.0 (Fig. 3). The ferrous oxidation ratio of strain SY decreases slowly with increasing pH values from 2.0 to 5.0. Strain SY could easily grow from pH 2.0 to 5.0 but a significant inhibition of its growth was observed at pH 1.0. The optimum pH of culture medium in the range tested was 2. The growth status of the isolates on ferrous ions substrate culture media under condition of optimal growth pH values and different temperature were studied. The optimal growth temperature was about 30 ℃. The ferrous oxidation ratio increased with the increment from 1% to 15% of inoculation amount at the initial phrase (Fig. 4), but the time for complete oxidation of ferrous ions was approximative except for 1% inoculation. Thus 5% inoculation was suggested for further study.
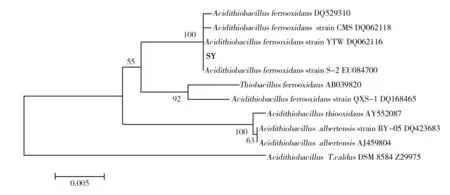
Fig.2 The phylogenetic analysis of strains SY

Fig.3 Effect of pH on ferrous ions oxidation by strain SY
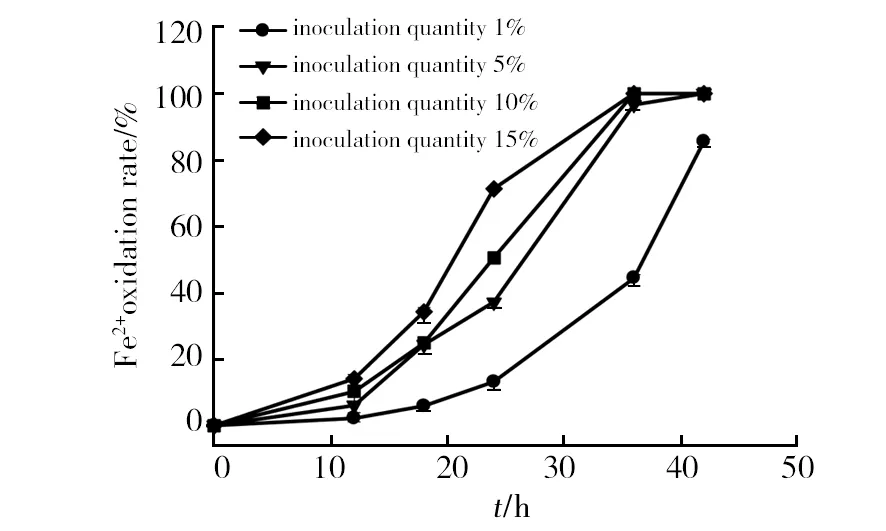
Fig.4 Effect of inoculation quantity on ferrous ions oxidation by strain SY
2.4 Tolerance of Cu, Cd, Ni and Zn
The tolerance ofA.ferrooxidansto metal ions is well documented in previous studies. Table 1 showed the highest levels at which the organism be able to oxidize iron in this work and selected reference data from leaching studies. For these data, it is possible to make a comparison between MTCs found in the literature and MTCs obtained in this work.
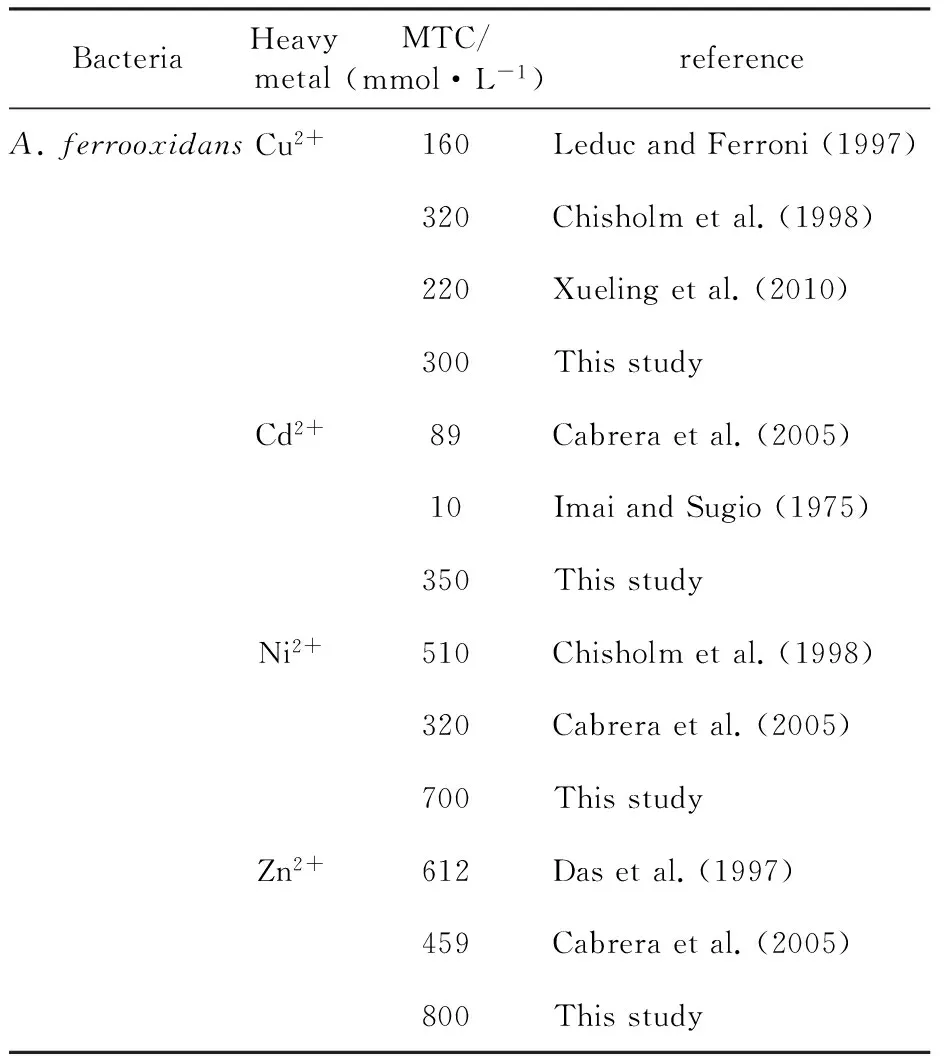
Table 1 MTC values to some heavy metals for pure cultures of A. ferrooxidans in literature and this study
Fig. 5 shows that the presence of copper decreased the oxidative capacity of strain SY and the cellular growth was inhibited. The effect of copper at 100 mmol/L on ferrous ions oxidation by strain SY was significant, the time necessary to oxidise of the initial ferrous content increased from 2 (control culture) to 5 days. Longer lag phases were observed for increasing copper concentrations (200-300) mmol/L, however, only a slight oxidation of ferrous ions was observed in the presence of copper at 400 mmol/L. strain SY was performed by successive subculturing, which resulted in a copper-adapted strain after consecutive transfers. The copper-adapted strain was able to completely oxidize ferrous ions at 400 mmol/L Cu2+with 20% (v/v) inoculation amount after two days of incubation.

Fig.5 The activity of bacteria in the presence of different concentrations of Cu2+
The tolerance study of Thiobacillus ferrooxidans with respect to cadmium was carried out in cultures supplemented with 200, 250, 300, 350, 400 mmol/L Cd2+(Fig.6). Growth was not observed at all at the higher concentration tested. The cadmium ion proved to be the most toxic of the metal ions in this study. The appearance of ferrous ions oxidation, which normally took place in two days in the absence of metals, while in the presence of 300 mmol/L Cd2+the lag period was extended to as much as 10 days. The maximum tolerated concentration was 350 mmol/L and there was about 50% oxidation of the initial ferrous content after 11 days cultivation.
The study in the presence of nickel was performed in cultures with concentrations in the range of 500-800 mmol/L. There was a high resistance of strain SY to Ni2+. strain SY showed growth up to 700 mmol/L Ni2+and for these cultures the time to complete oxidation of the initial ferrous increased from 2 days to 11 days (Fig. 7). The maximum tolerated concentrations (MTC) is higher than those found in literature for other strains of strain SY[20-21].

Fig.6 The activity of bacteria in the presence of different concentrations of Cd2+

Fig.7 The activity of bacteria in the presence of different concentrations of Ni2+
Concerning zinc tolerance, the results obtained in the present work suggest that the ion gives rise to the lowest toxit effect to strain SY.A.ferrooxidanstolerated 800 mmol/L Zn2+and the time required for substrate oxidation was only 6 days (Fig. 8). At 600 mmol/L, only a slight effect of Zn ions on ferrous oxidation by strain SY was observed as compared to the control.
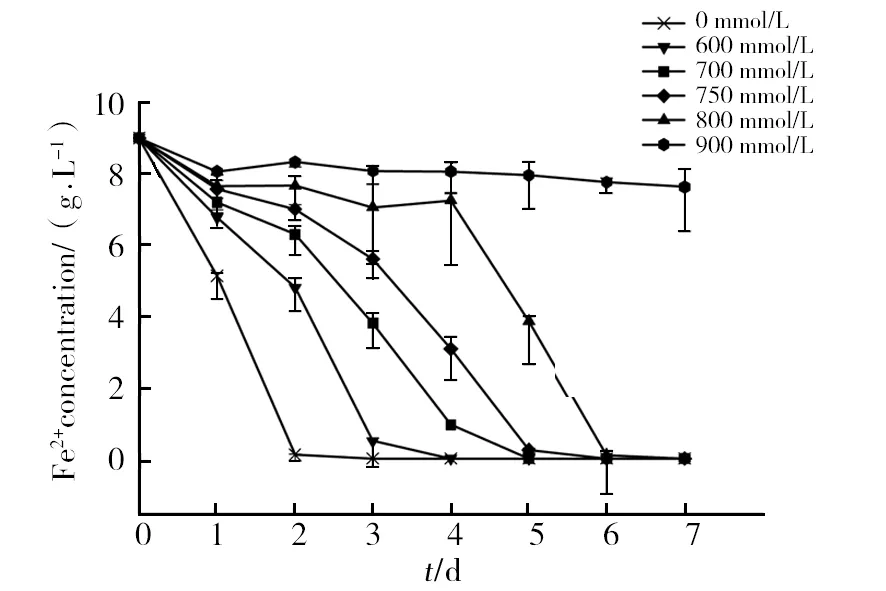
Fig.8 The activity of bacteria in the presence of different concentrations of Zn2+
2.5 Bioleaching of three minerals by strain SY
The bioleaching rates of metals copper after 20 d incubation of strain SY are listed in Table 2. The results indicated that the extraction rates were very low with the ores sample from Yunnan(about 33%) while there were higher extraction rates in the other two minerals, especially for the ores sample from Daye. The native microbes appear to be more efficient in the bioleaching of native minerals with a leaching rate up to 80%. The high copper dissolution in biotic and abiotic flasks was probably related to the solubilization of oxide ore in the mineral sample.
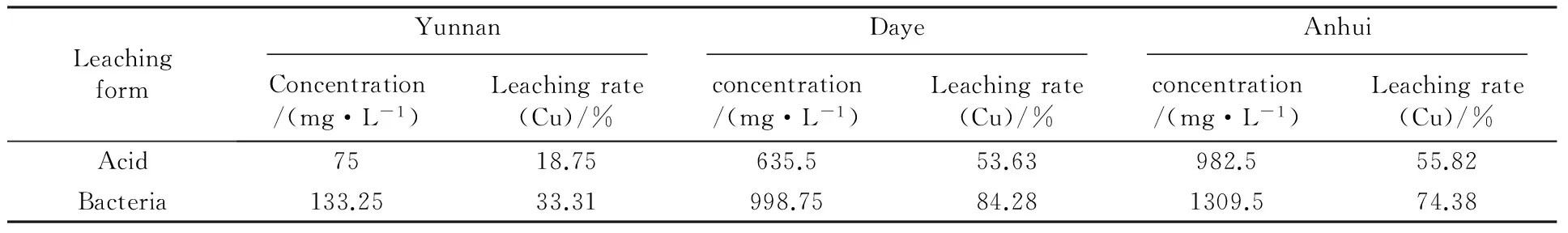
Table 2 Leaching solution concentration of measured copper and the leaching rates of several minerals after 20 days of bacterial leaching
3 Discussion
In the present study, eight strains were isolated from the ancient mine area of Daye in Hubei province. In order to obtain the strains with high activity, ferrous iron oxidation activity was compared. Strain SY was the most active on ferrous iron oxidation among theA.ferrooxidansstrains tested. The strain was rod-shaped, Gram-negative, motive, acidophlic, the optimal growth pH and growth temperature were respectively about 2.0 and 30 ℃. Some studies suggested that the physiological characteristics differences ofA.ferrooxidansstrains isolated from different environments[22-23]. Characteristic of some strains isolated from different sites include strain SY has summarized in Table 1. The data reveal a high degree of variability in the resistance to heavy metals for different strains of the same bacteria. This agree with the previous reports that strains isolated from various ecological niches have differences in the optimal pH and temperature for growth, resistance to heavy metal ions and toxic elements. This may be due to the adaptability of the strains to their isolated environment. That is why we isolated iron-oxidizing bacteria from the ancient mine area. The use of native microbes found in the locality of mineral deposits has resulted in an increased metal dissolution during bioleaching[18,24]and have proved to be more efficient than many effective consortia constructed in the laboratries. Strain SY isolated from the ancient mine area with high Fe2+oxidation activity and resistance to heavy metal ions,can extract metal from ancient mine with a high extraction rate by unadapted. There is clear economic advantages to acquire excellent bioleaching bacteria via screening from the locality of mineral deposits.
Strain SY tolerates high levels of the metals tested: 300 mmol/L Cu2+, 350 mmol/L Cd2+, 700 mmol/L Ni2+and 800 mmol/L Zn2+. These results and the data presented in the literature for iron oxidizers (Table 1) show that strain SY are very tolerant to the four heavy metal ions. Strain SY with multi-metal ion tolerance may be the best suited for bioleaching of multi-metal concentrates involving the four metals, namely, Cu2+, Cd2+, Ni2+, Zn2+. The bacterial tolerance to metal ions is caused by some metal resistance gene that are probably present on the chromosomes of most isolates of a bacterial species and mobile genes acquired by specific isolates of a species. The metal resistance mechanisms of several acidophilic micro-organisms to As2+, Cu2+, Zn2+, Cd2+and Ni2+have recently been reviewed, suggesting that the five metal resistance mechanisms identified in neutrophiles are also present in acidophiles, in some cases utilizing homologous proteins, but in many cases the degree of resistance is greater in acidophiles[25]. Increased resistance to metal ions could arise from two main sources: the occurrence of mutations in genes that are already present in the cell or the acquisition of new genes from other metal-resistant organisms, via the socalled horizontal gene pool. Bacterial evolution is often promoted by horizontal gene flow among different species and genera. Mobile genes are present on plasmid, transposons or viruses that may move from one microorganism to another and develop the tolerance level of the latter[26-30]. For example, continuous selection for arsenic resistance over many years had made the bacterium resistant to high concentrations of arsenic. Sequence analysis indicated that a transposon carrying a set of arsenic-resistance genes was arised. When the plasmid contains TnAtcArs was conjugated into a recipient strain. TnAtcArs conferred resistance to arsenite and arsenate uponE.colicells[28]. Micro-organisms inhabiting AMD environments contain high level metal ions in the ancient mine area in Daye encounter considerable selective pressure to develop resistance mechanisms to metal ions, providing them with a competitive selective advantage. Metal resistance gene should transfer among different bacteria in the ecological niches when the bacteria need. In the ancient mine area, there are high multi-metal concentrations that are toxic to most life. The transfer of metal resistance gene operons will create multi-metal resistant strains. In addition, the openness and non-sterile environment which permitting new organisms to enter, allows for the possibility of new genes to improve cell resistance to be selected from the horizontal gene pool. The metal resistance genes on the chromosomes also confer the tolerance to metals[31-33]. Resistance genes for a given metal ion might always present on the chromosomes and lack of use has resulted in the accumulation of mutations that have inactivated or reduced the activity of the resistance genes. But when exposure to a metal ion, the bacteria will increase resistance as a result of internal changes to a cell[34-37]. The gene expression profile of the ars operon under induction at different concentrations of arsenite was obtained via real-time PCR (TaqMan), by correlating the threshold cycle (C-t) values of induced and uninduced (control) samples. Through linear regression analysis, the gene expression profile of the ars operon showed clearly that the 0.125 mmol/L concentration of As3+was sufficient to provoke a 4-fold increase in the resistance system, and a further increase in concentration resulted in an increase of up to 53-fold in transcription rates[36]. In the ancient mine area, the bacteria always exposure to high concentrations muti-metal ions and reactivate on the resistance to many metals. Therefore, to get strains with high activity and resistance via screening from ancient mine is possible.
The bioleaching experiment of sulfide ores show that Strain SY was more efficient in the bioleaching native minerals than the others. Strain SY was isolated from the ancient mine area and adapted to the mineralogical and chemical composition of the ores to be treated. This particular advantage could lead to an increase in the metal extraction values during bioleaching process of native minerals. It is not difficult to understand there is a high extraction rate in the bioleaching of the Daye ore sample. In contrast, the extraction rates were very low with the ores sample from Yunnan (about 33%). In China, the majorities of the low-grade sulfuric ores contain varieties of metal ions and some of them are highly germicidal. A possible reason is that there is some special high concentration heavy metal ions or organic matter released from ores which does not exist in the Daye ore sample inhibit the activity of strain SY in the Yunnan samples. Therefore, the process of bioleaching must be taken into consideration of the interaction between environmental factors in the area with microbiological activities.
4 Conclusions
In this work, a high activity and multi-metal resistance native iron-oxidizing bacteria was isolated from the ancient mine area in Daye. According to the physiological features, 16S rDNA gene sequence analysis and the ability of bioleaching, the new isolate falls into the speciesA.ferrooxidans. The heavy metal resistance experiment reveals that strain SY have high tolerance to many metals which always exist in the process of bioleaching. Fundamental leaching experiment indicated that the native microbes are more efficient in the bioleaching of native minerals. There is a high extraction rates in the Daye native mine than the Yunnan minerals which may be induced by the adaptability of the isolates to the minerals. Strain SY is suitable to be used in the bioleaching of ores near to the ancient mine area. In our study, we highlight the importance about the adaptability of the strains to the environment. A cooperative bioleaching and a large-scale industry practice deserve further study.
Acknowledgments The authors thank tonglv mountain mine for supplying acid mine drainage to isolate iron-oxidizing bacteria from the ancient mine area in Daye, Hubei province, China, and for fruitful discussion.
Reference
[1] Rawlings D E. Characteristics and adaptability of iron-and sulfur-oxidizing microorganisms used for the recovery of metals from minerals and their concentrates[J]. Microbial Cell Factories, 2005, 4:13. DOI: 10.1186/1475-2859-4-13.
[2] Olson G J, Brierley J A, Brierley C L. Bioleaching review part B: Progress in bioleaching: applications of microbial processes by the minerals industries[J]. Applied Microbiology and Biotechnology, 2003, 63:249-257.
[3] Rawlings D E, Dew D, du Plessis C. Biomineralization of metal-containing ores and concentrates[J]. Trends in Biotechnology, 2003, 21:38-44.
[4] Mishra D, Rhee Y H. Microbial leaching of metals from solid industrial wastes[J]. Journal of Microbiology, 2014, 52:1-7.
[5] LI Hong-xu, WANG Dian-zuo, QIU Guan zhou, et al. Growth kinetics ofThiobacillusferrooxidansin bioelectrochemical cell [J]. Journal of Central South University of Technology, 2004,11(1):36-40.
[6] Rawlings D E. Heavy metal mining using microbes[J]. Annual Review of Microbiology, 2002, 56:65-91.
[7] Silverman M P. Mechanism of bacterial pyrite oxidation[J]. Journal of Bacteriology, 1967, 94:1046-1051.
[8] Ko M S, Park H S, Kim K W, et al. The role ofAcidithiobacillusferrooxidansandAcidithiobacillusthiooxidansin arsenic bioleaching from soil[J]. Environmental Geochemistry and Health, 2013,35:727-733.
[9] Sand W, Gehrke T, Jozsa P G, et al. (Bio) chemistry of bacterial leaching-direct vs. indirect bioleaching[J]. Hydrometallurgy, 2001, 59:159-175.
[10]Tributsch H. Direct versus indirect bioleaching[J]. Hydrometallurgy, 2001, 59:177-185.
[11]Das A, Modak J M, Natarajan K A. Technical note studies on multi-metal ion toleerance ofThiobacillusferrooxidans[J]. Minerals Engineering, 1997, 10:743-749.
[12]Watling H R. The bioleaching of nickel-copper sulfides[J]. Hydrometallurgy, 2008, 91:70-88.
[13]Kamalov M R, Krenes R Z, Ilialetbinov A N. Bacterial leaching of copper from the ores ofKounraddeposits[J]. Microbiology, 1969, 38:505-510.
[14]Lavalle L, Chiacchiarini P, Pogliani C, et al. Isolation and characterization of acidophilic bacteria from Patagonia, Argentina[J]. Process Biochemistry, 2005, 40:1095-1099.
[15]Sugio T, Wakabayashi M, Kanao T, et al. Isolation and characterization ofAcidithiobacillusferrooxidansstrain D3-2 active in copper bioleaching from a copper mine in Chile[J]. Bioscience Biotechnology and Biochemistry, 2008, 72:998-1004.
[16]Keeling S E, Davies K L, Palmer M L, et al. Utilisation of native microbes from a spent chalcocite test heap[J]. Hydrometallurgy, 2006, 83:124-131.
[17]Mousavi S M, Yaghmaei S, Vossoughi M, et al. Bacterial leaching of low-grade ZnS concentrate using indigenous mesophilic and thennophilic strains[J]. Hydrometallurgy, 2007, 85:59-65.
[18]Lavalle L, Giaveno A, Pogliani C, et al. Bioleaching of a polymetallic sulphide mineral by native strains ofLeptospirillumferrooxidansfrom Patagonia Argentina[J]. Process Biochemistry, 2008, 43:445-450.
[19]Kai M, Yano T, Fukumori Y, et al. Cytochrome oxidase of an acidophilic iron-oxidizing bacterium,Thiobacillusferrooxidans, functions at pH 3.5[J]. Biochemical and Biophysical Research Communications, 1989, 160:839-843.
[20]Cabrera G, Gomez J M, Cantero D. Kinetic study of ferrous sulphate oxidation ofAcidithiobacillusferrooxidansin the presence of heavy metal ions[J]. Enzyme and Microbial Technology, 2005, 36:301-306.
[21]Chisholm I A, Leduc L G, Ferroni G D. Metal resistance and plasmid DNA inThiobacillusferrooxidans[J]. Antonie van Leeuwenhoek, 1998, 73:245-254.
[22]Ageeva S N, Kondrat’eva T F, Karavaiko G E. Phenotypic characteristics ofThiobacillusferrooxidansstrains[J]. Microbiology, 2001, 70:226-234.
[23]CHEN Hong, YANG Bo, CHEN Xin-hua. Identification and characterization of four strains ofAcidithiobacillusferrooxidansisolated from different sites in China[J]. Microbiol Res, 2007, 164: 613-623.
[24] Barreira R P R, Villar L D, Garcia O. Tolerance to copper and zinc ofAcidithiobacillusthiooxidansisolated from sewage sludge [J]. World Journal of Microbiology & Biotechnology, 2005, 21:89-91.
[25]Dopson M, Baker-Austin C, Koppineedi P R, et al. Growth in sulfidic mineral environments: metal resistance mechanisms in acidophilic micro-organisms[J]. Microbiology-Sgm, 2003, 149:1959-1970.
[26]Ageeva S N, Kondrat’eva T F, Karavaiko G I. Plasmid profiles ofAcidithiobacillusferrooxidansstrains adapted to different oxidation substrates[J]. Microbiology, 2003, 72:579-584.
[27]Tuffin I M, Hector S B, Deane S M, et al. Resistance determinants of a highly arsenic-resistant strain ofLeptospirillumferriphilumisolated from a commercial biooxidation tank[J]. Applied and Environmental Microbiology, 2006, 72:2247-2253.
[28]Tuffin I M, de Groot P, Deane S M, et al. An unusual Tn21-like transposon containing an ars operon is present in highly arsenic-resistant strains of the biomining bacteriumAcidithiobacilluscaldus[J]. Microbiology-Sgm, 2005, 151:3027-3039.
[29]Orellana L H, Jerez C A. A genomic island provides Acidithiobacillus ferrooxidans ATCC 53993 additional copper resistance: a possible competitive advantage[J]. Applied Microbiology and Biotechnology, 2011, 92:761-767.
[30]Ghosh S, Mahapatra N R, Banerjee P C. Metal Resistance in Acidocella Strains and Plasmid-Mediated Transfer of This Characteristic toAcidiphiliummultivorumandEscherichiacoli[J]. Applied and Environmental Microbiology, 1997, 63:4523-4527.
[31] Lee Y A, Hendson M, Panopoulos N J, et al. Molecular cloning, chromosomal mapping, and sequence analysis of copper resistance genes fromXanthomonascampestrispv.juglandis: homology with small blue copper proteins and multicopper oxidase[J]. Journal of bacteriology,1994,176:173-188.
[32]Legatzki A, Grass G, Anton A, et al. Interplay of the Czc system and two P-type ATPases in conferring metal resistance to Ralstonia metallidurans[J]. Journal of bacteriology, 2003, 185:4354-4361.
[33] Dopson M, Lindstrom E B, Hallberg K B. Chromosomally encoded arsenical resistance of the moderately thermophilic acidophileAcidithiobacilluscaldus[J]. Extremophiles, 2001, 5:247-255.
[34] Natarajan K A, Sudeesha K, Rao G R. Stability of copper tolerance inThiobacillusferrooxidans[J]. Antonie van Leeuwenhoek, 1994, 66:303-306.
[35]Sugio T, Fujii M, Takeuchi F, et al. Volatilization of mercury by an iron oxidation enzyme system in a highly mercury-resistantAcidithiobacillusferrooxidansstrain MON-1[J]. Bioscience Biotechnology and Biochemistry, 2003, 67:1537-1544.
[36]Azevedo J S N, Silva-Rocha R, Silva A, et al. Gene expression of the arsenic resistance operon inChromobacteriumviolaceumATCC 12472[J]. Canadian Journal of Microbiology, 2008, 54:137-142.
[37]ZHANG Yun-jing, WU Xue-ling, LIU Dai-gang, et al. Sequencing and bioinformatics analysis of the metal-related genes inAcidithiobacillusferrooxidansstrain DC[J]. Folia Microbiol (Praha), 2013, 58:551-560.
冯光志 男,硕士,实验师。研究方向为应用微生物。 Tel:027-81730684, E-mail: fenggz2004@126.com
古矿区高活性高抗性氧化亚铁硫杆菌的分离鉴定及其特性研究
冯光志, 廖李明, 刘子莹, 石 玉*
(武汉设计工程学院 食品与生物科技学院,湖北 武汉 430205)
从湖北省大冶市古铜矿区域分离得到1株具有较高Fe2+氧化活性的嗜酸氧化亚铁硫杆菌SY,16S rDNA 序列分析表明,该菌与Acidithiobacillusferrooxidans(DQ 062116.1)的序列相似性最高。生理生化性质测定表明,菌株SY化能自养型,最适pH值为2.0,最适生长温度为30 ℃。菌株SY对Cu2+、Cd2+、Ni2+、Zn2+等重金属离子抗性的最低抑菌浓度分别为300、350、700和800 mmol/L,表明其对多种重金属离子均有很高的抗性。SY浸矿数据表明该菌对湖北大冶本土矿石浸出率为84.28%,高于其他地区矿石浸出率,在本土矿石生物浸出中显示出很大的优势。
嗜酸氧化亚铁硫杆菌;生理特性;生物氧化;重金属抗性;生物浸出
Q935;TF18
A
1005-7021(2017)03-0077-10
* 通讯作者。女 博士,讲师。研究方向为环境微生物。 E-mail: shiy2005@126.com
10.3969/j.issn.1005-7021.2017.03.013
Author profile: FENG Guang-zhi male, master, experimentalist. Research direction: applied microbiology.
Tel: 027-81730684,E-mail:fenggz2004@126.com
*Corresponding author:female, PhD, Lecturer. Research direction: environmental microbiology. E-mail: shiy2005@126.com
Draft accepted date:2016-05-17;Revision returned date:2016-07-28
