不同碳材料增强有机酸对土壤重金属的去除及对土壤化学性质的影响
2017-09-03马小杰张世熔王怡君王贵胤徐小逊李婷
马小杰,张世熔*,王怡君,王贵胤,徐小逊,李婷
(1.四川农业大学环境学院,成都 611130;2.四川农业大学资源学院,成都 611130)
不同碳材料增强有机酸对土壤重金属的去除及对土壤化学性质的影响
马小杰1,张世熔1*,王怡君1,王贵胤1,徐小逊1,李婷2
(1.四川农业大学环境学院,成都 611130;2.四川农业大学资源学院,成都 611130)
为了解碳材料和有机酸去除土壤重金属的效果,通过振荡淋洗法分别研究4种碳材料与柠檬酸、乙酸以及它们的复合淋洗剂对土壤重金属的去除。结果表明,单一纳米碳黑、水稻秸秆生物炭、木屑生物炭和果壳生物炭对重金属的去除率均低于0.5%。总体上,碳材料对柠檬酸去除重金属效果的提升作用高于乙酸,其中纳米碳黑对有机酸去除重金属的提升率最高,可达10.1%~11.1%,水稻秸秆次之,果壳和木屑生物炭对两种酸的提升率均低于5.8%。碳材料的加入,主要使酸溶态、可还原态和部分可氧化态的重金属在复合淋洗后显著降低(P<0.05),相对于单一有机酸淋洗,复合淋洗更有利于减缓有机酸对土壤速效养分的损失。研究结果表明,纳米碳黑和水稻秸秆生物炭是能改进有机酸淋洗效果的两种材料。
土壤淋洗;纳米碳黑;生物炭;有机酸;复合淋洗;重金属
近年来,采矿、冶炼和化工等人类活动的增长导致土壤重金属污染成为全球面临的严重环境问题之一[1-2]。在污染土壤的重金属中,Cd和Pb因具有高毒性、非生物降解和潜在致癌性,对生态环境构成严重威胁[3];锌是生物必需营养元素,但土壤中的Zn含量过度时会抑制生物生长,并通过食物链危害人体健康[4]。因此,重金属污染土壤的修复具有重要的现实意义。
目前,在众多重金属修复技术中,土壤淋洗因其高效和快速的特点而受到研究者的关注[5-7]。该技术应用的关键是选择效率较高且环境友好的淋洗剂,常用的淋洗剂包括无机酸[8]、螯合剂[9]和表面活性剂[10]等。然而,无机酸易于破坏土壤的理化性质,人工螯合剂和表面活性剂的生物降解性差而且难以回收[11]。近年来,研究发现低分子有机酸是环境友好的土壤重金属淋洗剂,但其去除率有限[12]。因此,筛选某些材料或者试剂来增强有机酸对重金属污染土壤的修复能力具有重要的实用价值。
生物炭和纳米碳黑是具有较大比表面积、大量微孔结构和活跃有机含氧官能团的材料[13-14]。它们被广泛用于吸附废水中重金属[15-17]和钝化土壤重金属[18-19],并可改良土壤结构和提升土壤肥力[20]。但是,生物炭和纳米碳黑与低分子有机酸改性后修复土壤重金属的研究却鲜见报道。因此有必要探究这些碳材料与低分子有机酸组合改性后对土壤重金属Cd、Pb和Zn的去除效果。
本研究以纳米碳黑、水稻秸秆生物炭、木屑生物炭和果壳生物炭为碳材料,选用柠檬酸和乙酸两种低分子有机酸,探究其组合对矿区污染土壤的修复效果,以期为重金属污染土壤的实际修复提供一定的参考依据。
1 材料与方法
1.1 供试土壤与淋洗材料
1.1.1 供试土壤
供试土样采自四川省汉源县唐家铅锌矿区表层土壤(0~20 cm)。样品经风干后,碾碎过2 mm尼龙筛,混匀后密封装袋保存,其理化性质见表1。
1.1.2 淋洗材料
柠檬酸(C6H8O7)和乙酸(C2H4O2),分析纯,均产自成都科龙化工试剂厂。纳米碳黑,粉状,粒径为7~9 nm,产自天津天一世纪化工产品科技发展有限公司;水稻秸秆生物炭、木屑生物炭、果壳生物炭,粉状,粒径均小于80 μm,均产自江苏溧阳市德胜活性炭厂。
1.2 试验方法
1.2.1 单一碳材料对土壤Cd、Pb和Zn的淋洗试验
分别称取2.00 g土样于50 mL离心管中,加入20 mL浓度为0.3、0.7、1.1、1.5 g·L-1的纳米碳黑、水稻秸秆生物炭、木屑生物炭和果壳生物炭,并用0.1 mol·L-1的HNO3和NaOH调节溶液pH至4.0。以水溶液作空白对照。随后将离心管放入振荡器中,在200 r·min-1条件下恒温(25℃)振荡1 h后,在3500 r· min-1下离心3 min。离心后的上清液经0.45 μm微孔滤膜过滤,再用火焰分光光度法(FAAS,M6型)测定溶液中Cd、Pb和Zn的含量。每个处理重复3次。
1.2.2 单一有机酸对土壤Cd、Pb和Zn的淋洗试验
分别称取2.00 g土样于50 mL离心管中,加入20 mL浓度为0.1、0.2、0.3 mol·L-1的柠檬酸或乙酸溶液,并用0.1 mol·L-1的HNO3和NaOH调节溶液pH 至4.0。以水溶液作空白对照。其余步骤同1.2.1。
1.2.3 土壤Cd、Pb和Zn的复合淋洗试验
分别称取60、140、220、300 mg纳米碳黑、水稻秸秆生物炭、木屑生物炭、果壳生物炭于50 mL离心管中,分别加入20 mL浓度为0.3 mol·L-1的柠檬酸、乙酸,在25℃、200 r·min-1条件下恒温振荡改性24 h,再分别称取2.00 g土样加入改性后的复合淋洗剂中,用0.1 mol·L-1的HNO3和NaOH调节pH至4.0。其余步骤同1.2.1。
1.3 土壤理化性质及重金属形态的测定
土壤pH值测定采用酸度pH计法;土壤阳离子交换量测定采用乙酸铵交换法[21];土壤颗粒组成测定用比重计法。土壤营养元素按照《土壤农化分析》[22]进行测定,其中土壤有机碳测定采用重铬酸钾外加热法,全氮测定采用半微量开氏法,全磷测定采用氢氧化钠熔融-钼锑抗比色法,全钾测定采用氢氧化钠熔融-火焰光度法,速效磷测定采用碳酸氢钠浸提-钼锑抗比色法,速效钾测定采用醋酸铵提取-火焰光度法,无机氮测定采用氯化钾浸提-靛酚蓝比色法(NH+4-N)和紫外分光光度法(NO-3-N)。

表1 供试土壤的基本理化性质Table 1 Physical-chemical characteristics of the contaminated soil
重金属元素全量分析采用HNO3/HCIO4/HF(体积比为1∶2∶2)三酸硝化法[2]。土壤中重金属形态测定采用改进BCR连续提取法[23-24]。
1.4 数据处理
数据采用SPSS 19.0进行统计分析,采用Origin 9.0绘图。数据平均值与标准差采用单因素方差分析,差异显著性分析采用S-N-K法(P<0.05)。
2 结果与讨论
2.1 单一碳材料对土壤Cd、Pb和Zn的去除率
4 种碳材料对土壤中Cd、Pb和Zn的去除率无明显变化趋势,且均小于0.5%(表1)。因此,单一碳材料难以直接用于土壤中Cd、Pb和Zn的去除。
2.2 单一有机酸对土壤Cd、Pb和Zn的去除率
3 种土壤重金属Cd、Pb和Zn的去除率均随有机酸浓度的增加而升高(图1)。在同一浓度下,柠檬酸对土壤中Cd、Pb和Zn的去除率显著高于乙酸(P<0.05),在浓度为0.3 mol·L-1时,柠檬酸对土壤Cd、Pb和Zn的去除率分别比乙酸高15.6%、35.2%和25.7%。这是因为柠檬酸是三元酸,乙酸是一元酸,前者比后者能提供更多的阴离子,其与重金属阳离子发生螯合反应,所以柠檬酸去除率高于乙酸[25]。
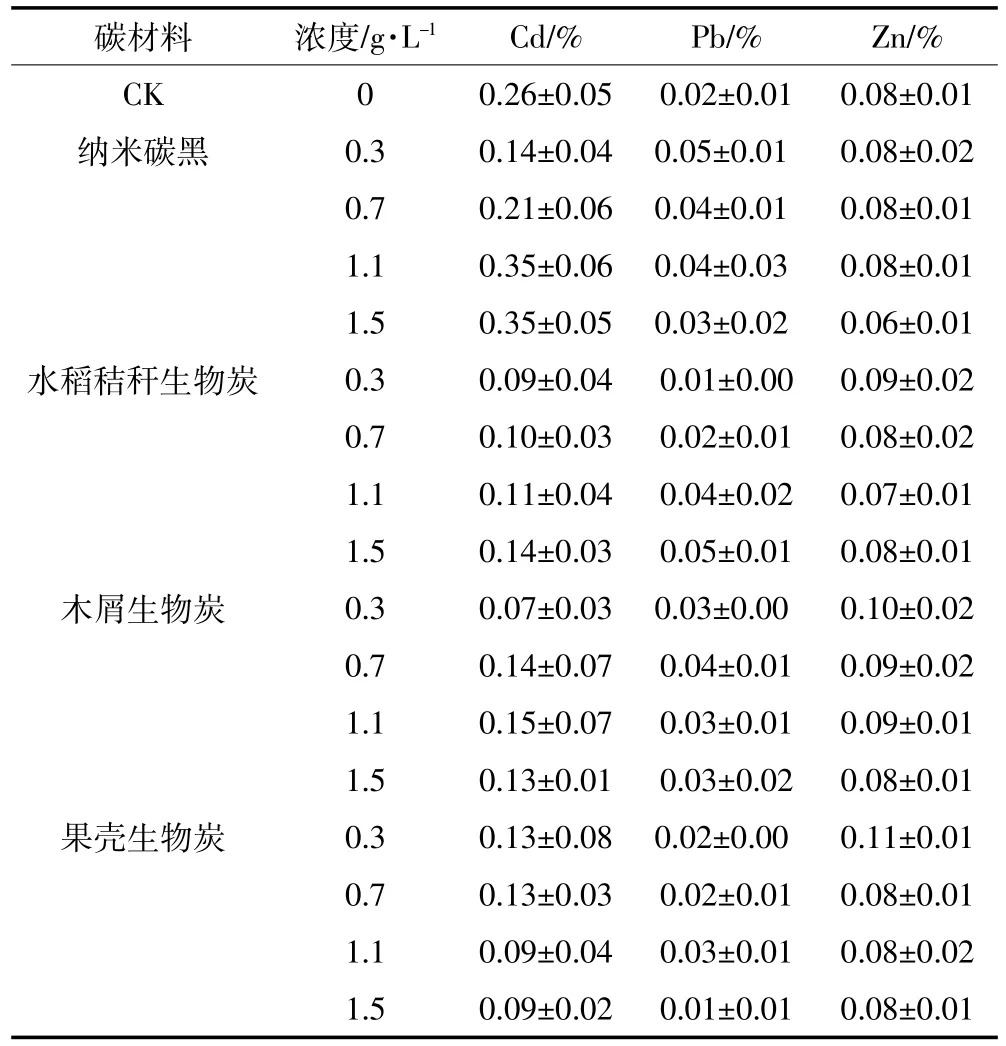
表2 单一碳材料对土壤Cd、Pb和Zn的去除率Table 2 Effects of carbon materials alone on the removal of soil Cd,Pb and Zn

图1 单一柠檬酸(CA)和乙酸(HAc)对土壤Cd、Pb和Zn的去除率Figure 1 Effects of single citric acid(CA)and acetic acid(HAc)on the removal of soil Cd,Pb and Zn
2.3 复合淋洗剂对土壤Cd、Pb和Zn的去除率
与碳材料或有机酸单独使用相比,两种有机酸分别与4种碳材料构成的复合淋洗剂对3种土壤重金属的去除率随碳材料浓度的增加呈现不同的变化趋势(图2)。
柠檬酸除与水稻秸秆生物炭改性后对重金属的去除率随着生物炭浓度增加而缓慢增加外,与其余3种碳材料复合改性后随着碳材料浓度的增加均呈现先增加后下降的趋势,并在浓度为0.7 g·L-1时达到最大去除率。总体上,4种碳材料与柠檬酸构成的复合淋洗剂对重金属的去除率增加值以纳米碳黑为最高(10.1%~11.1%),水稻秸秆生物炭次之(7.8%~10.3%)。这些现象可能是由于碳材料表面大量的含氧官能团与金属离子反应生成盐或者复合物吸附于其上,并在酸性环境下解吸进入淋洗液中,从而提高了去除率[26]。某些研究者认为柠檬酸能与土壤胶体中的铁氧化合物反应,形成LaFeO3复合物,进而破坏土壤胶膜,释放其包裹的金属离子[27],碳材料巨大的比表面积可能会增加这一反应的接触面积[14,17],促进去除率提高。当碳材料浓度大于临界值时,淋洗率降低的原因可能是由于碳材料上的PO3-4和CO2-3与溶液中的重金属离子形成沉淀[28]。
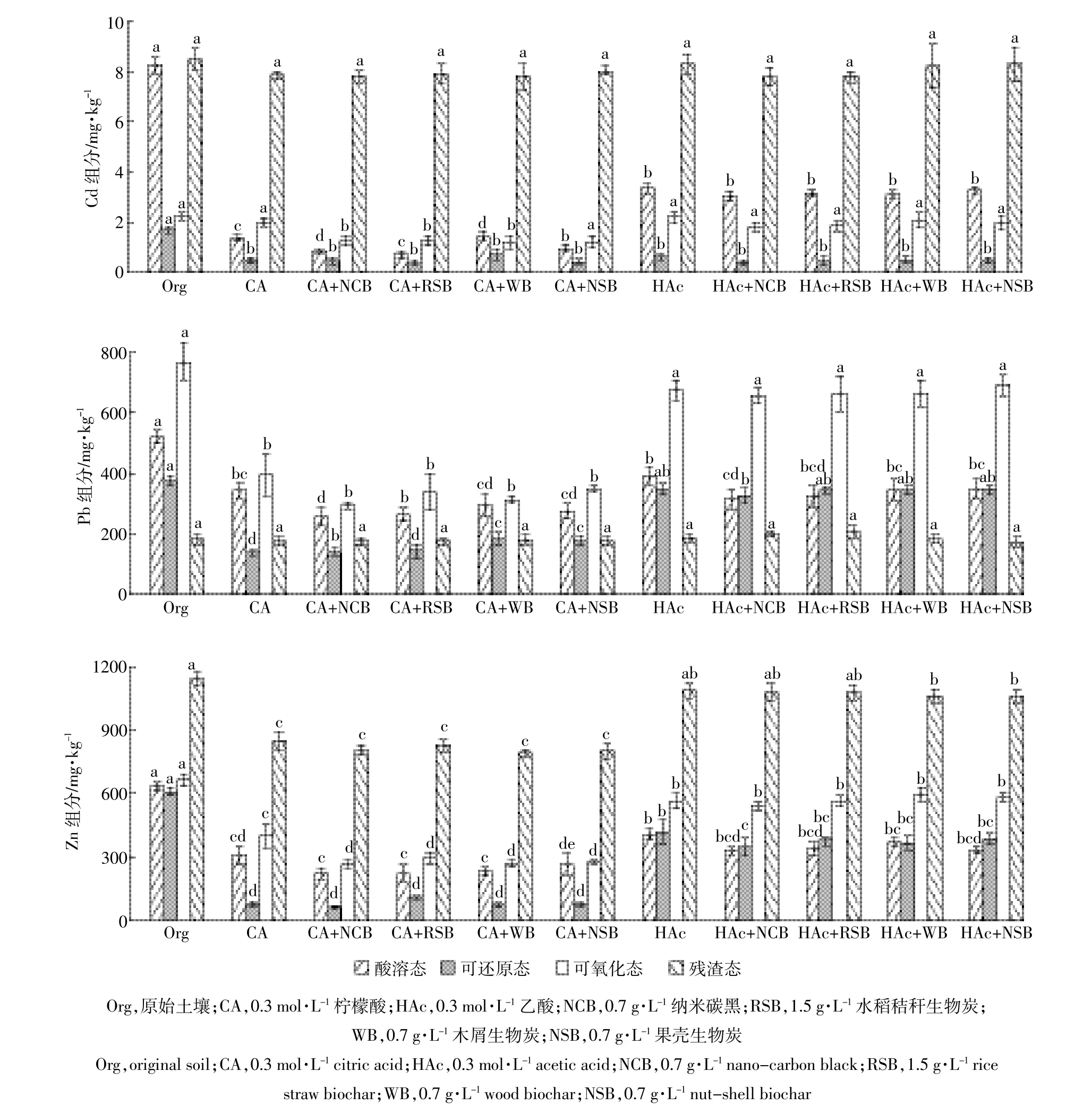
图3 不同淋洗处理后土壤的Cd、Pb和Zn的组分分布Figure 3 Distribution of fraction of Cd,Pb and Zn in soil before and after varieties washing treatments
乙酸与不同碳材料复合改性后对3种重金属的去除与柠檬酸相似,但其去除率和提升率均明显低于柠檬酸。总体上,4种碳材料与乙酸构成的复合淋洗剂对Cd、Pb和Zn的去除效果提升率下降至1.6%~6.6%。这可能是由于同一浓度下,柠檬酸比乙酸具有更多羧基官能团,能在改性后生成更多的配位体与重金属发生螯合作用[25]。
2.4 淋洗前后土壤中重金属组分分析
土壤中重金属的组分分布如图3所示。经有机酸和碳材料复合淋洗之后,土壤中各形态的Cd、Pb和Zn均有不同程度的变化。
淋洗前土壤中Cd以残渣态和酸溶态为主,含量分别为8.5、8.2 mg·kg-1,其次是可氧化态和可还原态,含量分别为2.2、1.7 mg·kg-1。柠檬酸和乙酸与4种碳材料复合淋洗之后,酸溶态含量分别下降80%~90%和50%~65%,表明在淋洗初始阶段,大量的酸溶态金属被迅速酸解进入淋洗液中,从而被去除[2]。值得关注的是,有机酸与碳材料复合淋洗后可还原态的Cd显著降低。此外,除含乙酸的复合淋洗外,柠檬酸与4种碳材料复合淋洗后的可氧化态也显著降低。这表明复合淋洗剂能将可还原态和部分可氧化态的Cd转化为酸溶态,通过淋洗过程去除[29-30]。
土壤中Pb以可氧化态和酸溶态为主,含量分别为769.4、520.9 mg·kg-1,其次是可还原态,含量为375.7 mg·kg-1。与Cd组分变化相似,Pb的酸溶态、可还原态和可氧化态均显著降低(P<0.05),差异在于,柠檬酸与碳材料复合淋洗后,可还原态和可氧化态降低幅度均明显高于乙酸。
与Cd和Pb不同,Zn的残渣态含量最多(1 143.2 mg·kg-1),其余3种形态所占比例接近,含量均在600~660 mg·kg-1之间,其组分变化也与Cd相似,但有明显差异的是,残渣态的Zn有显著降低(P<0.05),下降幅度在25%~30%之间。这表明柠檬酸与碳材料复合淋洗对残渣态的Zn有较好的去除效果。
综上所述,4种碳材料对柠檬酸去除重金属效果的提升作用可能是基于它们促进了重金属的可还原态和部分可氧化态向酸溶态转化。
2.5 淋洗前后土壤的化学性质分析
土壤淋洗可能通过溶解土壤中原有的氧化物、碳酸盐和有机质造成土壤化学性质的改变[31-32]。从表3可知,柠檬酸及其与碳材料淋洗后土壤中的有机碳含量显著升高(P<0.05),乙酸及其与碳材料淋洗后的土壤有机碳含量也增加了8.7%~12.4%。这是由于残留在土壤中的有机酸和碳材料会一定程度地造成有机碳的增加[33]。土壤中氮、磷、钾是植物生长所必需的营养元素,经淋洗后,全氮和全磷的含量均无显著变化(表3),全钾含量降低7%~15%。这表明淋洗剂在淋洗过程中会带走部分营养元素[10]。除乙酸及其与碳材料复合淋洗后的速效磷含量有所增加外,其余淋洗处理后的土壤速效养分均显著下降(P<0.05)。乙酸组处理后土壤速效磷增加的原因,可能是有机酸活化了土壤中原有的磷[34];柠檬酸组处理后造成速效磷含量显著下降的原因,可能是在淋洗过程中大量的有机阴离子从土壤胶体吸附点位上取代磷进入淋洗液中,从而使土壤速效磷下降[35]。与单一柠檬酸相比,碳材料的加入使土壤中无机氮和速效钾的含量分别提升3.1%~5.3%和2.7%~10.4%;而与单一乙酸相比,碳材料的加入使土壤无机氮和速效钾含量也均提升1.3%~6.3%。
3 结论
(1)4种碳材料对有机酸去除重金属的效果均有不同程度的提升作用。总体上,柠檬酸对重金属去除率的提升值高于乙酸;对柠檬酸去除率的提升值以纳米碳黑为最高,水稻秸秆生物炭次之,木屑和果壳生物炭最低。

表3 不同处理前后土壤的化学性质Table 3 Chemical characteristics of soil samples before and after varieties leaching treatments
(2)经有机酸和碳材料复合淋洗后,土壤中酸溶态、可还原态和部分可氧化态的重金属能被有效去除,这表明复合淋洗剂中的碳材料能促进重金属的各形态向酸溶态转化。
(3)土壤经淋洗后,有机碳含量升高8.7%~26.5%,全氮、全磷含量无显著变化,全钾含量显著下降(P<0.05)。与单一有机酸相比,碳材料的加入可以减缓淋洗过程对速效养分的损失。
[1]Mukwaturi M,Lin C.Mobilization of heavy metals from urban contaminated soils under water inundation conditions[J].Journal of Hazardous Materials,2015,285:445-452.
[2]Wang G Y,Zhang S R,Xu X X,et al.Heavy metal removal by GLDA washing:Optimization,redistribution,recycling,and changes in soil fertility[J].Science of the Total Environment,2016,569/570:557-568.
[3]Bolan N,Kunhikrishnan A,Thangarajan R,et al.Remediation of heavy metal(loid)s contaminated soils:To mobilize or to immobilize[J].Journal of Hazardous Materials,2014,266:141-166.
[4]Chasapis C T,Loutsidou A C,Spiliopoulou C A,et al.Zinc and human health:An update[J].Archives of Toxicology,2012,86(4):521-534.
[5]Kulikowska D,Gusiatin Z M,Bułkowska K,et al.Humic substances from sewage sludge compost as washing agent effectively remove Cu and Cd from soil[J].Chemosphere,2015,136:42-49.
[6]Mukhopadhyay S,Mukherjee S,Adnan N F,et al.Ammonium-based deep eutectic solvents as novel soil washing agent for lead removal[J]. Chemical EngineeringJournal,2016,294:316-322.
[7]Boente C,Sierra C,Rodríguez-Valdés E,et al.Soil washing optimization by means of attributive analysis:Case study for the removal of potentially toxic elements from soil contaminated with pyrite ash[J].Journal of Cleaner Production,2017,142:2693-2699.
[8]Alghanmi S I,Sulami A F A,El-Zayat T A,et al.Acid leaching of heavy metals from contaminated soil collected from Jeddah,Saudi Arabia:Kinetic and thermodynamics studies[J].International Soil and Water Conservation Research,2015,3(3):196-208.
[9]Zhang T,Wei H,Yang X H,et al.Influence of the selective EDTA derivative phenyldiaminetetraacetic acid on the speciation and extraction of heavy metals from a contaminated soil[J].Chemosphere,2014, 109:1-6.
[10]Chen Y,Zhang S R,Xu X X,et al.Effects of surfactants on lowmolecular-weight organic acids to wash soil zinc[J].Environmental Science and Pollution Research,2016,23(5):4629-4638.
[11]Yao Z T,Li J H,Xie H H,et al.Review on remediation technologies of soil contaminated by heavy metals[J].ProcediaEnvironmental Sciences, 2012,16:722-729.
[12]Wang G Y,Zhang S R,Xu X X,et al.Efficiency of nanoscale zero-valent iron on the enhanced low molecular weight organic acid removal Pb from contaminated soil[J].Chemosphere,2014,117:617-624.
[13]Figarol A,Pourchez J,Boudard D,et al.In vitro toxicity of carbon nanotubes,nano-graphite and carbon black,similar impacts of acid functionalization[J].Toxicology in Vitro,2015,30(1):476-485.
[14]Prete M C,Oliveira F M D,Tarley C R T.Assessment on the performance of nano-carbon black as an alternative material for extraction of carbendazim,tebuthiuron,hexazinone,diuron and ametryn[J].Journal of Environmental Chemical Engineering,2017,5(1):93-102.
[15]Tounsadi H,Khalidi A,Farnane M,et al.Experimental design for the optimization of preparation conditions of highly efficient activated carbon from Glebionis coronaria L.and heavy metals removal ability[J]. Process Safety and Environmental Protection,2016,102:710-723.
[16]Ruparelia J P,Duttagupta S P,Chatterjee A K,et al.Potential of carbon nanomaterials for removal of heavy metals from water[J].Desalination, 2008,232(1/2/3):145-156.
[17]Kołodyńska D,Krukowska J,Thomas P.Comparison of sorption and desorption studies of heavy metal ions from biochar and commercial active carbon[J].Chemical EngineeringJournal,2017,307:353-363.
[18]Igalavithana A D,Park J,Ryu C,et al.Slow pyrolyzed biochars from crop residues for soil metal(loid)immobilization and microbial community abundance in contaminated agricultural soils[J].Chemosphere, 2017,177:157-166.
[19]Lu K P,Yang X,Gielen G,et al.Effect of bamboo and rice straw biochars on the mobility and redistribution of heavy metals(Cd,Cu,Pb and Zn)in contaminated soil[J].Journal of Environmental Management,2017,186:285-292.
[20]Tang J C,Zhu W Y,Kookana R,et al.Characteristics of biochar and its application in remediation of contaminated soil[J].Journal of Bioscience and Bioengineering,2013,116(6):653-659.
[21]Gogo S,Shreeve T G,Pearce D M E.Geochemistry of three contrasting British peatlands:Complex patterns of cation availability and implications for microbial metabolism[J].Geoderma,2010,158(3/4):207-215.
[22]鲍士旦,江荣风,杨超光,等.土壤农化分析[M].三版.北京:中国农业出版社,2008:30-158.
BAO Shi-dan,JIANG Rong-feng,YANG Chao-guang,et al.Soil agricultural and chemical analysis method[M].3th Edition.Beijing:China Agricultural Press,2008:30-158.
[23]Pueyo M,Mateu J,Rigol A,et al.Use of the modified BCR three-step sequential extraction procedure for the study of trace element dynamics in contaminated soils[J].Environmental Pollution,2008,152(2):330-341.
[24]Nemati K,Bakar N K A,Abas M R,et al.Speciation of heavy metals by modified BCR sequential extraction procedure in different depths of sediments from Sungai Buloh,Selangor,Malaysia[J].Journal Hazardous Materials,2011,192(1):402-410.
[25]An C J,Huang G H,Wei J,et al.Effect of short-chain organic acids on the enhanced desorption of phenanthrene by rhamnolipid biosurfactant in soil-water environment[J].Water Research,2011,45(17):5501-5510.
[26]Wang H J,Zhou A L,Peng F,et al.Adsorption characteristic of acidified carbon nanotubes for heavy metal Pb(Ⅱ)in aqueous solution[J]. Materials Science and Engineering:A,2007,466(1/2):201-206.
[27]Khalil K M S,Elhamdy W A,Said A E A,et al.Porous LaFeO3/Silica nanocomposites via Sol-Gel mixing involving citric acid[J].Colloids and Surfaces A:Physicochemical and EngineeringAspects,2016,506:840-848.
[28]李瑞月,陈德,李恋卿,等.不同作物秸秆生物炭对溶液中Pb2+、Cd2+的吸附[J].农业环境科学学报,2015,34(5):1001-1008.
LI Rui-yue,CHEN De,LI Lian-qing,et al.Adsorption of Pb2+and Cd2+in aqueous solution by biochars derived from different crop residues[J]. Journal of Agro-Environment Science,2015,34(5):1001-1008.
[29]Begum Z A,Rahman I M M,Tate Y,et al.Remediation of toxic metal contaminated soil by washing with biodegradable aminopolycarboxylate chelants[J].Chemosphere,2012,87(10):1161-1170.
[30]Li Y J,Hu P J,Zhao J,et al.Remediation of cadmium-and lead-contaminated agricultural soil by composite washing with chlorides and citric acid[J].Environmental Science Pollution Research,2015,22(7):5563-5571.
[31]Tsang D C W,Zhang W H,Lo I M C.Copper extraction effectiveness and soil dissolution issues of EDTA-flushing of artificially contaminated soils[J].Chemosphere,2007,68(2):234-243.
[32]Jelusic M,Grcman H,Vodnik D,et al.Functioning of metal contaminated garden soil after remediation[J].Environmental Pollution,2013, 174:63-70.
[33]Liu C C,Lin Y C.Reclamation of copper-contaminated soil using EDTA or citric acid coupled with dissolved organic matter solution extracted from distillery sludge[J].Environmental Pollution,2013,178:97-101.
[34]章爱群,贺立源,赵会娥,等.有机酸对土壤无机态磷转化和速效磷的影响[J].生态学报,2009,29(8):4061-4069.
ZHANG Ai-qun,HE Li-yuan,ZHAO Hui-e,et al.Effect of organic acids on inorganic phosphorus transformation in soils and its readily available phosphate[J].Acta Ecologica Sinica,2009,29(8):4061-4069.
[35]胡红青,贺纪正,李学垣,等.有机酸对酸性土壤吸附磷的影响[J].华中农业大学学报,1997,16(1):37-42.
HU Hong-qing,HE Ji-zheng,LI Xue-yuan,et al.Effect of organic acids on phosphate adsorption by acid soil[J].Journal of Huazhong A-gricultural University,1997,16(1):37-42.
Effects of different carbon materials for enhancement of organic acid-mediated removal of heavy metals and nutrient retention in soil
MA Xiao-jie1,ZHANG Shi-rong1*,WANG Yi-jun1,WANG Gui-yin1,XU Xiao-xun1,LI Ting2
(1.College of Environmental Sciences,Sichuan Agricultural University,Chengdu 611130,China;2.College of Resources,Sichuan Agricultural University,Chengdu 611130,China)
Four carbon materials,including single nano-carbon black(NCB),rice straw biochar(RSB),wood biochar(WB),and nut-shell biochar(NSB),and two low molecular weight organic acids(citric acid and acetic acid)were tested alone and in combination to determine their efficacy for removal of soil Cd,Pb,and Zn.Single carbon materials showed removal efficiency of less than 0.5%.Generally,citric acid enhanced the removal of heavy metals by carbon materials more than acetic acid.Compared with citric acid alone,NCB significantly improved the efficiency of Cd,Pb,and Zn removal(10.1%~11.1%),whereas RSB enhanced removal efficiency less than NCB(7.8%~10.3%),and WB and NSB weakly increased the efficiency(1.8%~5.8%).The exchangeable,reducible,and partly oxidizable fractions of Cd,Pb,and Zn decreased markedly after washing with the mixed solutions(P<0.05).Soil organic carbon content increased by 8.7%~26.5%for all treatments,whereas total potassium decreased significantly relative to unwashed soils(P<0.05).The total nitrogen and phosphorus contents in different washed soils did not change significantly in comparison with original soils.Compared with single organic acids,the loss rates of available nitrogen,phosphorus,and potassium were reduced by 1.4%~6.1%,4.4%~11.5%,and 1.3%~10.4%,respectively,in the presence of carbon materials.Therefore,addition of carbon materials,especially nano-carbon black and rice straw biochar,to organic acids could effectively enhance the efficiency of removal of soil heavy metals,as well as alleviate the loss of available soil nutrients.
soil leaching;nano-carbon black;biochar;organic acid;composite leaching;heavy metals
X53
A
1672-2043(2017)08-1518-08
10.11654/jaes.2017-0347
2017-03-12
马小杰(1993—),女,四川宜宾人,硕士研究生,从事土壤重金属修复研究。E-mail:xiaojiema_z77@163.com
*通信作者:张世熔E-mail:rsz01@163.com
四川省科技支撑计划项目(2014NZ0044);四川农业大学科研兴趣培养计划项目(20150154)
Project supported:The Science and Technology Support Program of Sichuan Province,China(2014NZ0044);The Scientific Training Program of Sichuan Agricultural University(20150154)
马小杰,张世熔,王怡君,等.不同碳材料增强有机酸对土壤重金属的去除及对土壤化学性质的影响[J].农业环境科学学报,2017,36(8):1518-1525.
MA Xiao-jie,ZHANG Shi-rong,WANG Yi-jun,et al.Effects of different carbon materials for enhancement of organic acid-mediated removal of heavy metals and nutrient retention in soil[J].Journal of Agro-Environment Science,2017,36(8):1518-1525.
