Adult neural stem cell dysfunction in the subventricular zone of the lateral ventricle leads to diabetic olfactory defects
2017-08-07YuhongJingChuchuQiLiYuanXiangwenLiuLipingGaoJieYin
Yu-hong Jing, Chu-chu Qi Li Yuan Xiang-wen Liu Li-ping Gao, Jie Yin
1 Institute of Anatomy and Histology & Embryology and Neuroscience, School of Basic Medical Sciences, Lanzhou University, Lanzhou, Gansu Province, China
2 Key Laboratory of Preclinical Study for New Drugs of Gansu Province, Lanzhou University, Lanzhou, Gansu Province, China
3 Institute of Biochemistry and Molecular Biology, School of Basic Medical Sciences, Lanzhou University, Lanzhou, Gansu Province, China
Adult neural stem cell dysfunction in the subventricular zone of the lateral ventricle leads to diabetic olfactory defects
Yu-hong Jing1,2, Chu-chu Qi1, Li Yuan1, Xiang-wen Liu1, Li-ping Gao3, Jie Yin1,*
1 Institute of Anatomy and Histology & Embryology and Neuroscience, School of Basic Medical Sciences, Lanzhou University, Lanzhou, Gansu Province, China
2 Key Laboratory of Preclinical Study for New Drugs of Gansu Province, Lanzhou University, Lanzhou, Gansu Province, China
3 Institute of Biochemistry and Molecular Biology, School of Basic Medical Sciences, Lanzhou University, Lanzhou, Gansu Province, China
How to cite this article:Jing YH, Qi CC, Yuan L, Liu XW, Gao LP, Yin J (2017) Adult neural stem cell dysfunction in the subventricular zone of the lateral ventricle leads to diabetic olfactory defects. Neural Regen Res 12(7):1111-1118.
Graphical Abstract
orcid: 0000-0002-3131-5254 (Jie Yin)
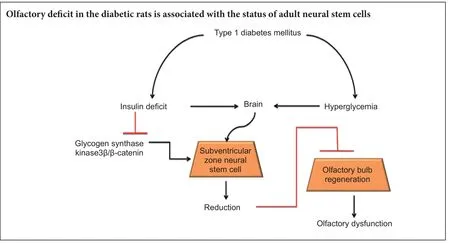
Sensitive smell discrimination is based on structural plasticity of the olfactory bulb, which depends on migration and integration of newborn neurons from the subventricular zone. In this study, we examined the relationship between neural stem cell status in the subventricular zone and olfactory function in rats with diabetes mellitus. Streptozotocin was injected through the femoral vein to induce type 1 diabetes mellitus in Sprague-Dawley rats. Two months aer injection, olfactory sensitivity was decreased in diabetic rats. Meanwhile, the number of BrdU-positive and BrdU+/DCX+double-labeled cells was lower in the subventricular zone of diabetic rats compared with agematched normal rats. Western blot results revealed downregulated expression of insulin receptor β, phosphorylated glycogen synthase kinase 3β, and β-catenin in the subventricular zone of diabetic rats. Altogether, these results indicate that diabetes mellitus causes insulin deficiency, which negatively regulates glycogen synthase kinase 3β and enhances β-catenin degradation, with these changes inhibiting neural stem cell proliferation. Further, these signaling pathways a ff ect proliferation and di ff erentiation of neural stem cells in the subventricular zone. Dysfunction of subventricular zone neural stem cells causes a decline in olfactory bulb structural plasticity and impairs olfactory sensitivity in diabetic rats.
nerve regeneration; diabetic encephalopathy; adult neural stem cells; olfactory function; subventricular zone; proliferation; glycogen synthase kinase 3 beta; β-catenin; di ff erentiation; rats; insulin; type 1 diabetes mellitus; neural regeneration
Introduction
In many mammalian species, newborn neurons continue to be integrated into the olfactory bulb. In rodents, the subventricular zone (SVZ) near the lateral ventricle wall generates newborn neurons that migrate to the olfactory bulb where they differentiate into local neurons (Whitman and Greer, 2009). Recently, several studies suggest that adult olfactory neurogenesis may be involved in regulation of olfactory behavior in rodents (Kageyama et al., 2012; Manzini, 2015). Moreno et al. (2009) reported that olfactory learning improves odor distinction and is damaged by infusion of cytosine-β-D-arabinofuranoside (AraC), which inhibits neural stem cell proliferation and survival. Additionally, a previous study showed that AraC infusion decreases short-term olfactory memory and odor detection sensitivity in mice (Breton-Provencher and Saghatelyan, 2012). Furthermore, longterm olfactory memory retention was impaired with AraC treatment, although basic olfactory functions were unaltered (Sultan et al., 2010).
A study has shown that olfactory dysfunction may be an early sign of brain changes in Alzheimer’s disease or cognitive impairment because it appears to precede clinical signs. In addition, mild cognitive impairment is accompanied by olfactory dysfunction in patients (Devanand et al., 2000). Several clinical studies also revealed association between olfactory dysfunction and cognitive impairment in the older population (Wilson et al., 2006; Schubert et al., 2008). Some patients with type 2 diabetes mellitus (DM) also su ff er from olfactory dysfunction (Le Floch et al., 1993; Infante-Garcia et al., 2015). Indeed, several studies have proposed that olfactory dysfunction in diabetic patients is due to, or at least aggravated by, secondary pathologies (Naka et al., 2010; Brady et al., 2013; Gouveri et al., 2014). Interestingly, epidemiological surveys suggest that diabetes is associated with increased prevalence of Alzheimer’s disease (Sahay et al., 2011). Diabetic encephalopathy is characterized by brain atrophy, reactive oxygen species accumulation, reduced synaptic plasticity, and cognitive impairment.ese changes are similar to those that occur during acceleration of brain ageing (Biessels et al., 2002; Baquer et al., 2009). We previously showed that aberrant metabolism following insulin de fi ciency (including hyperglycemia and hyperlipidemia) causes hippocampal atrophy, neurodegeneration, amyloid beta deposition, and declined dendritic spine density in streptozotocin (STZ)-induced diabetic rats (Wang et al., 2014).
Signaling molecules of the Wnt family play important roles in maintaining cellular proliferation, di ff erentiation, migration, and axon guidance during neural development (Ille and Sommer, 2005). Increased β-catenin due to virally transduced expression of a stabilized form of this protein increases proliferation of Ascl1-expressing SVZ cells and olfactory bulb neurogenesis. As the modulator, insulin is implicated in modi fi cation of β-catenin signaling (Kim et al., 2013). Additionally, type 1 diabetes mellitus (T1DM) is characterized by absolute insulin deficiency. Therefore, in this study, we determined whether T1DM negatively affects proliferation and di ff erentiation of neural stem cells in SVZ, and explored olfaction changes in this process.
Materials and Methods
Animals
Eight- to 10-week male Sprague-Dawley rats were obtained from the Animal Center of Lanzhou University of China (license No. SCXK (Gan) 2009-0004). Rats were fed in an animal house at 22 ± 2°C and relative humidity of 55 ± 10% on 12-hour light-dark cycle. Rats were allowed free access to food and water. Experimental procedures were approved by the Animal Ethics Committee, Lanzhou University, China. The experiment followed the National Guidelines for the Care and Use of Laboratory Animals, and Consensus Author Guidelines for Animal Use formulated by the International Association of Veterinary Editors (IAVE).e article was prepared in accordance with the Animal Research: Reporting of In Vivo Experiments Guidelines (ARRIVE Guidelines).
Overnight-fasted rats were injected once with 65 mg/kg STZ (Sigma, St. Louis, MO, USA) through the femoral vein to induce DM. Age-matched normal rats received 0.2 mL normal saline. One week aer STZ injection, blood samples were collected through the tail vein, and plasma glucose levels measured by plasma glucose test fi lms (Sinocare Inc., Changsha, China) and enzymatic diagnostic kits (Shanghai Rongsheng Biotech Co., Ltd., Shanghai, China). Rats with plasma glucose levels ≥ 300 mg/dL and symptoms of polyuria, polyphagia, and polydipsia were considered diabetic and used in the present study. Diabetic rats (DM group) and age-matched rats (normal group) were raised for 2 months.
Olfactory function evaluated by buried food pellet test
At 55 days after treatment, rats were evaluated for their ability to find food (lab regular diet) hidden underneath bedding as previously described (Montani et al., 2013). Before the test, rats were food deprived for 12 hours with free access to water. A scented pellet was placed at one corner of the clean cage and the time taken to reach the visible pellet recorded. Rats were then removed from the cage and a scented pellet buried underneath a 7 cm-thick layer of bedding. Time from introduction of the animal to the cage until the food pellet was retrieved with its front paws was measured in seconds up to a maximum of 300 seconds. Failure to fi nd the food pellet within the allocated time was represented as 300 seconds. Time to fi nd the buried pellet was recorded.e trial was repeated three times, separated by 10-minute intervals. Latency to fi nd visible food in three trials was averaged.
Olfactory sensitivity test

Figure 1 Schematic diagrams.(A) Experimental fl ow chart: time of streptozotocin injection, time of behavioral testing, time course of BrdU injection. (B) Three coronal serial sections at 2.16, 1.08, and 0.12 mm from bregma (according to the Brain Atlas, 5thversion) were selected from each rat brain. Rectangle frames indicate areas of cell counting in the subventricular zone. Lv: Lateral ventricle; CPu: caudate putamen (striatum); AcbC: nucleus accumbens, core; BrdU: bromodeoxyuridine.
Injection of bromodeoxyuridine (BrdU)
Biochemistry assay
Immunohistochemistry
Western blot assay
Five rats from each group were decapitated, and their brains removed and placed on ice plates. Bilateral SVZ were dissected and frozen in liquid nitrogen. Total protein was extracted in lysates containing protein inhibitor cocktail. Protein (30 μg) was fractionated on 10% sodium dodecyl sulfate-polyacrylamide gels for electrophoresis, and then transferred onto polyvinylidene fluoride membranes. Membranes were blotted overnight with anti-insulin receptor β (IRβ) (1:1,000), anti-glycogen synthase kinase 3 beta (GSK3β) (1:1,000; Cell Signaling, Boston, MA, USA), anti-phospho-glycogen synthase kinase 3 beta (p-GSK3β) (1:1,000; Cell Signaling), anti-β-catenin (1:1,000; Santa Cruz Biotechnology, Santa Cruz, CA, USA), and anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (1:5,000; Santa Cruz Biotechnology) at 4°C. Membranes were washed with Tris-buffered saline containing Tween 20 and then blotted with corresponding horseradish peroxidase-conjugated sec-ondary antibodies (1:5,000). Blotted bands were visualized by enhanced chemiluminescence and analyzed by Image J soware (NIH, Bethesda, MD, USA). All western blot experiments were performed at least three times. Lanes were scanned and optical density normalized using GAPDH as an internal control.

Figure 2 Metabolic parameters from rats in 2 consecutive months aer streptozotocin injection.

Figure 3 Olfactory sensitivity in normal and diabetic rats aer 2 months of treatment.
Statistical analysis
All data are expressed as the mean ± SEM, and were analyzed with SPSS 17.0 software (SPSS, Chicago, IL, USA). One-way analysis of variance was used for multiple-group comparisons; Tukey’spost-hocanalysis was performed for unpaired-group comparisons.Pvalues < 0.05 were consid-ered statistically signi fi cant.

Figure 4 E ff ect of DM on neural stem cell status in SVZ.

Figure 5 E ff ect of DM on IRβ, p-GSK3β, and β-catenin expression in SVZ 8 weeks aer treatment (western blot assay).
Results
Metabolic parameters
Consecutive metabolic testing showed reduced body weight (Figure 2D) in diabetic rats compared with age-matched rats (P< 0.01). Consecutive plasma examination showed higher glucose levels (Figure 2A), but lower insulin (Figure 2C) in diabetic rats compared with age-matched rats (P< 0.01). Consistently, glucose levels increased in cerebrospinal fl uid of diabetic rats compared with normal rats (Figure 2B) (P<0.01).
Diabetes impaired olfactory function
Two months after STZ injection, olfactory sensitivity was evaluated using the buried food test and odor discrimination. As shown in Figure 3A, diabetic rats showed no di ff erence compared with normal rats in time taken to reach the visible pellet, indicating no speed difference. In the buried pellet trial, diabetic rats required a notably longer time to reach the pellet than age-matched normal rats (Figure 3B) (P< 0.01), indicating impaired olfactory performance. Time spent exploring high-concentration amyl acetate (1:1) showed signi fi cant increases compared with the water stimulus in both diabetic rats and normal rats, but the increase was smaller in the diabetic group compared with the normal group (P< 0.05; Figure 3C). Time exploring low-concentration amyl acetate (1:50) odor showed signi fi cant increases in the diabetic and normal groups, with a higher increase in the normal group compared with the diabetic group (P< 0.05;Figure 3D).
Diabetes reduced proliferation and di ff erentiation of neural stem cells in SVZ
BrdU labeling was used to examine proliferation of neural stem cells in SVZ.e number of BrdU-positive cells in SVZ was lower in diabetic rats (120.3 ± 23.2) than age-matched normal rats (202.2 ± 10.8;P< 0.05; Figure 4A, B). Immuno-double labeling, cell counting, and confocal microscopy were performed to examine di ff erentiation ability of neural precursor cells in SVZ of diabetic rats. Cells labeled with BrdU and DCX were identified as immature, newly generated neurons.e number of BrdU+/DCX+cells in SVZ was signi fi cantly lower in diabetic rats than in age-matched rats (P< 0.05; Figure 4C, D).
Changes in insulin/GSK3β/β-catenin signaling in SVZ of diabetic rats
Insulin signals play important roles in maintaining energy balance and neuronal survival in the central nervous system. In the central nervous system, most insulin is produced by pancreatic islets and transferred into the central nervous system across the blood-brain barrier (Schwartz et al., 1991). T1DM is characterized by an absolute insulin deficit throughout the whole body, including the brain. To determine whether insulin signaling is impaired in SVZ, IRβ protein levels were tested by western blot assay. Our results show that IRβ expression levels in SVZ were lower by approximately 60% in diabetic rats compared with agematched normal rats (P< 0.05; Figure 5A, B). Many downstream signals are regulated by insulin including GSK3β and β-catenin. Cell cycle and proliferation are regulated by β-catenin, and β-catenin activity is negatively regulated by GSK3β.us, we measured GSK3β activity. Our results show that GSK3β phosphorylation (at lysine 9) was significantly lower in SVZ of diabetic rats than age-matched normal rats (P< 0.05; Figure 5C, D), suggesting increased GSK3β activity. Consistently, β-catenin expression levels were lower in SVZ of diabetic rats than age-matched normal rats (P< 0.05;Figure 5E, F).
Discussion
Here, we show that proliferation of adult neural stem cells is markedly lower in SVZ of diabetic rats compared with normal rats. Particularly, the number of BrdU-positive cells located in the lateral ventricle margin was significantly reduced. It has been shown that the lateral ventricle wall is principally comprised of ependymal cells, which possess neural stem cell characteristics (Tong et al., 2014). In our experiments, SVZ cell types were identi fi ed by transmission electron microscopy. We found that in DM rats, proliferated SVZ cells are mainly ependymal cells located along the lateral ventricle. Nevertheless, in the normal group, proliferated SVZ cells include ependymal cells, astrocytes, and neuroblasts. Our results suggest that di ff erential localization of proliferated cells between normal and DM groups may contribute to divergent cell types. DCX is a microtubule-associated protein implicated in neuronal migration during development and adulthood. DCX expression is transitory during adult neurogenesis, dropping o ff with the emergence of mature neuronal markers, and primarily localized to areas of continuous neurogenesis and rarely elsewhere (Brown et al., 2003; Keays, 2007; von Bohlen und Halbach, 2011). Dramatically, compared with other markers (such as nestin and GFAP), DCX is particular to the neuronal lineage. Our results show that BrdU/DCX double-positive cells located in SVZ decrease signi fi cantly in DM rats compared with the normal group. Similarly, BrdU/GFAP double-positive cells also decreased in diabetic rats.is suggests that progenitor cell differentiation in SVZ is impaired under the diabetic condition.
In brief, the SVZ niche is changed in DM, exhibiting disturbed status of neural stem cells, which decrease the proliferation and differentiation abilities of adult neural stem cells in SVZ.e mechanisms underlying DM-induced abnormalities of adult neural stem cells are involved in deregulation of GSK3β and β-catenin signals. Diabetes-induced olfactory de fi cits are partly associated with neural stem cell impairments in SVZ.
Author contributions:JY, CCQ and YHJ planned experiments, and interpreted data. JY and CCQ performed most of the experiments and analyzed data. YHJ wrote the paper. LY and XWL participated in the animal experiment. LPG participated in acquisition of the study specimens. All authors read and approved the fi nal version of the paper.
Con fl icts of interest:None declared.
Research ethics:
Open access statement:
Contributor agreement:A statement of “Publishing Agreement” has been signed by an authorized author on behalf of all authors prior to publication.
Plagiarism check:This paper has been checked twice with duplication-checking soware ienticate.
Peer review:A double-blind and stringent peer review process has been performed to ensure the integrity, quality and signi fi cance of this paper.
Adachi K, Mirzadeh Z, Sakaguchi M, Yamashita T, Nikolcheva T, Gotoh Y, Peltz G, Gong L, Kawase T, Alvarez-Buylla A, Okano H, Sawamoto K (2007) Beta-catenin signaling promotes proliferation of progenitor cells in the adult mouse subventricular zone. Stem Cells 25:2827-2836.
Arum O, Boparai RK, Saleh JK, Wang F, Dirks AL, Turner JG, Kopchick JJ, Liu JL, Khardori RK, Bartke A (2014) Speci fi c suppression of insulin sensitivity in growth hormone receptor gene-disrupted (GHRKO) mice attenuates phenotypic features of slow aging. Aging Cell 13:981-1000.
Baquer NZ, Taha A, Kumar P, McLean P, Cowsik SM, Kale RK, Singh R, Sharma D (2009) A metabolic and functional overview of brain aging linked to neurological disorders. Biogerontology 10:377-413.
Biessels GJ, van der Heide LP, Kamal A, Bleys RL, Gispen WH (2002) Ageing and diabetes: implications for brain function. Eur J Pharmacol 441:1-14.
Brady S, Lalli P, Midha N, Chan A, Garven A, Chan C, Toth C (2013) Presence of neuropathic pain may explain poor performances on olfactory testing in diabetes mellitus patients. Chem Senses 38:497-507.
Breton-Provencher V, Saghatelyan A (2012) Newborn neurons in the adult olfactory bulb: unique properties for specific odor behavior. Behav Brain Res 227:480-489.
Brown JP, Couillard-Despres S, Cooper-Kuhn CM, Winkler J, Aigner L, Kuhn HG (2003) Transient expression of doublecortin during adult neurogenesis. J Comp Neurol 467:1-10.
Davidson G, Niehrs C (2010) Emerging links between CDK cell cycle regulators and Wnt signaling. Trends Cell Biol 20:453-460.
Devanand DP, Michaels-Marston KS, Liu X, Pelton GH, Padilla M, Marder K, Bell K, Stern Y, Mayeux R (2000) Olfactory de fi cits in patients with mild cognitive impairment predict Alzheimer’s disease at follow-up. Am J Psychiatry 157:1399-1405.
Eibenstein A, Fioretti AB, Lena C, Rosati N, Amabile G, Fusetti M (2005) Modern psychophysical tests to assess olfactory function. Neurol Res 26:147-155.
Gouveri E, Katotomichelakis M, Gouveris H, Danielides V, Maltezos E, Papanas N (2014) Olfactory dysfunction in type 2 diabetes mellitus: an additional manifestation of microvascular disease? Angiology 65:869-876.
Hall AC, Brennan A, Goold RG, Cleverley K, Lucas FR, Gordon-Weeks PR, Salinas PC (2002) Valproate regulates GSK-3-mediated axonal remodeling and synapsin I clustering in developing neurons. Mol Cell Neurosci 20:257-270.
Ille F, Sommer L (2005) Wnt signaling: multiple functions in neural development. Cell Mol Life Sci 62:1100-1108.
Infante-Garcia C, Ramos-Rodriguez JJ, Garcia-Alloza M (2015) Prediabetes and type 2 diabetes implication in central proliferation and neurogenesis. Neural Regen Res 10:28-29.
Kageyama R, Imayoshi I, Sakamoto M (2012)e role of neurogenesis in olfaction-dependent behaviors. Behav Brain Res 227:459-463.
Keays DA (2007) Neuronal migration: unraveling the molecular pathway with humans, mice, and a fungus. Mamm Genome 18: 425-430.
Kim MH, Hong SH, Lee MK (2013) Insulin receptor-overexpressing beta-cells ameliorate hyperglycemia in diabetic rats through Wnt signaling activation. PLoS One 8:e67802.
Korf ES, White LR, Scheltens P, Launer LJ (2006) Brain aging in very old men with type 2 diabetes: the Honolulu-Asia Aging Study. Diabetes Care 29:2268-2274.
Le Floch JP, Le Lievre G, Labroue M, Paul M, Peynegre R, Perlemuter L (1993) Smell dysfunction and related factors in diabetic patients. Diabetes care 16:934-937.
Li CL, Sathyamurthy A, Oldenborg A, Tank D, Ramanan N (2014) SRF phosphorylation by glycogen synthase kinase-3 promotes axon growth in hippocampal neurons. J Neurosci 34:4027-4042.
Manzini I (2015) From neurogenesis to neuronal regeneration: the amphibian olfactory system as a model to visualize neuronal development in vivo. Neural Regen Res 10:872-874.
Montani G, Tonelli S, Sanghez V, Ferrari PF, Palanza P, Zimmer A, Tirindelli R (2013) Aggressive behaviour and physiological responses to pheromones are strongly impaired in mice de fi cient for the olfactory G-protein -subunit G8. J Physiol 591:3949-3962.
Moreno MM, Linster C, Escanilla O, Sacquet J, Didier A, Mandairon N (2009) Olfactory perceptual learning requires adult neurogenesis. PNAS 106:17980-17985.
Naka A, Riedl M, Luger A, Hummel T, Mueller CA (2010) Clinical signi fi cance of smell and taste disorders in patients with diabetes mellitus. Eur Arch Otorhinolaryngol 267:547-550.
Real S, Meo-Evoli N, Espada L, Tauler A (2011) E2F1 regulates cellular growth by mTORC1 signaling. PLoS One 6:e16163.
Sahay A, Scobie KN, Hill AS, O’Carroll CM, Kheirbek MA, Burghardt NS, Fenton AA, Dranovsky A, Hen R (2011) Increasing adult hippocampal neurogenesis is su ffi cient to improve pattern separation. Nature 472:466-470.
Schubert CR, Carmichael LL, Murphy C, Klein BE, Klein R, Cruickshanks KJ (2008) Olfaction and the 5-year incidence of cognitive impairment in an epidemiological study of older adults. J Am Geriatr Soc 56:1517-1521.
Schwartz MW, Bergman RN, Kahn SE, Taborsky GJ, Jr., Fisher LD, Sipols AJ, Woods SC, Steil GM, Porte D, Jr. (1991) Evidence for entry of plasma insulin into cerebrospinal fl uid through an intermediate compartment in dogs. Quantitative aspects and implications for transport. J Clin Invest 88:1272-1281.
Sohrabi HR, Bates KA, Rodrigues M, Taddei K, Laws SM, Lautenschlager NT, Dhaliwal SS, Johnston AN, Mackay-Sim A, Gandy S, Foster JK, Martins RN (2009) Olfactory dysfunction is associated with subjective memory complaints in community-dwelling elderly individuals. J Alzheimers Dis 17:135-142.
Sultan S, Mandairon N, Kermen F, Garcia S, Sacquet J, Didier A (2010) Learning-dependent neurogenesis in the olfactory bulb determines long-term olfactory memory. FASEB J 24:2355-2363.
Tong CK, Chen J, Cebrian-Silla A, Mirzadeh Z, Obernier K, Guinto CD, Tecott LH, Garcia-Verdugo JM, Kriegstein A, Alvarez-Buylla A (2014) Axonal control of the adult neural stem cell niche. Cell Stem Cell 14:500-511.
von Bohlen und Halbach O (2011) Immunohistological markers for proliferative events, gliogenesis, and neurogenesis within the adult hippocampus. Cell Tissue Res 345:1-19.
Wang JQ, Yin J, Song YF, Zhang L, Ren YX, Wang DG, Gao LP, Jing YH (2014) Brain aging and AD-like pathology in streptozotocin-induced diabetic rats. J Diabetes Res 2014:796840.
Whitman MC, Greer CA (2009) Adult neurogenesis and the olfactory system. Prog Neurobiol 89:162-175.
Wilson RS, Arnold SE, Tang Y, Bennett DA (2006) Odor identi fi cation and decline in di ff erent cognitive domains in old age. Neuroepidemiology 26:61-67.
Xu F, Gu JH, Qin ZH (2012) Neuronal autophagy in cerebral ischemia. Neurosci Bull 28:658-666.
Copyedited by Wang J, Li CH, Qiu Y, Song LP, Zhao M
10.4103/1673-5374.211190
Accepted: 2017-04-05
*Correspondence to: Jie Yin, M.D., yinjie@lzu.edu.cn.
杂志排行
中国神经再生研究(英文版)的其它文章
- SoxC transcription factors in retinal development and regeneration
- Umbilical cord: an unlimited source of cells di ff erentiable towards dopaminergic neurons
- Targeting 14-3-3 adaptor protein-protein interactions to stimulate central nervous system repair
- RACK1 regulates neural development
- Schwann cell development, maturation and regeneration: a focus on classic and emerging intracellular signaling pathways
- BDNF pro-peptide: a novel synaptic modulator generated as an N-terminal fragment from the BDNF precursor by proteolytic processing
