Neural di ff erentiation of human Wharton’s jelly-derived mesenchymal stem cells improves the recovery of neurological function aft er transplantation in ischemic stroke rats
2017-08-07LeiZhangLinmeiWangWeiweiChenZhiMaXiaoHanChengmingLiuXiangChengWeiShiJingjingGuoJianbingQinXiaoqingYangGuohuaJinXinhuaZhang
Lei Zhang, Lin-mei Wang, Wei-wei Chen, Zhi Ma Xiao Han Cheng-ming Liu Xiang Cheng Wei Shi, Jing-jing Guo Jian-bing Qin Xiao-qing Yang, Guo-hua Jin, Xin-hua Zhang,
1 Department of Anatomy, Nantong University, Nantong, Jiangsu Province, China
2 Department of Radiation Oncology,ird People’s Hospital of Yancheng, Yancheng, Jiangsu Province, China
3 Department of Neurosurgery, the A ffi liated Hosptial of Nantong University, Nantong, Jiangsu Province, China
4 Department of Obstetrics and Gynecology, the A ffi liated Hosptial of Nantong University, Nantong, Jiangsu Province, China
5 Co-innovation Center of Neuroregeneration, Nantong University, Nantong, Jiangsu Province, China
Neural di ff erentiation of human Wharton’s jelly-derived mesenchymal stem cells improves the recovery of neurological function aft er transplantation in ischemic stroke rats
Lei Zhang1,#, Lin-mei Wang1,#, Wei-wei Chen2, Zhi Ma1, Xiao Han1, Cheng-ming Liu1, Xiang Cheng1, Wei Shi3, Jing-jing Guo1, Jian-bing Qin1, Xiao-qing Yang4, Guo-hua Jin1,5, Xin-hua Zhang1,5,*
1 Department of Anatomy, Nantong University, Nantong, Jiangsu Province, China
2 Department of Radiation Oncology,ird People’s Hospital of Yancheng, Yancheng, Jiangsu Province, China
3 Department of Neurosurgery, the A ffi liated Hosptial of Nantong University, Nantong, Jiangsu Province, China
4 Department of Obstetrics and Gynecology, the A ffi liated Hosptial of Nantong University, Nantong, Jiangsu Province, China
5 Co-innovation Center of Neuroregeneration, Nantong University, Nantong, Jiangsu Province, China
How to cite this article:Zhang L, Wang LM, Chen WW, Ma Z, Han X, Liu CM, Cheng X, Shi W, Guo JJ, Qin JB, Yang XQ, Jin GH, Zhang XH (2017) Neural di ff erentiation of human Wharton’s jelly-derived mesenchymal stem cells improves the recovery of neurological function aer transplantation in ischemic stroke rats. Neural Regen Res 12(7):1103-1110.
Graphical Abstract
#
orcid: 0000-0002-5702-6733 (Xin-hua Zhang)
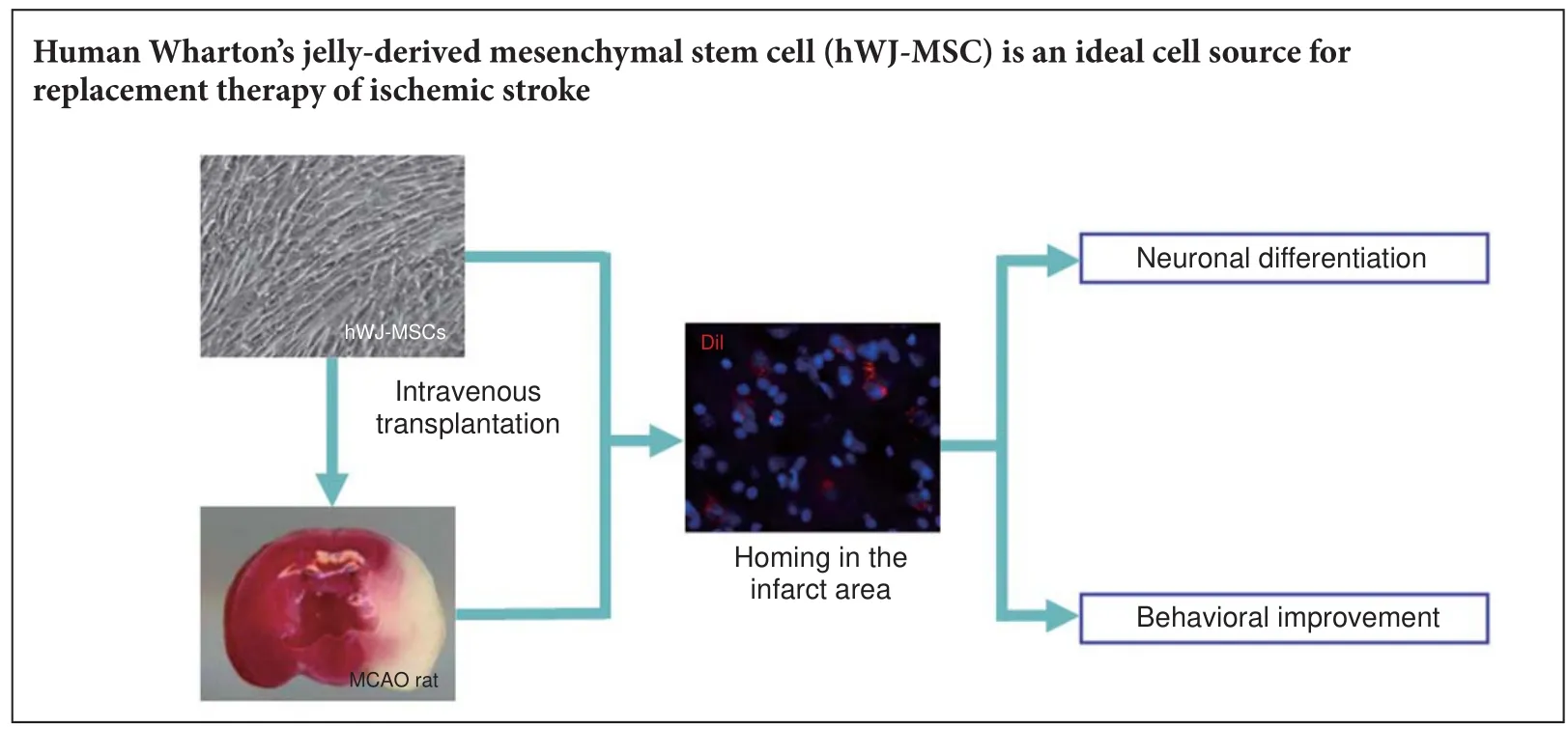
Human Wharton’s jelly-derived mesenchymal stem cells (hWJ-MSCs) have excellent proliferative ability, di ff erentiation ability, low immunogenicity, and can be easily obtained. However, there are few studies on their application in the treatment of ischemic stroke, therefore their therapeutic e ff ect requires further veri fi cation. In this study, hWJ-MSCs were transplanted into an ischemic stroke rat modelviathe tail vein 48 hours aer transient middle cerebral artery occlusion. Aer 4 weeks, neurological functions of the rats implanted with hWJMSCs were signi fi cantly recovered. Furthermore, many hWJ-MSCs homed to the ischemic frontal cortex whereby they di ff erentiated into neuron-like cells at this region.ese results con fi rm that hWJ-MSCs transplanted into the ischemic stroke rat can di ff erentiate into neuron-like cells to improve rat neurological function and behavior.
nerve regeneration; human Wharton’s jelly-derived mesenchymal stem cells; ischemic stroke; cell transplantation; middle cerebral artery occlusion; neural di ff erentiation; neurological function; neural regeneration
Introduction
Ischemic stroke is a primary cause of death and long-term disability and is of huge social and economic burden worldwide (Zhao et al., 2014; Auer et al., 2015; Kawle et al., 2015). Following ischemic stroke, penumbra apoptosis and core necrosis in the infarct region can be seen within minutes to days (Deshpande et al., 1987; Leist and Jaattela, 2001; Du et al., 2014). Neuronal death following ischemia is strongly linked to the interruption of blood supply to brain regions in which nutrients and oxygen cannot be delivered as a result of thrombus occlusion (Cui et al., 2012).
In the present study, we cultured hWJ-MSCsin vitroand transplanted them into a rat MCAO-invoked stroke model. We evaluated their survival and di ff erentiationin vivo, and their potential to improve behavioral de fi ciencies to provide evidence for using hWJ-MSCs as an ideal cell source for replacement therapy following ischemic stroke.
Materials and Methods
Animals
Forty-eight healthy adult female specific-pathogen-free Sprague-Dawley rats weighing 200–250 g and three pregnant Sprague-Dawley rats were provided by the Experimental Animal Center of Nantong University of China (license No. SYXK (Su) 2015-0031). All rats were caged in an approved animal facility with free access to food and water, and were kept in a temperature-controlled environment in a 12-hour light/dark cycle. All animal experiments were conducted in accordance with the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1996). The rats were randomly divided into sham group (sham operation;n= 12), saline group (MCAO + saline;n= 18) and transplantation group (MCAO + cell transplantation;n= 18).
Ischemic stroke model establishment by MCAO
2,3,5-Triphenyltetrazolium chloride (TTC) staining
At 24 hours after surgery, the rats were decollated and the brain was rapidly removed. Aer being frozen for 10 minutes at –20°C, brains were sectioned into 2 mm-thick slices using a vibratome, immersed for 15 minutes in 1.5% TTC solution at 4°C, then subjected to histological analysis and observed using a phase contrast microscope (Leica, Heidelberg, Germany).
Isolation of hWJ-MSCs
Preparation of hWJ-MSCs was approved by the Research Ethics Committee at the Affiliated Hospital of Nantong University of China. Informed consent was obtained from all pregnant women and their family members. Cells were isolated and cultured at Beike Biotechnology (Taizhou, Jiangsu Province, China). Fresh human umbilical cords were obtained from the A ffi liated Hospital of Nantong University of China, and stored in iced Hanks’ balanced salt solution(Gibco, Grand Island, NY, USA) at 4°C and processed within 2 hours aer birth. Aer being rinsed in 75% ethanol for 30 seconds, umbilical cords were cut into segments (2–3 cm long). Aerwards, the umbilical cord arteries and veins were gently removed to avoid contamination with endothelial cells.e mesenchymal tissue (Wharton’s jelly) was dissected into small pieces of approximately 0.5 cm3and transferred into a fl ask containing StemPro® MSC serum-free medium (Life Technologies, Invitrogen, Carlsbad, CA, USA) with antibiotics (penicillin 100 IU/mL, streptomycin 100 μg/mL; Invitrogen) and 10% fetal bovine serum (Invitrogen). The explants were cultured in a humidi fi ed 95% air 5% (v/v) CO2incubator at 37°C for 3–4 days without disturbance to allow migration from the explants. Following cell migration from the explants, the tissue masses were removed, and the media were half replaced by fresh media twice weekly. After approximately 2 weeks, the cells reached 90–100% con fl uence and were passaged. Cells at passage 3 were used experimentally or stored in liquid nitrogen for further use.
Identi fi cation of hWJ-MSCs
hWJ-MSCs at passage 3 were harvested and stained with the following antibodies: phycoerythrin-conjugated mouse anti-human CD34, CD73, CD105, and HLA-DR; allophycocyanin-conjugated mouse anti-human CD79a and CD90 (BD Pharmingen, San Diego, CA, USA). The isotype-matched immunoglobulins IgG1-phycoerythrin and IgG1-allophycocyanin were used as negative controls under the same conditions. All steps were performed according to the manufacturer’s instructions.e pro fi les of hWJ-MSCs were analyzed by fl ow cytometry (FACSCalibur, Becton, Dickinson and Company, Franklin Lakes, NJ, USA).
Identi fi cation of adipogenic and osteogenic di ff erentiation
hWJ-MSCs at passage 3 were used to detect adipogenic and osteogenic di ff erentiation potential using di ff erent media. Adipogenic di ff erentiation medium contained 1 μM dexamethasone, 0.5 mM 3-isobutyl-1-methylxanthine, 10 μg/mL insulin, 100 μM indomethacin, and 10% fetal bovine serum in DMEM/ F12. Osteogenic differentiation medium contained 0.1 μM dexamethasone solution, 0.2 mM ascorbic acid 2-phosphate solution, 10 mM glycerol 2-phosphate, and 10% fetal bovine serum in DMEM/F12. Cells at 100% confluence were incubated in these di ff erent media. Two weeks later, the cells were incubated with 0.375% oil red O (Sigma, St. Louis, MO, USA) or 0.5% Alizarin red S (Sigma) for 30 minutes to identify their potentiality to di ff erentiate into adipocytes and osteoblasts, respectively.e oil red O and Alizarin red S were removed and imaging was performed aer the cells were air-dried.
Transplantation of hWJ-MSCs
hWJ-MSCs at passage 3 were harvested and incubated with Cell Tracker CM-Dil (3 μM; Invitrogen) for 5 minutes at 37°C, and an additional 15 minutes at 4°C.e cells were washed in phosphate bu ff ered saline and fi ltered through a 100-μm fi lter. Cells were resuspended in saline and placed in an ice bath before cell transplantation. Subsequently, approximately 1 × 107cells in 200 μL cell suspension were injected into the MCAO model rat through the tail vein 2 days after surgery (Figure 2A). An identical volume (200 μL) of saline was given to the saline group through the tail vein 2 days aer surgery.
Behavioral studies
Functional behavior following transplantation was monitored in all groups as the schedule shown in Figure 2A. All behavioral tests were performed by an experimenter who was blinded to the experimental protocol.
Longa scoring
According to the 5-grade scoring standard of Longa et al. (1989), all rats were evaluated at 6, 72 hours, 7, 20 and 30 days aer cell transplantation.e scoring criteria were as follows: 0 = Normal, no neurological function defect; 1 = forelimb fl exion; 2 = unidirectional circling; 3 = falling to the contralateral side; 4 = decreased level or lack of consciousness.
Rotarod test
Morris water maze test
The learning and memory abilities of the rats were assessed using the Morris water maze test. The Morris water maze test (de Bruin et al., 1997) was performed at 30 days aer cell transplantation.e rats learned to locate a circular platform at a fi xed location every day (5 trials per day). In each trial, the rat was placed into the water at one of three designated startpoints on the wall of the tank. Escape latency (time to fi nd the platform) and time in each quadrant were measured using an auto-tracking system. If rats were unable to fi nd the platform within 120 seconds, the escape latency was recorded as 120 seconds.e average escape latency and time in each quadrant of fi ve tests per day was used for statistical analysis.
Immuno fl uorescence staining
At 35 days after hWJ-MSCs transplantation, the rats were sacri fi ced and the brains were collected and fi xed in 4% formalin.e coronal sections (15 μm thickness) through the area of ischemia were prepared using a cryostat (CM1900; Leica, Heidelberg, Germany).e sections were blocked in 10% goat serum in phosphate-buffered saline/Tween (0.01 M sodium phosphate bu ff er, pH 7.4, 0.05% Tween 20) for 1 hour at room temperature, and incubated with the primary antibody diluted in blocking buffer at 4°C for 24 hours, followed by incubation with secondary antibody overnight at 4°C. Immuno fl uorescence signals were observed under a fl uorescence microscope (DMIRB; Leica). Primary antibodies were as follows: guinea pig anti-doublecortin (1:1,000; Millipore, Boston, MA, USA), mouse anti-microtubule-associated protein 2 (MAP2) (1:1,000; Millipore) or mouse anti-Tuj1 (1:400; Sigma). Secondary antibodies were as follows:Alexa fl uor 488-conjugated goat anti-guinea pig IgG (1:1,000; Invitrogen, Carlsbad, CA, USA) and FITC-conjugated goat anti-mouse IgG (1:1,000; Millipore). Aer that, sections were counterstained with Hoechst (1:1,000) to indicate cell nuclei.

Figure 1 Model establishment and con fi rmation of ischemic stroke.
Statistical analysis
Statistical analysis was performed using GraphPad Prism v4.0 soware (GraphPad Soware, San Diego, CA, USA). Data are expressed as the mean ± standard error of the mean (SEM). The differences between groups were analyzed using the unpairedt-test.P< 0.05 was considered statistically signi fi cant.
Results
Infarct area in rat models of ischemic stroke
Culture and identi fi cation of hWJ-MSCs
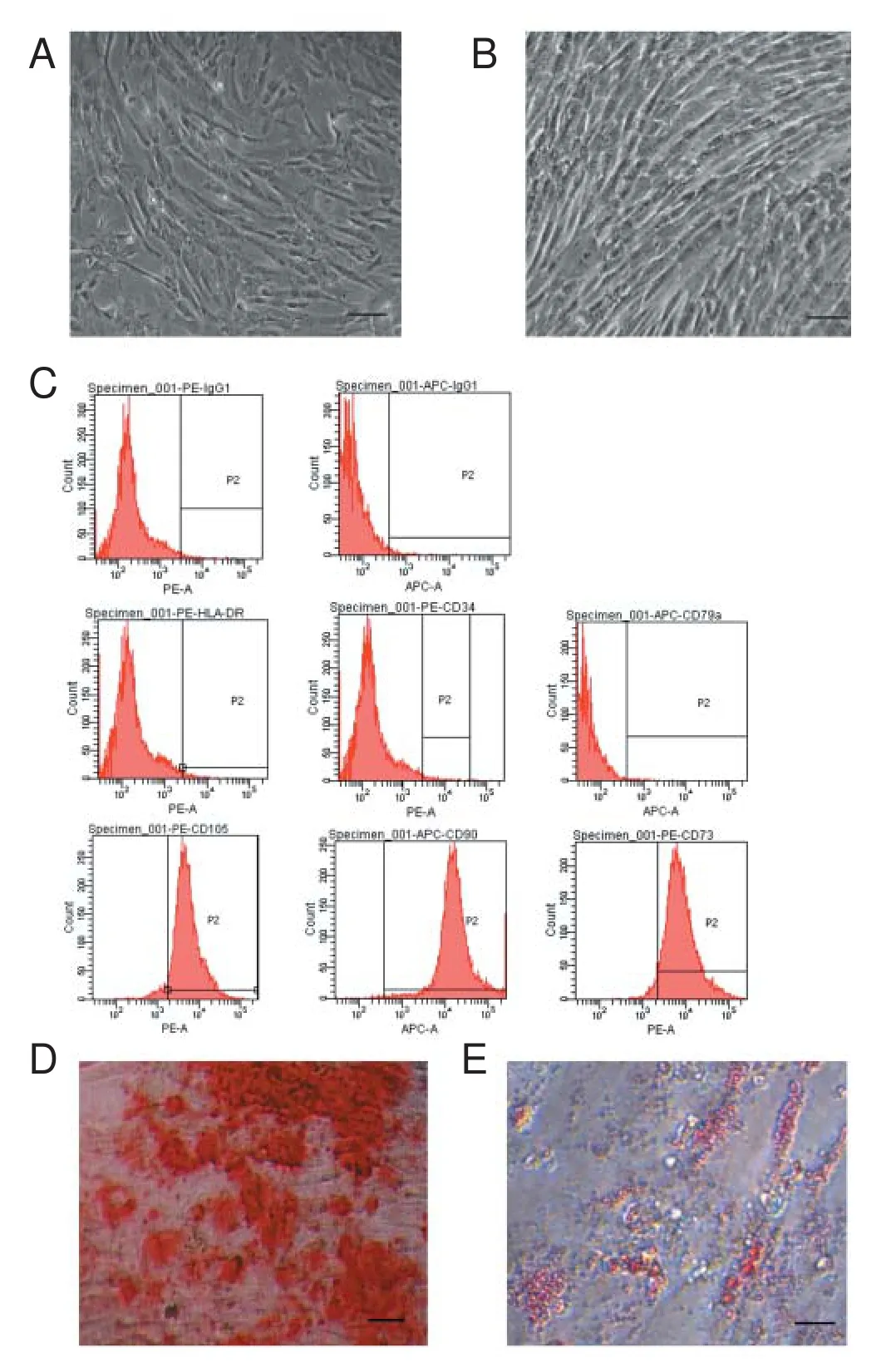
Figure 3 Identi fi cation of mesenchymal stem cells from human Wharton’s jellyin vitro.
On day 4 after primary culture, cells grew as a monolayer and exhibited a shape with a fl at and polygonal morphology (Figure 3A). When cells at passage 3 reached approximately 90% con fl uence, they mostly presented spindle-shaped and were arranged in a whirlpool pattern (Figure 3B). Flow cytometric analysis demonstrated that the third passage cells were positive for MSC markers CD73, CD90, and CD105, and negative for the hematopoietic and endothelial markers CD34, CD79a, and HLA-DR (Figure 3C).
To investigate multipotential di ff erentiation of the cultured cells, osteogenic and adipogenic di ff erentiation experiments were carried out. Following treatment with standard osteogenic and adipogenic differentiation media, most adherent cells were positive for Alizarin red S (Figure 3D) and Oil red O (Figure 3E) staining, respectively. These results demonstrated that the cells had the phenotype and di ff erentiation characteristics of MSCs.

Figure 4 Transplanted hWJ-MSCs in the infarct and corresponding areas of the ischemic stroke model rat.
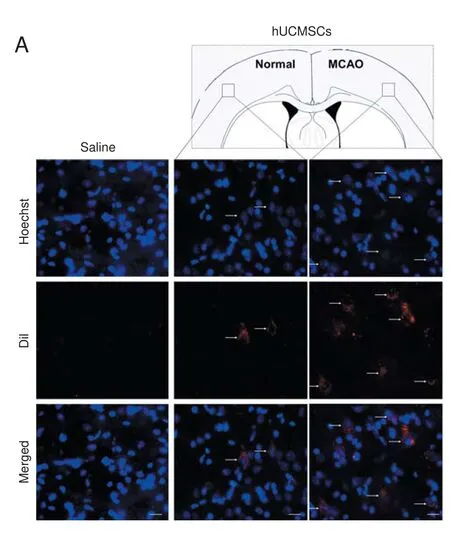

Figure 5 Detection of neuronal di ff erentiation of the transplanted CM-Dil-labeled hWJ-MSCs in the cortex of the ischemic stroke model rat.
Behavioral improvement of the ischemic stroke model rat aer hWJ-MSC transplantation
Behavioral tests were performed timely as exhibited in Figure 2A. Longa scoring results showed that there was no signi fi cant di ff erence between the transplantation group and the saline group at 6 and 72 hours aer cell transplantation. However, at 7, 20 and 30 days after transplantation, the Longa scores of rats in the transplantation group were signi fi cantly lower than those in the saline group at the corresponding time points (P< 0.05), although they did not reach the levels of the sham rats (P> 0.05) (Figure 2B).

Figure 2 Behavioral improvement of MCAO rats aer hWJ-MSC transplantation.
In the rotarod test, rats were trained for 3 consecutive days before MCAO and evaluated at 20 days aer cell transplantation. Results showed that the rotarod latency in the transplantation group was longer than that in the saline group (P< 0.01; Figure 2C).
The Morris water maze test results indicated that rats transplanted with hWJ-MSCs showed a decrease in escape latency compared with the saline group (Figure 2D) and the time they stayed in the target quadrant also increased signi fi -cantly (P< 0.01; Figure 2E).
Transplanted hWJ-MSC location and survival in the infarct area of the ischemic stroke model rat
To verify whether transplanted hWJ-MSCs could reach the infarct area and survive, we performed a tracing experiment.e transplanted hWJ-MSCs were labeled with CMDil before tail vein injection. Immuno fl uorescence staining results showed that the number of hWJ-MSCs in the infarct area was greater than that in the corresponding area of the controlateral side in the transplantation group (P< 0.01). In the saline group, there were no hWJ-MSCs detected on the MCAO side (Figure 4).
In vivoneuronal di ff erentiation of transplanted hWJ-MSCs in the ischemic stroke model rat
At 35 days post-transplantation, immuno fl uorescence staining results showed that approximately 25.17 ± 1.2%, 18.13 ± 0.57% and 12.36 ± 1.39% of the implanted cells expressed the neuronal markers doublecortin, Tuj1 and MAP2 (Ng et al., 2012; Castaño et al., 2014), respectively, in the infarct area (Figure 5A−C). Quanti fi cation indicated that the numbers of doublecortin-, Tuj1- and MAP2-positive cells labeled with CM-Dil were greater in the MCAO region compared with the normal brain (P< 0.05) (Figure 5D). However, neuronal differentiation was seldom observed in the corresponding area on the normal side.
Discussion
The MSC is an important member of the stem cell family, characterized by strong self-renewal and multi-differentiation potentials. Romanov et al. (2003) successfully isolated and cultured human MSCs from umbilical cord vasculature and Wharton’s jelly, and verified that these cells contain MSC-like properties, so named them hWJ-MSCs. Since then, more studies have indicated that hWJ-MSCs can differentiate into neurons under specific conditions (Mitchell et al., 2003; Balasubramanian et al., 2013). hWJ-MSCs are in abundant supply and easy to obtain with no ethical limits. Furthermore, hWJ-MSCs possess almost all characteristics of MSCs and have minimal immunogenicity, which is bene fi cial for their long-term survival in the host brain.erefore, hWJ-MSCs are expected to become a promising source for treatment of neurodegenerative diseases (Porada et al., 2006; Noël et al., 2007).
In this study, hWJ-MSCs were isolated from Wharton’s jelly of human umbilical cord and expandedin vitro. Flow cytometric analysis demonstrated that these cells had char-acteristics of MSCs, with positive expression of the markers CD105, CD73 and CD90, and negative expression of CD34, HLA-DR and CD79a. CD105, CD73 and CD90 are not ‘speci fi c’ to MSCs, but their expression pro fi le helps to identify them. Intravenous transplantation was deemed to be a suitable method (Chen et al., 2001; Doeppner and Hermann, 2014; Zhang et al., 2014). In this study, the cells were transplanted into rat MCAO modelsviathe tail vein. A comparative study indicates that the CM-DiI cell tracker is much less diffuse than other standard Dil analogues (Daubeuf et al., 2009), and was therefore used to label the cells prior to transplantation (Qiao et al., 2015). We observed that hWJMSC transplantation signi fi cantly improved the neurological function of MCAO rats compared with the saline control at 7 days after transplantation. Furthermore, we found more implanted hWJ-MSCs in the infarct region than the normal side, and some cells in the infarct area differentiated into neurons at 35 days aer transplantation. Furthermore, neurological damages in behavior were also partially alleviated. These results indicate that exogenous hWJ-MSCs injectedviathe tail vein can migrate into the infarct area of the MCAO rat, survive and even di ff erentiate into neurons to partially rescue the damaged motor function. However, the mechanisms by which the implanted hWJ-MSCs improved neurological behavior of the MCAO models remain unclear. Some researchers consider that MSCs transplanted into the MCAO rat can differentiate into mature neurons, which can form a local neural circuit with the host nervous cells and replace the damaged neurons to some extent (Kim et al., 2008). Other researchers speculate that the transplanted MSCs promote endogenous neural stem cell proliferation (Yoo et al., 2008), which is partially responsible for the recovery of neurological function. Xin et al. (2013) reported that aer injecting MSCs through the tail vein, expression of transforming growth factor β-1 decreased in microglia and macrophages of MCAO rats and the inhibitor of plasminogen activator was also reduced, resulting in the activation of plasminogen activator and matrix metalloproteinase (Adibhatla and Hatcher, 2008). Subsequently, proliferation of astrocytes was reduced, and migration and neurite extension of neurons was promoted (Hosomi et al., 2001).erefore, the effects on the regulation of glial cells may play an important role in the treatment of MCAO rats with hWJ-MSC transplantation. In addition, we observed that the neurological function of MCAO rats in the saline group could be partially recovered with prolonged time.
Orito et al. (2010) reported that cerebrospinal fluid extracted from rats 15 minutes after MCAO could promote proliferation of BMSCsin vitro. Yang et al. (2010) injected BMSCs into MCAO rats through the tail vein at 1 day aer surgery and found that some factors, such as interleukin 13, vascular endothelial growth factor and nerve growth factor receptor, tended to be up-regulated and e ffi ciently promoted the recovery of neurological function. All of these studies indicate that in a certain period of time after MCAO, the internal environment of rats and the exogenous MSCs can form a complementary relationship. In the present study, we found that the transplanted hWJ-MSCs were more dynamic in migration into the infarct area than to the corresponding area on the normal side.is prompted that MCAO injury may stimulate the model animal to secrete factors to bene fi t migration, survival and proliferation of the transplanted hWJ-MSCs, and that these cells may continuously stimulate the host to secrete factors for the improvement of neurological function.e factors need to be studied further in future.
In summary, exogenous hWJ-MSCs transplanted into MCAO rats through tail vein injection can locate and survive in the infarct area. Furthermore, some of these cells di ff erentiate into mature neurons and lead to signi fi cant recovery of the function of the MCAO rats.ese fi ndings suggest that hWJMSC transplantation has signi fi cant potential for clinical application in the treatment of ischemic stroke.
Author contributions:XHZ conceived the study. XHZ and LZ wrote the paper. XHZ and GHJ polished the paper. LZ, LMW and XC performed most experiments with the help of ZM, XH, CML, XC, WS, JBQ and XQY. All authors approved the fi nal version of the paper.
Con fl icts of interest:None declared.
Research ethics:
Open access statement:
Contributor agreement:A statement of “Publishing Agreement” has been signed by an authorized author on behalf of all authors prior to publication.Plagiarism check:This paper has been checked twice with duplication-checking soware ienticate.
Peer review:A double-blind and stringent peer review process has been performed to ensure the integrity, quality and signi fi cance of this paper.
Adibhatla RM, Hatcher JF (2008) Tissue plasminogen activator (tPA) and matrix metalloproteinases in the pathogenesis of stroke: therapeutic strategies. CNS Neurol Disord Drug Targets 7:243-253.
Ali H, Al-Yatama MK, Abu-Farha M, Behbehani K, Al Madhoun A (2015) Multi-lineage differentiation of human umbilical cord Wharton’s Jelly Mesenchymal Stromal Cells mediates changes in the expression profile of stemness markers. PLoS One 10:e0122465.
Auer PL, Nalls M, Meschia JF, Worrall BB, Longstreth WT, Seshadri S, Kooperberg C, Burger KM, Carlson CS, Carty CL, Chen W-M, Cupples LA, DeStefano AL, Fornage M, Hardy J, Hsu L, Jackson RD, Jarvik GP, Kim DS, Lakshminarayan K, et al. (2015) Rare and Coding Region Genetic Variants Associated With Risk of Ischemic Stroke:e NHLBI Exome Sequence Project. JAMA Neurol 72:781-788.
Borhani-Haghighi M, Talaei-Khozani T, Ayatollahi M, Vojdani Z (2015) Wharton’s Jelly-derived mesenchymal stem cells can differentiate into hepatocyte-like cells by HepG2 cell line extract. Iran J Med Sci 40:143-151.
Castaño J, Menendez P, Bruzos-Cidon C, Straccia M, Sousa A, Zabaleta L, Vazquez N, Zubiarrain A, Sonntag KC, Ugedo L, Carvajal-Vergara X, Canals Josep M, Torrecilla M, Sanchez-Pernaute R, Giorgetti A (2014) Fast and e ffi cient neural conversion of human hematopoietic cells. Stem Cell Reports 3:1118-1131.
Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, Chopp M (2001)erapeutic bene fi t of intravenous administration of bone marrow stromal cells aer cerebral ischemia in rats. Stroke 32:1005-1011.
Cheng Y, Zhang J, Deng L, Johnson NR, Yu X, Zhang N, Lou T, Zhang Y, Wei X, Chen Z, He S, Li X, Xiao J (2015) Intravenously delivered neural stem cells migrate into ischemic brain, di ff erentiate and improve functional recovery after transient ischemic stroke in adult rats. Int J Clin Exp Pathol 8:2928-2936.
Cui J, Chen S, Zhang C, Meng F, Wu W, Hu R, Hadass O, Lehmidi T, Blair GJ, Lee M, Chang M, Mobashery S, Sun GY, Gu Z (2012) Inhibition of MMP-9 by a selective gelatinase inhibitor protects neurovasculature from embolic focal cerebral ischemia. Mol Neurodegener 7:21.
Daubeuf S, Bordier C, Hudrisier D, Joly E (2009) Suitability of various membrane lipophilic probes for the detection of trogocytosis by fl ow cytometry. Cytometry A 75:380-389.
de Bruin JP, Swinkels WA, de Brabander JM (1997) Response learning of rats in a Morris water maze: involvement of the medical prefrontal cortex. Behav Brain Res 85:47-55.
Deshpande JK, Siesjo BK, Wieloch T (1987) Calcium accumulation and neuronal damage in the rat hippocampus following cerebral ischemia. J Cereb Blood Flow Metab 7:89-95.
Dharmasaroja P (2009) Bone marrow-derived mesenchymal stem cells for the treatment of ischemic stroke. J Clin Neurosci 16:12-20.
Doeppner TR, Hermann DM (2014) Stem cell-based treatments against stroke: observations from human proof-of-concept studies and considerations regarding clinical applicability. Front Cell Neurosci 8:357.
Du S, Guan J, Mao G, Liu Y, Ma S, Bao X, Gao J, Feng M, Li G, Ma W, Yang Y, Zhao RC, Wang R (2014) Intra-arterial delivery of human bone marrow mesenchymal stem cells is a safe and e ff ective way to treat cerebral ischemia in rats. Cell Transplant 23 Suppl 1:S73-82.
Goel RK, Kaur D, Pahwa P (2016) Assessment of anxiolytic effect of nerolidol in mice. Indian J Pharmacol 48:450-452.
Goldmacher GV, Nasser R, Lee DY, Yigit S, Rosenwasser R, Iacovitti L (2013) Tracking transplanted bone marrow stem cells and their effects in the rat MCAO stroke model. PLoS One 8:e60049.
Goodwin HS, Bicknese AR, Chien SN, Bogucki BD, Quinn CO, Wall DA (2001) Multilineage di ff erentiation activity by cells isolated from umbilical cord blood: expression of bone, fat, and neural markers. Biol Blood Marrow Transplant 7:581-588.
Hosomi N, Lucero J, Heo JH, Koziol JA, Copeland BR, del Zoppo GJ (2001) Rapid di ff erential endogenous plasminogen activator expression aer acute middle cerebral artery occlusion. Stroke 32:1341-1348.
Jensen MB, Yan H, Krishnaney-Davison R, Al Sawaf A, Zhang SC (2013) Survival and di ff erentiation of transplanted neural stem cells derived from human induced pluripotent stem cells in a rat stroke model. J Stroke Cerebrovasc Dis 22:304-308.
Kakinuma S, Tanaka Y, Chinzei R, Watanabe M, Shimizu-Saito K, Hara Y, Teramoto K, Arii S, Sato C, Takase K, Yasumizu T, Teraoka H (2003) Human umbilical cord blood as a source of transplantable hepatic progenitor cells. Stem Cells 21:217-227.
Kawle AP, Nayak AR, Lande NH, Kabra DP, Chandak NH, Badar SR, Raje DV, Taori GM, Daginawala HF, Kashyap RS (2015) Comparative evaluation of risk factors, outcome and biomarker levels in young and old acute ischemic stroke patients. Ann Neurosci 22:70-77.
Kim SS, Yoo SW, Park TS, Ahn SC, Jeong HS, Kim JW, Chang DY, Cho KG, Kim SU, Huh Y, Lee JE, Lee SY, Lee YD, Suh-Kim H (2008) Neural induction with neurogenin1 increases the therapeutic e ff ects of mesenchymal stem cells in the ischemic brain. Stem Cells 26:2217-2228.
Leist M, Jaattela M (2001) Four deaths and a funeral: from caspases to alternative mechanisms. Nat Rev Mol Cell Biol 2:589-598.
Li D, Zhang M, Zhang Q, Wang Y, Song X, Zhang Q (2015) Functional recovery aer acute intravenous administration of human umbilical cord mesenchymal stem cells in rats with cerebral ischemia-reperfusion injury. Intractable Rare Dis Res 4:98-104.
Longa EZ, Weinstein PR, Carlson S, Cummins R (1989) Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 20:84-91.
Mitchell KE, Weiss ML, Mitchell BM, Martin P, Davis D, Morales L, Helwig B, Beerenstrauch M, Abou-Easa K, Hildreth T, Troyer D, Medicetty S (2003) Matrix cells from Wharton’s jelly form neurons and glia. Stem Cells 21:50-60.
Ng SY, Johnson R, Stanton LW (2012) Human long non-coding RNAs promote pluripotency and neuronal di ff erentiation by association with chromatin modi fi ers and transcription factors. EMBO J 31:522-533.
Noël D, Djouad F, Bou ffi C, Mrugala D, Jorgensen C (2007) Multipotent mesenchymal stromal cells and immune tolerance. Leuk Lymphoma 48:1283-1289.
Orito K, Harada H, Hara M, Yamashita S, Kikuchi K, Shigemori M (2010) Cerebrospinal fl uid following cerebral ischemia accelerates the proliferation of bone marrow stromal cells in vitro. Kurume Med J 57:21-28.
Pelizzo G, Avanzini MA, Icaro Cornaglia A, Osti M, Romano P, Avolio L, Maccario R, Dominici M, De Silvestri A, Andreatta E, Costanzo F, Mantelli M, Ingo D, Piccinno S, Calcaterra V (2015) Mesenchymal stromal cells for cutaneous wound healing in a rabbit model: pre-clinical study applicable in the pediatric surgical setting. J Transl Med 13:219.
Porada CD, Zanjani ED, Almeida-Porad G (2006) Adult mesenchymal stem cells: a pluripotent population with multiple applications. Curr Stem Cell Reser 1:365-369.
Qiao PF, Yao L, Zhang XC, Li GD, Wu DQ (2015) Heat shock pretreatment improves stem cell repair following ischemia-reperfusion injury via autophagy. World J Gastroenterol 21:12822-12834.
Rao MS, Mattson MP (2001) Stem cells and aging: expanding the possibilities. Mech Ageing Dev 122:713-734.
Romanov YA, Svintsitskaya VA, Smirnov VN (2003) Searching for alternative sources of postnatal human mesenchymal stem cells: candidate MSC-like cells from umbilical cord. Stem Cells 21:105-110.
Subramanian A, Fong CY, Biswas A, Bongso A (2015) Comparative characterization of cells from the various compartments of the human umbilical cord shows that the Wharton’s jelly compartment provides the best source of clinically utilizable mesenchymal stem cells. PLoS One 10:e0127992.
Tao J, Chen B, Gao Y, Yang S, Huang J, Jiang X, Wu Y, Peng J, Hong Z, Chen L (2014) Electroacupuncture enhances hippocampal NSCs proliferation in cerebral ischemia-reperfusion injured rats via activation of notch signaling pathway. Int J Neurosci 124:204-212.
Toyoshima A, Yasuhara T, Kameda M, Morimoto J, Takeuchi H, Wang F, Sasaki T, Sasada S, Shinko A, Wakamori T, Okazaki M, Kondo A, Agari T, Borlongan CV, Date I (2015) Intra-arterial transplantation of allogeneic mesenchymal stem cells mounts neuroprotective e ff ects in a transient ischemic stroke model in rats: analyses of therapeutic time window and its mechanisms. PLoS One 10:e0127302.
Xin H, Chopp M, Shen LH, Zhang RL, Zhang L, Zhang ZG, Li Y (2013) Multipotent mesenchymal stromal cells decrease transforming growth factor β1 expression in microglia/macrophages and down-regulate plasminogen activator inhibitor 1 expression in astrocytes aer stroke. Neurosci Lett 542:81-86.
Xue X, Liu Y, Zhang J, Liu T, Yang Z, Wang H (2015) Bcl-xL genetic modi fi cation enhanced the therapeutic e ffi cacy of mesenchymal stem cell transplantation in the treatment of heart infarction. Stem Cells Int 2015:176409.
Yang C, Liu H, Liu D (2014) Mutant hypoxia-inducible factor 1alpha modi fi ed bone marrow mesenchymal stem cells ameliorate cerebral ischemia. Int J Mol Med 34:1622-1628.
Yang M, Wei X, Li J, Heine LA, Rosenwasser R, Iacovitti L (2010) Changes in host blood factors and brain glia accompanying the functional recovery aer systemic administration of bone marrow stem cells in ischemic stroke rats. Cell Transplant 19:1073-1084.
Yoo SW, Kim SS, Lee SY, Lee HS, Kim HS, Lee YD, Suh-Kim H (2008) Mesenchymal stem cells promote proliferation of endogenous neural stem cells and survival of newborn cells in a rat stroke model. Exp Mol Med 40:387-397.
Zhang X, Zhang Q, Li W, Nie D, Chen W, Xu C, Yi X, Shi J, Tian M, Qin J, Jin G, Tu W (2014)erapeutic e ff ect of human umbilical cord mesenchymal stem cells on neonatal rat hypoxic-ischemic encephalopathy. J Neurosci Res 92:35-45.
Zhao J, Zhang X, Dong L, Wen Y, Cui L (2014) The many roles of statins in ischemic stroke. Curr Neuropharmacol 12:564-574.
Copyedited by Yu J, Li CH, Qiu Y, Song LP, Zhao M
10.4103/1673-5374.211189
Accepted: 2017-06-10
*Correspondence to: Xin-hua Zhang, Ph.D., zhangxinhua@ntu.edu.cn.
杂志排行
中国神经再生研究(英文版)的其它文章
- SoxC transcription factors in retinal development and regeneration
- Umbilical cord: an unlimited source of cells di ff erentiable towards dopaminergic neurons
- Targeting 14-3-3 adaptor protein-protein interactions to stimulate central nervous system repair
- RACK1 regulates neural development
- Schwann cell development, maturation and regeneration: a focus on classic and emerging intracellular signaling pathways
- BDNF pro-peptide: a novel synaptic modulator generated as an N-terminal fragment from the BDNF precursor by proteolytic processing
