Corrosion Inhibition by Co-Immobilized Lysozyme and Lipase in Circulating Cooling Water System
2017-08-07LiuFangYucuiJiangGuofeiChenXiaoruiSunJuanZhaoChaocheng
Liu Fang; Lü Yucui; Jiang Guofei; Chen Xiaorui; Sun Juan; Zhao Chaocheng
(State Key Laboratory of Petroleum Pollution Control, Department of Environmental and Safety Engineering, China University of Petroleum, Qingdao 266580)
Corrosion Inhibition by Co-Immobilized Lysozyme and Lipase in Circulating Cooling Water System
Liu Fang; Lü Yucui; Jiang Guofei; Chen Xiaorui; Sun Juan; Zhao Chaocheng
(State Key Laboratory of Petroleum Pollution Control, Department of Environmental and Safety Engineering, China University of Petroleum, Qingdao 266580)
The corrosion inhibition performance of co-immobilized lysozyme and lipase was investigated in a recirculating cooling water system. Four methods were carried out in co-immobilization, and the operating parameters were optimized by using the respond surface methodology (RSM). The corrosion inhibition performance of co-immobilized lipase and lysozyme was evaluated by weight loss measurements and electrochemical measurements. The results revealed that the optimal co-immobilization method should be the sequential immobilization of lysozyme and then lipase. The inhibition effciency was 86.10% under the optimal co-immobilized conditions. Electrochemical data showed that co-immobilized lysozyme and lipase was a mixed-type inhibitor and the corrosion inhibition effciency was 81%.
circulating cooling water; co-immobilization; lysozyme; lipase; corrosion inhibition
1 Introduction
Heat exchanger corrosion in circulating cooling water system is a common problem at the petroleum refneries of China. The use of chemical inhibitors is one of the most effective methods for preventing metal corrosion. Various organic and non-organic compounds have been researched as inhibitors. The efficiency of organic corrosion inhibitors is related to the presence of polar functional groups containing S, O or N atoms, which are centers for the establishment of the adsorption process[1-3]. Nevertheless, the negative effect of excessive use of chemical inhibitors is worthy of attention. Most organic inhibitors are poisonous and cannot be biodegraded[4], and inorganic inhibitors with heavy metal can contaminate the aquatic environment[5-6].
Nowadays, the restrictive environmental regulations have made researchers focus on the need to develop nontoxic and natural corrosion inhibitors. Enzyme corrosion inhibitor is a kind of novel and environmentally friendly agent[7]. Enzymes have received extensive attention in the fields of wastewater treatment because of their high selectivity and specificity, high activity, and moderate reaction conditions[8-9].
Lysozyme (murein enzyme or mureinN-acetyl polysaccharide hydrolase, EC3.2.1.17) is a kind of hydrolases which has special effect on the microbial cell wall[10], and it is an effective antibacterial agent which can cause bacterial lysis[11]. Liu, et al. found lysozyme had great corrosion inhibition performance in the carbon steel corrosion experiment when it was combined with ATMP and laccase[12]. However, the stability of free lysozyme is low, and it is easily subject to deactivation under environmental conditions. Therefore, the immobilized lysozyme is an effective way to achieve its widespread use.
Lipase (acylglycerol hydrolase, EC3.1.1.3) is a special kind of ester bond hydrolase that breaks down a variety of natural oils[13-14]. In our previous research, the corrosion inhibition property of free lipase was verified in the cooling water system[7].
Enzymes can be immobilized on solid supports by four methods, namely: ① enzyme attachment to matrix by adsorption process; ② covalent bonds between the enzyme and matrix; ③ cross-linking by multifunctional reagents between enzyme and matrix; and④ entrapment of enzyme molecules within a porous hollow fber[15]. They are mainly based on chemical and physical mechanisms.
As for the immobilization support, the mesoporous silica materials exhibit a tunable and uniform pore size in the nanometer scale, along with large pore volume, high surface area and excellent biocompatibility that are beneficial to enzyme immobilization. Due to their higher selectivity, higher yield, mild reaction parameters, and environmental compatibility[16-17], the immobilized enzymes have been applied in industrial and environmental protection felds[18]. Nevertheless, few studies have been done to investigate the application in the corrosion inhibition feld.
Therefore, the objective of this work was to study the corrosion inhibition performance of immobilized enzyme inhibitor in the cooling water system. The coimmobilization of lipase and lysozyme was carried out by means of four immobilization methods. The co-immobilization parameters were optimized by using the single factor experiment and the response surface methodology (RSM). The corrosion inhibition performance of optimal co-immobilized lysozyme and lipase was investigated by using the electrochemical method.
2 Experimental
2.1 Materials
Mesoporous silica particles MCM-41, which were used as the carrier, were purchased from the Nankai University. The bio-enzymes included lysozyme (>20 000 units/ mg of protein, Shanghai Huixing Biochemical Reagent Company, Ltd.) and lipase (>40 000 units/mg of protein, Nanjing Aoduofuni Biotechnology Company, Ltd.).
2.2 Characterization methods
The morphology of MCM-41 was observed by a field emission scanning electron microscope (SEM, Hitachi S-4800). MCM-41 was also observed by transmission electron microscopy (TEM, JEM-2100). The nitrogen sorption isotherms were measured at 77 K with a Micromeritics ASAP 2020 physisorption analyzer. The surface area of MCM-41 was calculated by means of the Brunauer-Emmett-Teller (BET) method. The Barrett–Joyner–Halenda (BJH) method was utilized to measure the mesopore size distribution. And the micropore size distribution was analyzed by the Horvath Kawazoe method.
2.3 Selection of co-immobilization methods
There were four methods for co-immobilizing lysozyme and lipase on MCM-41. The first method was the sequential immobilization of lysozyme and then lipase. 0.3 g of MCM-41 was added into 10 mL of Na2HPO4-KH2PO4buffer solution containing 0.8 g/L of lysozyme (with a pH value of 6.5). The mixture was immobilized for 10 h at 30oC. The resulting solid particles were filtered and then added to 10 mL of Na2HPO4-KH2PO4buffer solution (with a pH value of 8) containing 1.5 g/L of lipase. The mixture was immobilized again for 10 h at 30oC. The resulting powder was fltered and washed with 50 mL of distilled water to remove free enzyme. After having been dried at 30oC for 6 h, the co-immobilized sample was obtained.
The second method was the sequential immobilization of lipase and then lysozyme. The third method was the simultaneous immobilization of lysozyme and lipase at a pH value of 6.5. The fourth method was the simultaneous immobilization of lysozyme and lipase at a pH value of 8. The detailed operation steps are same as the frst method.
2.4 Activity Determination of activity of lipase and lysozyme
Lysozyme breaks the β-1,4-glycosidic bonds of chitosan, so the lysozyme activity can be indirectly obtained by measuring the concentration of reducing sugar in solution[19].
The lipase activity was measured through the volumetric method, which was based on the titrimetric determination of the free acids released from the lipase catalyzed hydrolysis. About 50 μL of tributyrin, 6.0 mL of phosphate buffer (with a pH value of 8.0, a concentration of 25 mM) and a certain amount of immobilized enzymes were incubated for 15 min in a shaker incubator at 40oC and a rotation speed of 140 r/min. The hydrolysis reaction was stopped by adding 10 mL of methanol (95%) and the released free acids were titrated with a 0.05M NaOH solution, using phenolphthalein as the indicator. A unit of activity (U) was defned as the amount of enzyme needed to release 1 μmol of free acids per minute. All enzyme activity experiments were replicated at least three times. The activity can be calculated by Eq. (1):
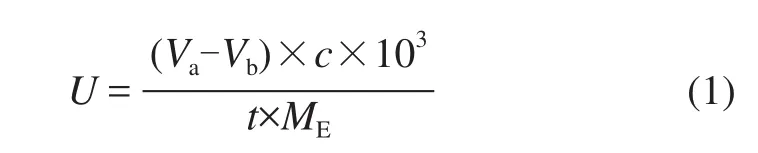
wherecis the concentration of NaOH (mol/L),Va-Vbrepresents the titrimetric volume (mL),trepresents the reaction time (min) andMErepresents the amount of enzyme (mg).
2.5 Immobilization efficiency
The immobilization efficiency was defined as the ratio of enzyme concentration immobilized on MCM-41 to the added enzyme concentration, as shown in Eq. (2). The enzyme concentration was expressed by protein concentration.

in whichωis the immobilization efficiency (%),c0is the protein concentration in the solution containing enzyme before immobilization (g/L),ctis the protein concentration in the solution after immobilization (g/L).
2.6 Optimization of co-immobilization operating conditions
The co-immobilization operating conditions were optimized by the Response Surface Methodology (RSM). The factors and levels of Box-Behnken experimental design are listed in Table 1.
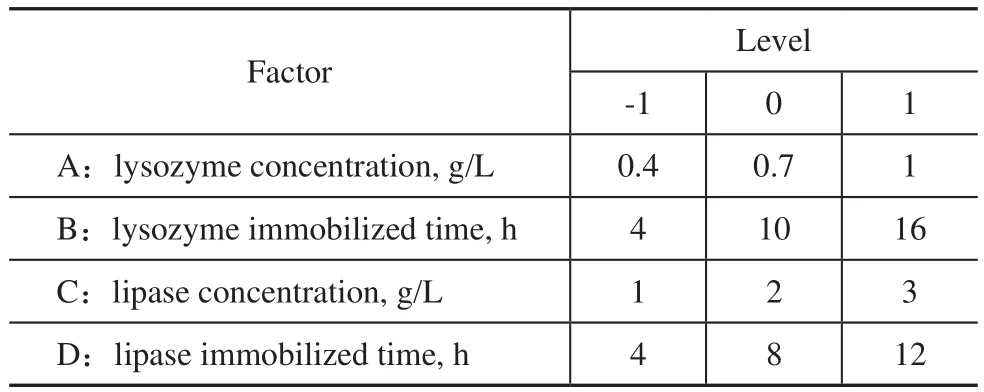
Table 1 Factors and levels of Box-Behnken experimental design
2.7 Thermal stability and pH stability of immobilized enzymes and free enzymes
The thermal stability of immobilized lysozyme or lipase and free lysozyme or lipase was determined in two stages. The frst stage is introducing 0.5 g of immobilized enzyme or free enzyme in 50 mM Na2HPO4-KH2PO4buffer solution (with a pH of 6.5 for lysozyme and a pH of 8 for lipase) at different temperatures for 1h. The second stage is introducing 0.5 g of immobilized enzymes or free enzymes in Na2HPO4-KH2PO4buffer solution (with a pH value of 6.5 for lysozyme and a pH value of 8 for lipase) at 70oC over a different time duration. Then these samples were withdrawn, and their residual activity was assayed. In this work, the enzyme activity was expressed in terms of the relative activity, which was calculated by Eq. (3) (percentage of the maximum activity obtained):

whereRis the relative activity (%),Aiis the activity of immobilized enzyme or free lysozyme (U/mg), andAmaxis the maximum value ofAi(U/mg).
The pH stability of immobilized enzyme and free enzyme was determined by introducing 0.5 g of immobilized enzyme or free enzyme in 50 mM Na2HPO4-KH2PO4buffer solution for 1 h. Then these samples were withdrawn and their residual activity was assayed.
2.8 Corrosion inhibition test by weight loss method
The weight loss measurements were carried out for determining the corrosion inhibition efficiency of immobilized enzyme[12]. The carbon steel coupon (50 mm×25 mm×2 mm) was used as the corrosion object (with a composition of: 0.16%C; 0.30%Si; 0.53%Mn; <0.035% P; <0.04%S and a balance of Fe (in mass fractio)). After being polished, cleaned and dried, the coupons were weighed for recording their original weight. The inhibitors were added into the laboratory circulating cooling water. The characterization of cooling water was analyzed in a previous research[12].
2.9 Electrochemical measurements
Electrochemical measurements, including potentiodynamic polarization curves and electrochemical impedance spectroscopy (EIS), were performed in a three-electrode cell which was connected to a CHI660E electrochemical workstation. A saturated calomel electrode (SCE) was used as the reference electrode. A platinum foil with an area of 1 cm2was used as the auxiliary electrode. A carbon steel coupon was used as the working electrode. All the reported potentials were measured versus SCE. Measurements were performed in the cooling water containing different tested inhibitors by changing the electrode potential automatically from -250 mV to +250 mV versus the open circuit potential at a scan rate of 1.0 mV/s. The electrochemical impedance spectroscopy measurements (EIS) were performed in a frequency range of 100 kHz to 10 mHz with an amplitudeof 10 mV peak-to-peak, using the AC signal atEcorr. The experiments were measured after 1 h of immersion in the testing cooling water with different inhibitors (without de-aeration and stirring).
3 Results and Discussion
3.1 Characterization of mesoporous silica particles MCM-41
The appearance of MCM-41 observed by SEM and TEM is shown in Figure 1. The club shaped morphology of MCM-41 with a uniform particle size is shown in Figure 1a. The TEM image of MCM-41 gives the regular pores in Figure 1b. The TEM image shows that MCM-41 has a uniform pore distribution and a regular hexagonal array of uniform channels.
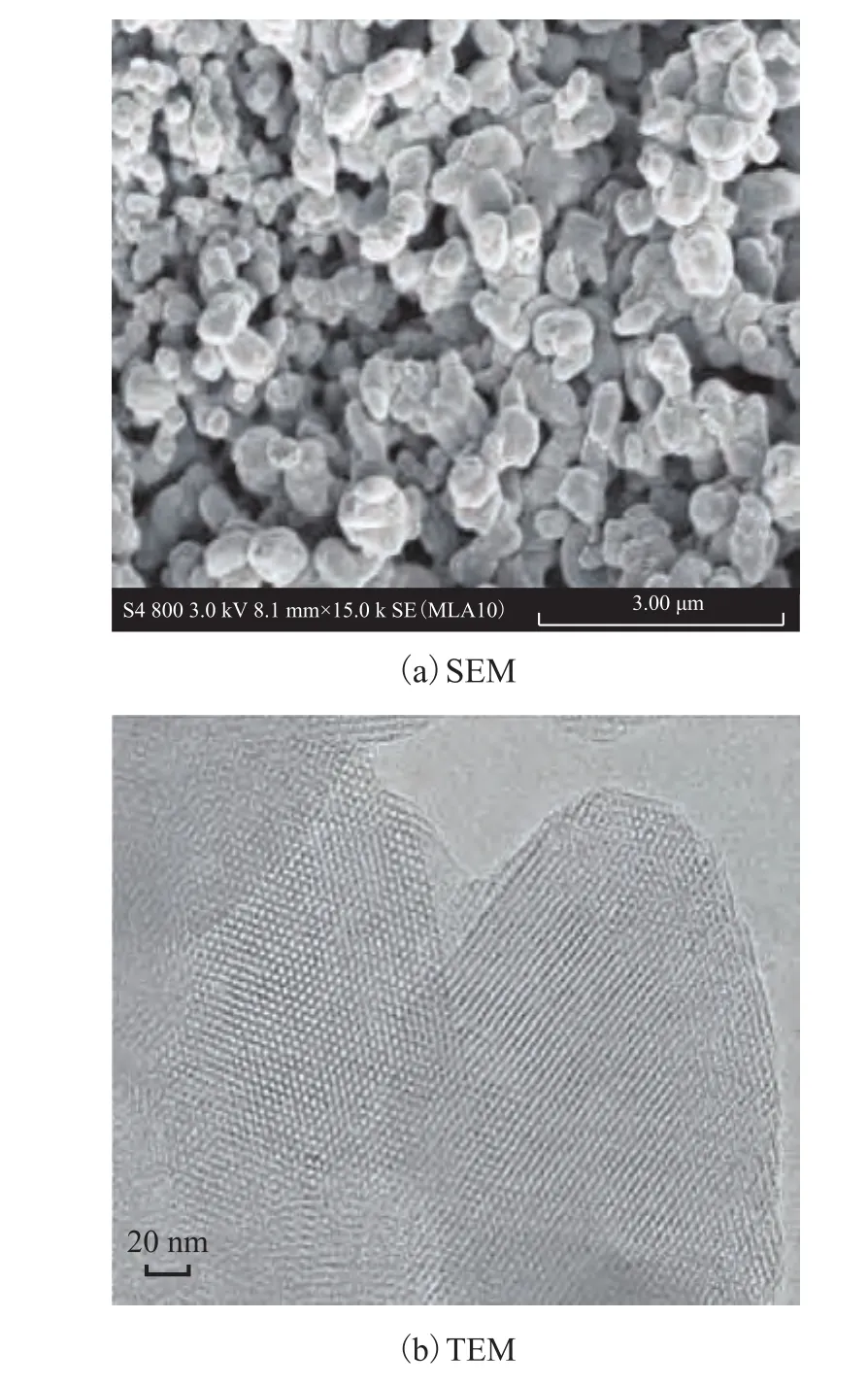
Figure 1 SEM and TEM images of MCM-41
Figure 2a presents the nitrogen adsorption isotherms of MCM-41. The uniform pronounced capillary condensation step of MCM-41 exhibits a type IV isotherm according to IUPAC classifcation[20]. Figure 2b depicts the pore size distribution of MCM-41. According to the data shown in Figure 2, the specific surface area is 1 043.1 m2/g and MCM-41 possesses a vast amount of mesopores with a diameter of around 2.85 nm. The characterization of MCM-41 shows that it has big specifc surface area and uniform pore distribution, so MCM-41 is suitable for use as the carrier for enzyme immobilization.

Figure 2 Nitrogen adsorption-desorption isotherms and pore size distribution of MCM-41
3.2 Optimization of co-immobilized methods
Figure 3 shows that the lysozyme activity of the coimmobilized lysozyme and lipase is 927.57 U/mg, 608.67 U/mg, 689.54 U/mg, and 408.48 U/mg, respectively, and the lipase activity of the co-immobilized lysozyme and lipase is 668.32 U/mg, 734.89 U/mg, 486.45 U/mg, and 576.78 U/mg, respectively, in four co-immobilized processes. The activity of the immobilized lysozyme and immobilized lipase is 897.59 U/mg and 708.46 U/ mg, respectively. In comparison, the frst co-immobilized method (sequential immobilization of lysozyme and then lipase) is the optimal method. So in the later experiments, the co-immobilized lysozyme and lipase is prepared by using the first method. The lysozyme activity in the frst method is even higher than that in the ffth method(the immobilized lysozyme). The reasons may be that lysozyme is adsorbed on the surface of MCM-41, after adding lipase, lipase and lysozyme can combine together through covalent bonds or hydrophobic and hydrophilic groups, because they are biological macromolecules. The molecular conformation of lysozyme molecules may be changed, and the active sites may be exposed. As a result, the enzyme can easily combine with the substrate, thereby improving the enzyme activity[21].
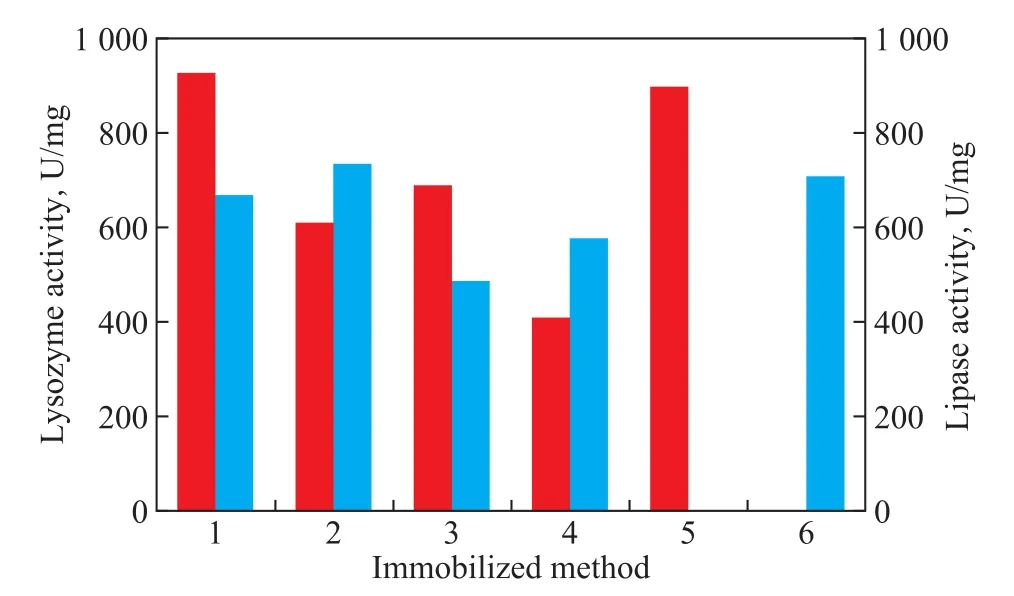
Figure 3 Effect of immobilized method on enzyme activity
3.3 Effects of co-immobilization operating factors on enzyme activity and immobilization efficiency
The optimal co-immobilized method is the sequential immobilization of lysozyme and then lipase. In order to further optimize the operating parameters in this co-immobilized process, the effects of lysozyme concentration, lysozyme immobilization time, lipase concentration and lipase immobilization time on the activity of co-immobilized lysozyme and lipase and the immobilization effciency were investigated.
With the increase of lysozyme concentration from 0.2 g/L to 1.0 g/L, the lysozyme activity of the coimmobilized lysozyme and lipase clearly increases from 174.21 U/mg to 897.57 U/mg and then slightly rises to 904.87 U/mg. On the contrary, the lipase activity of the co-immobilized lysozyme and lipase slightly reduces from 778.76 U/mg to 693.14 U/mg (Figure 4). Lysozyme is frstly immobilized onto MCM-41 and the majority of MCM-41 surface is occupied by lysozyme, which results in the decrease of the amount of immobilized lipase. So the lipase activity of the coimmobilized lysozyme and lipase slightly declines. In addition, the lysozyme immobilization efficiency decreases from 84.59% to 60.93%, notwithstanding the lysozyme activity clearly increases with the increase of lysozyme concentration from 0.2 g/L to 1.0 g/L. It may be occur due to the lysozyme molecular competition at high concentration. Judging from the infuence of the activity of lysozyme and lipase on co-immobilization of lysozyme and lipase, the lysozyme concentration of 0.7 g/L is the optimal value.
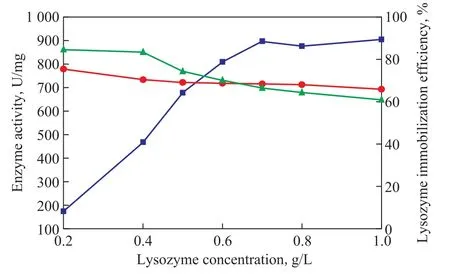
Figure 4 Effect of lysozyme concentration on the activity of co-immobilized enzyme and immobilization efficiency
Figure 5 shows that the lysozyme activity of the coimmobilized lysozyme and lipase evidently increases from 241.68 U/mg to 876.25 U/mg and then slightly rises to 916.32 U/mg with the increase of lysozyme immobilization time from 2 h to 12 h. However, when the time extends to 20 h, the lysozyme activity reduces to 854.87 U/mg. Meanwhile, with the increase of lysozyme immobilization time, the lipase activity of the coimmobilized lysozyme and lipase gradually decreases from 765.26 U/mg to 593.12 U/mg. The trend for variation of lysozyme immobilization effciency with an increasing immobilization time is nearly the same as that of lysozyme activity. When the lysozyme immobilization time increases from 2 h to 8 h, the immobilization efficiency increases from 38.96% to 70.12% and then remains at about 70% with the time being extended up to 20 h. The results illustrate that the time required forimmobilizing lysozyme onto the surface of MCM-41 should be at least 8 h, and a too long immobilization time is not conducive to the increase of lysozyme immobilization effciency. Based on the activity of the coimmobilized lysozyme and lipase and the immobilization effciency, the optimal value of lysozyme immobilization time is 10 h.

Figure 5 Effect of lysozyme immobilization time on the activity of co-immobilized enzyme and immobilization efficiency (at 0.7 g/L of lysozyme)
As shown in Figure 6, the lipase activity of the coimmobilized lysozyme and lipase clearly increases and then slightly decreases with the lipase concentration rising from 0.5 g/L to 3 g/L, and the maximum value is 709.16 U/mg when the lipase concentration reaches 2 g/L. The lysozyme activity remains at about 880—920 U/ mg, and a maximum value of 916.57 U/mg is obtained, with the lipase concentration reaching 1.5 g/L. It is worth noting that the lipase immobilization effciency obviously reduces from 60.17% to 22.28% with the increase of lipase concentration. This phenomenon may occur because of the steric hindrance[22]. Upon considering the activity of the co-immobilized lysozyme and lipase and the immobilization effciency, the optimal value of lipase concentration is 2 g/L.
The lipase activity of the co-immobilized lysozyme and lipase increases from 203.16 U/mg to 651.25 U/mg with the lipase immobilization time rising from 2 h to 8 h, and then keeps at about 670 U/mg with the time extending to 12 h (Figure 7). Similarly, the lipase immobilization efficiency increases from 24.16% to 50.91% and then remains at about 53%. The results show that the lipase immobilization time needs to be 8 h when the maximum value of lysozyme activity (917.27 U/mg) is achieved thereby. So the optimal lipase immobilization time is 8 h.

Figure 6 Effect of lipase concentration on the activity of co-immobilized enzyme and immobilization efficiency (at 0.7 g/L of lysozyme, and a lysozyme immobilization time of 10 h)
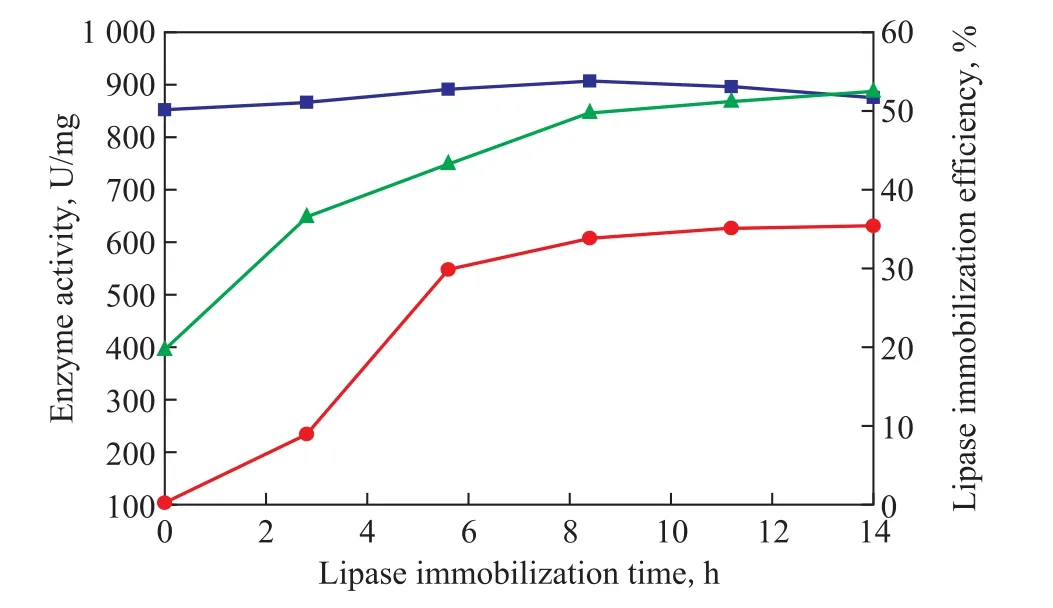
Figure 7 Effect of lipase immobilization time on the activity of co-immobilized enzyme and immobilization ef ficiency (at 0.7 g/L of lysozyme, a lysozyme immobilization time of 10 h, and 2 g/L of lipase)
3.4 Thermal stability and pH stability of immobilized lysozyme and lipase
The relative activity of both the free and immobilized lysozyme as a function of temperature is compared in Figure 8a. The relative activity of the free and immobilized lysozyme at 70oC is shown in Figure 8b. It can be seen that the activity of two enzyme samples was strongly dependent on the temperature. Figure 8a shows that the activity of both the immobilized and freelysozyme decreases with an increasing temperature. But the activity of the immobilized lysozyme is higher than that of free lysozyme at the same temperature. When the temperature is equal to 100oC, the relative activity of the immobilized lysozyme is about 36%, while the relative activity of free lysozyme is only 6%.
According to Figure 8b, the immobilized lysozyme exhibits lower inactivation rate than the free lysozyme. The free lysozyme only retains 1.5% of its initial activity after 3.5 h, while the immobilized lysozyme still retains 30% of initial activity after 4 h. These two sets of data suggest that the thermal activity of the immobilized lysozyme is higher than that of free lysozyme.

Figure 8 Thermal stability of immobilized and free lysozyme
Effects of pH value on the activity of the free lysozyme and immobilized lysozyme were investigated at different pH values ranging from 4.5 to 9 (Figure 9). With the increase of pH value, the activity of the free and immobilized lysozyme simultaneously reaches a maximum at a pH value of 6. The free lysozyme only exhibits high activity values (≥70%) from pH 4.5 to pH 6. By contrast, the immobilized lysozyme shows a broader pH-activity profile than the free lysozyme, and higher activity values (≥70%) of the immobilized lysozyme are obtained at pH values ranging from 4.5 to 8, indicating that immobilization can preserve the enzyme activity in a broader pH profle.

Figure 9 The stability of immobilized and free lysozyme in buffer with different pH value
It can be seen from Figure 10a that the heat resistance of free lipase is poor and the heat resistance of the immobilized lipase is improved obviously as compared with free lipase. When the temperature is 55oC, the activity of the immobilized lipase is retained at above 67%, while the free lipase activity is only 50% of its initial activity. The relative activity of the immobilized lipase is still equal to around 15% at 100oC for 1 h, while the activity of free lipase is close to zero after being incubated at 90oC. It can be found from Figure 10b that the relative activity of the immobilized lipase and free lipase decreases with time after being incubated at 70oC, but the activity of free lipase decreases more signifcantly as compared with that of immobilized lipase. After being incubated for 2.5 h, the activity of free lipase is reduced to zero, and the relative activity of the immobilized lipase is still over 30%. It can be concluded that the stability of the immobilized lipase improves signifcantly as compared to the free lipase.
Figure 11 shows that a maximum relative activity of the immobilized lipase is obtained at a pH value of 8, which is higher than that of free lipase. This reflects that after immobilization the suitable range of pH shifts towards the alkaline value.

Figure 10 Thermal stability of immobilized and free lipase
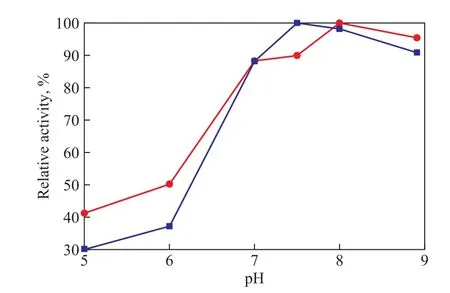
Figure 11 The stability of immobilized and free lipase at different pH values of buffer
3.5 Optimization of co-immobilized operating factors by RSM
According to the above results of single factor experiment, the co-immobilization operating factors are further optimized by using the Response Surface Methodology (RSM). The lysozyme concentration, the lysozyme immobilization time, the lipase concentration, and the lipase immobilization time are independent variables and the corrosion inhibition efficiency is regarded as the response value. The results are listed in Tables 2—3.
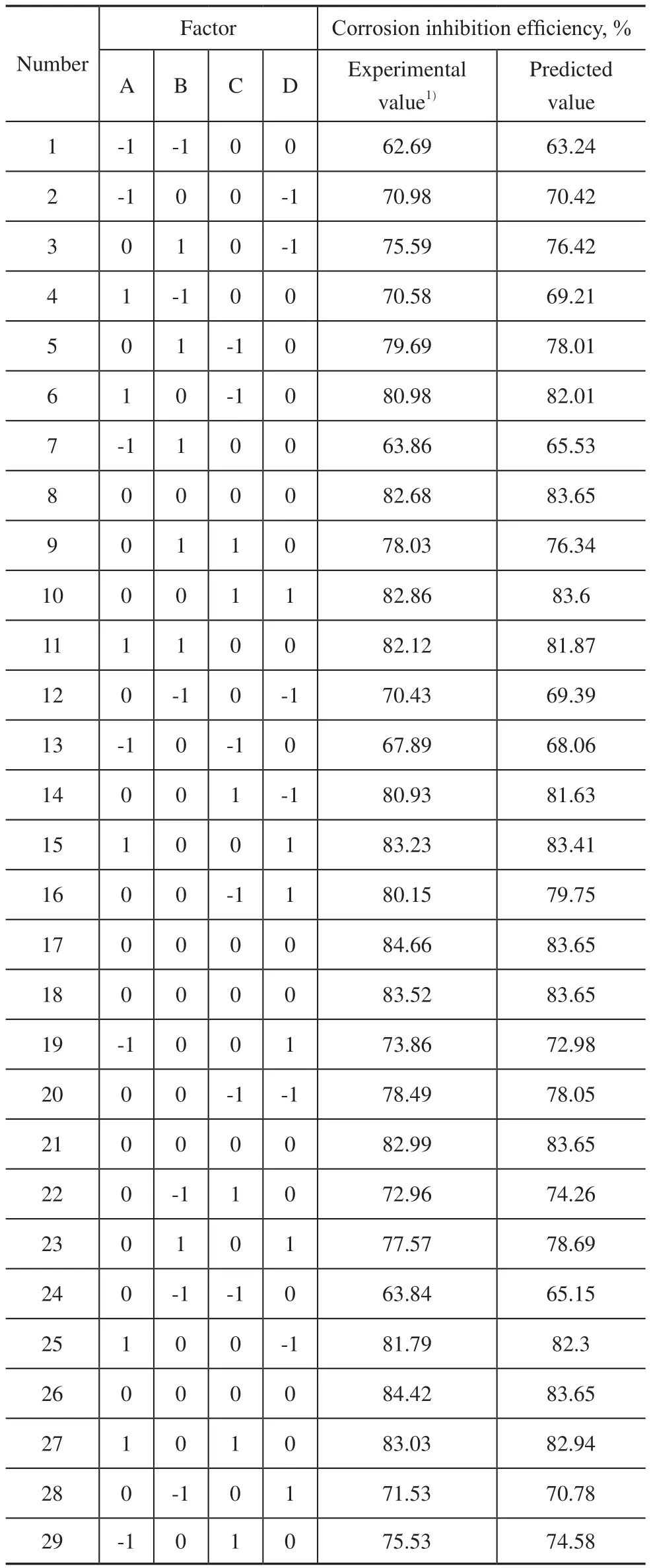
Table 2 Results of RSM

Table 3 Analysis of variance (ANOVA) for regression model
According to Tables 2-3, the ftting equation of factors A, B, C and D is shown in Eq. (5):

The Response Surface Methodology can evaluate the interaction between the signifcant factors of an experiment and optimize them[23-24]. Table 3 shows thatpvalue is less than 0.0001 and the value for lack of fit (0.1512) is greater than 0.05, which indicates that the model selection is appropriate. The lysozyme concentration (A), the lysozyme immobilization time (B), the lipase concentration (C), the interaction of lysozyme concentration (A) with lysozyme immobilization time (B), and the interaction of lysozyme immobilization time (B) with lipase concentration (C) have the extremely significant effects on corrosion inhibition efficiency, and the lipase immobilization time has a relatively signifcant effect on the corrosion inhibition efficiency. The correction coefficientis 0.9592, indicating that the model can explain the variation in 95.92% of response values. The correlation coeffcientR2of fitting equation is 0.9796, which reflects good correlation between the predicted value and the experimental value.
The optimal co-immobilization parameters are calculated by the experimental model. The optimal parameters cover a lysozyme concentration of 0.88 g/L, a lysozyme immobilization time of 11.81 h, a lipase concentration of 2.07 g/L, and a lipase immobilization time of 9.22 h. Under these conditions, the maximum corrosion inhibition efficiency (86.10%) is obtained through calculation. In order to verify the accuracy of models, the experiments have been carried out under the optimal conditions in the circulating cooling water system. As a result, the average corrosion inhibition efficiency (after 3 parallel experiments) is 85.98%, which is close to the result obtained from the model.
3.6 Corrosion inhibition evaluation by electrochemical method
According to the optimal co-immobilization parameters obtained by RSM, the co-immobilized lysozyme and lipase were prepared. The potentiodynamic anodic and cathodic polarization plots for carbon steel in cooling water, in the absence and presence of 2.5 g/L of coimmobilized lysozyme and lipase are shown in Figure 12. Figure 12 indicates that the anodic and cathodic reactions are all affected by the co-immobilized lysozyme and lipase. The results reveal that the addition of the co-immobilized lysozyme and lipase exhibits cathodic and anodic inhibition effects. The respective kinetic parameters including the corrosion current density (Icorr), the corrosion potential (Ecorr), the cathodic Tafel slope (βc), the anodic Tafel slope (βa), the polarization resistance (Rp), and the inhibition effciency (η) are given in Table 4.

Figure 12 Polarization curves and Nyquist plots for carbon steel in cooling water in the absence and in the presence of 2.5 g/L of co-immobilized lysozyme and lipase
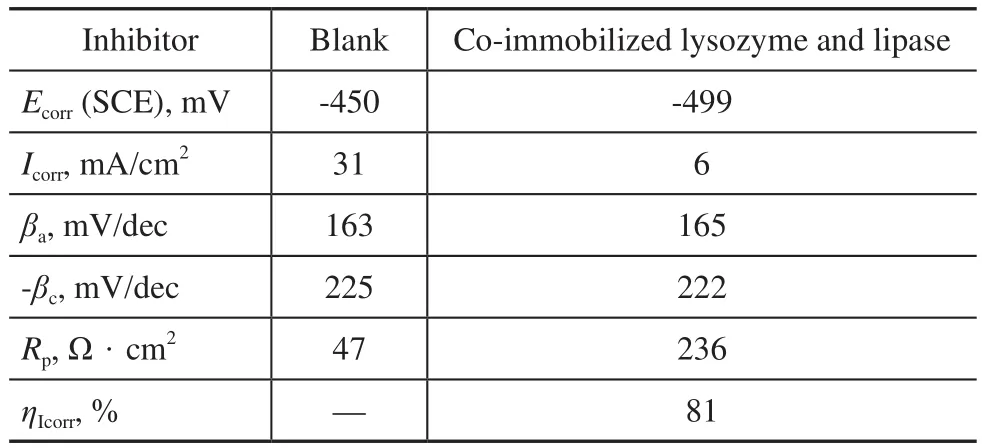
Table 4 Polarization parameters and corresponding inhibition efficiency for the corrosion of carbon steel in cooling water
The inhibition efficiency (ηIcorr) is calculated fromIcorrusing the following equation[25]:

in whichandare the corrosion current density values (mA/cm2) in the absence and in the presence of the co-immobilized lysozyme and lipase, respectively.
According to the data listed in Table 4, the corrosion current density decreased in the presence of coimmobilized lysozyme and lipase, while the polarization resistance increased. According to Eq. (6), the corrosion inhibition effciency is 81%. The data show that the coimmobilized lysozyme and lipase acted as effective inhibitors against the corrosion of carbon steel in the cooling water. By contrast with the blank, the cathodic Tafel slope (βc) and the anodic Tafel slope (βa) of the co-immobilized lysozyme and lipase changed. This observation suggests that the inhibitor molecules could control the two reactions and could be adsorbed on the metal surface by blocking the active sites on the metal surface to retard the corrosion reaction[26]. An inhibitor can be defined as the cathodic or the anodic type if the displacement in corrosion potential is more than 85 mV as compared with that of the blank solution[27]. In the presence of the co-immobilized lysozyme and lipase, the shift of corrosion potential is 49 mV (SCE) (Table 4), indicating that the co-immobilized lysozyme and lipase is a mixed-type inhibitor.
Figure 12 also shows the Nyquist plots obtained at the open-circuit potential after one hour of immersion. The curves consist of a large capacitive loop in the presence of the co-immobilized lysozyme and lipase. The depressed semicircles in impedance spectra indicate to a non-ideal electrochemical behavior at the solid/liquid interface[28]. The addition of the co-immobilized lysozyme and lipase does not change the type of the shape, indicating that the inhibitor controls the activity of corrosion reaction rather than infects its nature[29]. It can be seen obviously from Figure 12 that the diameter of capacitive loop signifcantly increases when the co-immobilized lysozyme and lipases are introduced into the corrosive media, indicating that the co-immobilized lysozyme and lipase can inhibit the carbon steel corrosion in the cooling water.
4 Conclusions
(1) In the present paper, the co-immobilized lipase and lysozyme are prepared by four immobilization methods. The optimal co-immobilization method is the sequential immobilization of lysozyme and then lipase.
(2) The optimal condition for co-immobilizing lysozyme and lipase covers: a lysozyme concentration of 0.88 g/L, a lysozyme immobilization time of 11.81 h, a lipase concentration of 2.07 g/L, and a lipase immobilization time of 9.22 h.
(3) Under the optimal conditions, the experimentalvalue and predicted value are consistent. By means of the electrochemical analysis, it is found that the coimmobilized lipase and lysozyme are a kind of mixedtype inhibitors and the corrosion inhibition effciency can reach 81%, which is lower than that of the weight loss method.
Acknowledgments: This research was financially supported by the National Natural Science Foundation of China (project 21077133), the Natural Foundation of Shandong Province and the Top Talent Project of China University of Petroleum (16RC17040003).
Reference
[1] Nam N D, Somers A, Mathesh M, et al. The behavior of praseodymium 4-hydroxycinnamate as an inhibitor for carbon dioxide corrosion and oxygen corrosion of steel in NaCl solutions [J]. Corrosion Science, 2014, 80: 128-138
[2] Torres V V, Rayol V A, Magalhaes M, et al. Study of thiourea derivatives synthesized from a green route as corrosion inhibitors for mild steel in HCl solution [J]. Corrosion Science, 2014, 79: 108-118
[3] Xu B, Yang W, Liu Y, et al. Experimental and theoretical evaluation of two pyridinecarboxaldehyde thiosemicarbazone compounds as corrosion inhibitors for mild steel in hydrochloric acid solution [J]. Corrosion Science, 2014, 78:260-268
[4] RameshKumar S, Danaee I, RashvandAvei M, et al. Quantum chemical and experimental investigations on equipotent effects of (+)R and (−)S enantiomers of racemic amisulpride as eco-friendly corrosion inhibitors for mild steel in acidic solution [J]. Journal of Molecular Liquids, 2015, 21: 168-186
[5] Umoren S A, M. Eduok U. Application of carbohydrate polymers as corrosion inhibitors for metal substrates in different media: A review [J]. Carbohydrate Polymers, 2016, 140: 314-341
[6] Zhang D Q, Gao L X, Zhou G D, et al. Undecyl substitution in imidazole and its action on corrosion inhibition of copper in aerated acidic chloride media [J]. Journal of Applied Electrochemistry, 2007, 38: 71-76
[7] Lu X H, Liu F, Lu J J, et al. The biological enzyme corrosion inhibitors in the circulating water system with diesel leak[J]. Acta Petrolei Sinica (Petroleum Processing Section), 2013, 29(6): 1090-1095 (in Chinese)
[8] Celikbicak O, Bayramoglu G, Yilmaz M, G. et al. Immobilization of laccase on hairy polymer grafted zeolite particles: Degradation of a model dye and product analysis with MALDI–ToF-MS [J]. Microporous and Mesoporous Materials, 2014, 199: 57-65
[9] Shi L, Ma F, Han Y, et al. Removal of sulfonamide antibiotics by oriented immobilized laccase on Fe3O4nanoparticles with natural mediators [J]. Journal of Hazardous Materials, 2014, 279: 203-211
[10] Callewaert L, Michiels C W. Lysozymes in the animal kingdom [J]. Journal of Biosciences, 2010, 35: 127-160
[11] Blake C, Koenig D, Mair G, et al. Structure of hen eggwhite lysozyme. A three-dimensional Fourier synthesis at 2 Angstrom resolution [J]. Nature, 1965, 206(4986): 757-761
[12] Liu F, Zhang L, Yan X, et al. Effect of diesel on corrosion inhibitors and application of bio-enzyme corrosion inhibitors in the laboratory cooling water system [J]. Corrosion Science, 2015, 93: 293-300
[13] Dos Santos P, Rezende C A, Martínez J. Activity of immobilized lipase from Candida antarctica (Lipozyme 435) and its performance on the esterifcation of oleic acid in supercritical carbon dioxide [J]. Journal of Supercritical Fluids, 2016, 107: 170-178
[14] Jaeger K E, Eggert T. Lipases for biotechnology [J]. Current Opinion in Biotechnology, 2002, 13: 390-397
[15] Durán N, Rosa M A, D’Annibale A, et al. Applications of laccases and tyrosinases (phenoloxidases) immobilized on different supports: A review [J]. Enzyme and Microbial Technology, 2002, 31(7): 907-931
[16] Mateo C, Palomo J M, Fernandez-Lorente G, et al. Improvement of enzyme activity, stability and selectivity via immobilization techniques [J]. Enzyme and Microbial Technology, 2007, 40(6): 1451-1463
[17] Hou C, Wang Y, Zhu H, et al. Construction of enzyme immobilization system through metal-polyphenol assisted Fe3O4/chitosan hybrid microcapsules [J]. Chemical Engineering Journal, 2016, 283: 397-403
[18] DiCosimo R, McAuliffe J, Poulose A J, et al. Industrial use of immobilized enzymes [J]. Chemical Society Reviews, 2013, 42(15): 6437-6474
[19] Imoto T, Yagishita K. A simple activity measurement of lysozyme [J]. Agricultural and Biological Chemistry, 2008,35(7): 1154-1156
[20] Gregg S J, Sing K S W. Adsorption, Surface Area and Porosity [M]. London: Academic Press, 1986
[21] Lee D H, Kim J M, Shin H Y, et al. Optimization of lipase pretreatment prior to lipase immobilization to prevent loss of activity [J](4). Journal of Microbiology and Biotechnology, 2007, 17: 650-654
[22] Zhang D H, Li Y Q, Peng L J, et al. Lipase immobilization on magnetic microspheres via spacer arms: Effect of steric hindrance on the activity [J]. Biotechnology and Bioprocess Engineering, 2014, 19(5): 838-843
[23] Pérez L, Salgueiro J L, Maceiras R, et al. Study of infuence of pH and salinity on combined focculation of Chaetoceros gracilis microalgae [J]. Chemical Engineering Journal, 2016, 286: 106-113
[24] Zheng H, Zhen G, Yin J, et al. Harvesting of microalgae by flocculation with poly (γ-glutamic acid) [J]. Bioresource Technology, 2012, 112: 212-220
[25] Oguzie E, Chidiebere M, Oguzie K, et al. Biomass Extracts for Materials Protection: Corrosion Inhibition of Mild Steel in Acidic Media by Terminalia chebula Extracts [J]. Chemical Engineering Communications, 2014, 201: 790-803
[26] Faustin M, Maciuk A, Salvin P, et al. Corrosion inhibition of C38 steel by alkaloids extract of Geissospermum laeve in 1 M hydrochloric acid: Electrochemical and phytochemical studies [J]. Corrosion Science, 2015, 92: 287-300
[27] Ferreira E S, Giancomelli C, Giacomelli F C, et al. Evaluation of the inhibitor effect of L-ascorbic acid on the corrosion of mild steel [J]. Materials Chemistry and Physics, 2004, 83: 129-134.
[28] Hosseini M, Mertens S F L, Ghorbani M, et al. Asymmetrical Schiff bases as inhibitors of mild steel corrosion in sulphuric acid media [J]. Materials Chemistry and Physics, 2003, 78: 800-808
[29] Zhang K, Xu B, Yang W, et al. Halogen-substituted imidazoline derivatives as corrosion inhibitors for mild steel in hydrochloric acid solution [J]. Corrosion Science, 2015, 90: 284-295
New Process of Low-temperature Fixed-bed Fisher-Tropsch Reaction Using JL-GX Catalyst
On April 17, 2017 the new process of low-temperature fixed-bed Fisher-Tropsch reaction using the JL-GX catalyst developed independently by the Beijing Jingli Clean Energy Technology Company, Ltd., has been realized through the commissioning of the commercial demonstration unit at the Xingtai Rising Sun Chemical Company. In comparison with the conventional Fisher-Tropsch process, this novel Fisher-Tropsch process can be adapted to a variety of feed gases, which besides natural gas can also use the industrial offgases (including the coke oven gas, the coal seam gas, the oilfield associated gas, and the marsh gas) to manufacture synthetic chemicals through Fisher-Tropsch process. Furthermore, this catalyst which is independently developed by the Beijing Jingli Clean Energy Technology Company can be regenerated many times, which has carved out a new route for upgrading and improving the quality of coal chemical products.
It is learned that in the course of pilot tests, under the conventional operating conditions the total conversion of feed gas can reach 93%, while the methane selectivity is not greater than 10%, and the C5hydrocarbons selectivity can exceed 80% (among which the Fischer-Tropsch synthetic wax yield accounts for 50%—60%). Such key parameters as the catalyst activity, the conversion of feed gas, the product selectivity, and the reactor heat transfer effciency are highly consistent with the model predicted values. The key indicators, including the feedstock utilization rate, the product selectivity and the space-time yield, all have reached or surpassed the internationally advanced level.
date: 2017-02-13; Accepted date: 2017-03-27.
Prof. Liu Fang, E-mail: liufangfw @163.com.
杂志排行
中国炼油与石油化工的其它文章
- Correlation of Deactivation of Ni-Mo-W/Al2O3during Ultra-Low-Sulfur Diesel Production with Surface Carbon Species
- Effect of Lithium Nitrate on the Structure and Property of α-Al2O3Platelets Prepared via Solid-state Reaction
- Effect of Preparation Process on Compressive Strength and Hydrogenation Performance of Raney-Ni/Al2O3Catalyst
- Preliminary Analysis of Diesel-Degrading Bacteria Immobilized on Organic Carriers in Seawater
- Synthesis of Cu/ZnO Flower-like Hierarchical Porous Structures and Investigation of Their Catalytic Performance for Dimethyl Oxalate Hydrogenation
- Facile Synthesis of Cu/Al2O3with High Copper Dispersion for Direct Synthesis of Dimethyl Ether from Syngas
