Putative adult neurogenesis in two domestic pigeon breeds (Columba livia domestica): racing homer versus utility carneau pigeons
2017-08-07PedzisaiMazengenyaAdhilBhagwandinPilaniNkomozepiPaulMangerAmadiIhunwo
Pedzisai Mazengenya, Adhil Bhagwandin, Pilani Nkomozepi, Paul R. Manger, Amadi O. Ihunwo
School of Anatomical Sciences, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
Putative adult neurogenesis in two domestic pigeon breeds (Columba livia domestica): racing homer versus utility carneau pigeons
Pedzisai Mazengenya, Adhil Bhagwandin, Pilani Nkomozepi, Paul R. Manger, Amadi O. Ihunwo*
School of Anatomical Sciences, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
How to cite this article:Mazengenya P, Bhagwandin A, Nkomozepi P, Manger PR, Ihunwo AO (2017) Putative adult neurogenesis in two domestic pigeon breeds (Columba livia domestica): racing homer versus utility carneau pigeons. Neural Regen Res 12(7):1086-1096.
Generation of neurons in the brains of adult birds has been studied extensively in the telencephalon of song birds and few studies are reported on the distribution of PCNA and DCX in the telencephalon of adult non-song learning birds. We report here on adult neurogenesis throughout the brains of two breeds of adult domestic pigeons (Columba livia domestica), the racing homer and utility carneau using endogenous immunohistochemical markers proliferating cell nuclear antigen (PCNA) for proliferating cells and doublecortin (DCX) for immature and migrating neurons. The distribution of PCNA and DCX immunoreactivity was very similar in both pigeon breeds with only a few minor di ff erences. In both pigeons, PCNA and DCX immunoreactivity was observed in the olfactory bulbs, walls of the lateral ventricle, telencephalic subdivisions of the pallium and subpallium, diencephalon, mesencephalon and cerebellum. Generally, the olfactory bulbs and telencephalon had more PCNA and DCX cells than other regions. Two proliferative hotspots were evident in the dorsal and ventral poles of the lateral ventricles. PCNA- and DCX-immunoreactive cells migrated radially from the walls of the lateral ventricle into the parenchyma. In most telencephalic regions, the density of PCNA- and DCX-immunoreactive cells increased from rostral to caudal, except in the mesopallium where the density decreased from rostral to middle levels and then increased caudally. DCX immunoreactivity was more intense in fi bres than in cell bodies and DCX-immunoreactive cells included small granular cells, fusiform bipolar cells, large round and or polygonal multipolar cells.e similarity in the distribution of proliferating cells and new neurons in the telencephalon of the two breeds of pigeons may suggest that adult neurogenesis is a conserved trait as an ecological adaptation irrespective of body size.
nerve regeneration; proliferating cell nuclear antigen; doublecortin; immunohistochemistry; avian brain; racing homer; utility carneau; brain evolution; neural regeneration
Accepted: 2017-07-03
Introduction
The process of adult neurogenesis occurs in both invertebrates and vertebrates, including humans (Eriksson et al., 1998; Bartkowska et al., 2010; Barnea and Pravosudov, 2011). In birds, generation of new neurons is limited to the dorsal and ventral reaches of the subventricular zone of the lateral ventricles (Alvarez-Buylla and Nottebohm, 1988; Alvarez-Buylla et al., 1998). Along these areas of the subventricular zone, proliferating cells form aggregates referred to as proliferative hotspots (Alvarez-Buylla et al., 1990a). From the walls of the lateral ventricles, new neurons migrate to various areas of the telencephalon which includes, but are not limited to, the high vocal centre, area X, the nidopallium caudale in song birds, and the hippocampus in both nonsong birds and song birds (Paton and Nottebohm, 1984; Nottebohm, 1985; Alvarez-Buylla et al., 1994; Lipkind et al., 2002; Sherry and Hoshooley, 2010; Melleu et al., 2013).
In adult song birds, such as canaries, and food caching birds, such as the black capped chickadees, seasonal variations in the recruitment of new neurons in the high vocal centre and hippocampus, respectively, have been observed (Kirn and Nottebohm, 1993; Barnea and Nottebohm, 1994).ere is evidence that adult neurogenesis varies in the members of the same species from di ff erent populations, for example in black capped chickadees (Chancellor et al., 2011) and mice (Kempermann et al., 1997).
Adult neurogenesis has been reported in the telencephalon of song birds including canaries (Alvarez-Buylla and Nottebohm, 1988; Balthazart et al., 2008) and zebra fi nches (Kim et al., 2006) and non-song birds such as chickens (Mezey et al., 2012), black capped chickadees (Sherry and Hoshooley, 2010), Japanese quails (Balthazart et al., 2010), ring doves (Ling et al., 1997) and rock pigeons (Melleu et al., 2013, 2016). Domestic pigeons (Columba livia domestica) of the order Columbiformes are descendants of the wild rock pigeon through domestication and selective breeding (Levi, 1986; Stringham et al., 2012).e racing homer possesses characteristics such as increased flight speed, large home ranges, improved spatial memory, and larger hippocampal formations and olfactory bulbs than other pigeon breeds (Bingman et al., 2006; Rehkämper et al., 2008; Mehlhorn and Rehkämper, 2009). Utility pigeons were selectively bred for their fast growth and large body size desirable for meat production. These different characteristics in the two breeds of domestic pigeons allowexamining and comparing the process of adult neurogenesis in closely related species with di ff erent behaviour repertoires.
Examination of multiple species with phylogenetically diverse traits and also in closely related species, or breeds, in different ecological niches may facilitate our understanding of the functions of adult neurogenesis and the factors contributing to variances in this neural trait amongst species (Jarvis et al., 2005; Amrein and Lipp, 2009; Bartkowska et al., 2010; Ihunwo and Olaleye, 2014). Based on this premise, we examined putative adult neurogenesis in the brains of two breeds of the adult domestic pigeon (Columba livia domestica), the racing homer pigeons and the utility carneau pigeons using the markers proliferating nuclear cell antigen (PCNA) and doublecortin (DCX) which label proliferating cells and immature neurones respectively (Hall et al., 1990; Brown et al., 2003; Melleu et al., 2013).
Materials and Methods
Animals and tissue processing
Four adult male domestic pigeon brains, two of each of racing homer and utility carneau pigeons were purchased from a local breeder and used in this study. The animals were bred in isolated cages according to the breeds in large social groups consisting of both males and females. The animals were supplied with water and foodad libitum.
Five minutes prior to being euthanized, all animals were given an intramuscular dose of heparin (0.5 mL) to prevent blood clotting. Animals were euthanized with an intraperitoneal injection of Euthapent (0.5–1 mL/kg) and the average body mass was 316.00 ± 23.33 g for racing homer pigeon and 542.35 ± 7.00 g for utility carneau pigeons. Animals were transcardially perfusion- fi xed, initially with a rinse of 0.9% saline solution, followed by 4% paraformaldehyde in 0.1 M phosphate bu ff er (PB; pH 7.4) solution. Brains were carefully removed from the skull, post fixed overnight in 4% paraformaldehyde in 0.1 M PB.e brains were then weighed 2.20 ± 0.00 g for racing homer pigeons and 2.80 ± 0.14 g for utility carneau pigeons, cryopreserved in 30% sucrose in 0.1 M PB at 4°C for 3 days and then stored in an antifreeze solution at –20°C. Before sectioning, the tissue was allowed to equilibrate in 30% sucrose in 0.1 M PB at 4°C.e brains were frozen in dry ice and sectioned along the coronal plane into 50 μm thick sections, on a sliding microtome (Microm HM 430, Thermo Scientific, Schaumburg, IL, USA). One of three series of sections from each animal were taken and stained for Nissl substance, PCNA and DCX.
Nissl staining for cytoarchitecture
Immunohistochemistry for PCNA and DCX
The second and third series of sections from each animal were used for free fl oating PCNA and DCX immunohistochemistry. The sections were incubated in a solution containing 1.6% H2O2, 49.2% methanol and 49.2% 0.1 M PB, for 30 minutes to reduce endogenous peroxidase activity, which was followed by three 10-minute rinses in 0.1 M PB. To block unspecific binding, the sections were then pre-incubated for 2 hours, at room temperature under gentle shaking, in a blocking bu ff er solution consisting of 3% normal horse serum (for PCNA sections) or 3% normal rabbit serum (for DCX sections), 2% bovine serum albumin, and 0.25% Triton X-100 in 0.1 M PB. Following pre-incubation, the primary antibodies were added to the blocking buffer solution and the sections were incubated for 48 hours at 4°C under gentle shaking (PCNA, 1:500 dilution of mouse anti-PCNA, NCLL-PCNA Leica Biosystems, Newcastle, United Kingdom; DCX, 1:300 dilution of goat anti-DCX antibody, C-18, Santa Cruz Biotechnology, Dallas, TX, USA) under gentle agitation.e primary antibody incubation was followed by three 10-minute rinses in 0.1 M PB and the sections were then incubated in a secondary antibody solution [PCNA sections, 1:1,000 dilution of biotinylated anti-mouse IgG (BA-2001, Vector Laboratories, Burlingame, CA, USA)] in 3% normal horse serum and 2% bovine serum albumin in 0.1 M PB; DCX sections, 1:1,000 dilution of anti-goat IgG (BA-5000, Vector Laboratories) in 3% normal rabbit serum and 2% bovine serum albumin in 0.1 M PB) for 2 hours at room temperature.is was followed by three 10-minute rinses in 0.1 M PB, aer which sections were incubated for 1 hour in an avidinbiotin solution (1:125 in 0.1 M PB); Vector Laboratories), followed by three 10-minute rinses in 0.1 M PB. Sections were then transferred to a solution consisting of 0.05% diaminobenzidinete trahydrochloride in 0.1 M PBs for 5 minutes at room temperature, after which 3.3 μL 30% H2O2/mL solution was added. With the aid of a low power objective lens on a stereomicroscope (Leica MZ 7.5, Meyer instruments, Houston, TX, USA), the progression of the staining was visually followed and allowed to continue until a level was reached where the background staining could assist in analysis by matching to architectonic borders from Nissl stained sections without obscuring the immunopositive structures. The tissue was then rinsed twice more in 0.1 M PB before being mounted on glass slides coated with 0.5% gelatine and allowed to dry overnight. Once dry, the slides were placed in a solution of 70% ethanol for 2 hours and then dehydrated, cleared in xylene, and coverslipped with DPX mountant. To test for non-specific staining of the immunohistochemical protocol, the primary and secondary antibodies were omitted from random sections and no speci fi c staining was evident.e observed immunostaining patterns support the speci fi city of the antibodies and are compatible with observations made in columbiformes and other avian species (Boseret et al., 2007; Charvet and Striedter, 2008; Melleu et al., 2013, 2016).
Data analysis
Results
General observations
We examined putative adult neurogenesis throughout the brains of the racing homer and utility carneau pigeon breeds using immunohistochemical techniques for the endogenous markers PCNA and DCX. The distribution of PCNA and DCX immunoreactivity was almost identical in both breeds, but a few minor di ff erences were observed (Table 1). Due to this extensive similarity, we depicted only the mapping of the distribution of the PCNA and DCX-immunoreactive cells in the racing homer pigeons (Figure 1).
In both pigeons, PCNA and DCX immunoreactivity was observed in the olfactory bulbs, subdivisions of the pallium (hippocampus, hyperpallium apicalle, hyperpallium intercalatum, hyperpallium densocellulare, mesopallium, nidopallium, entopallium, arcopallium, dorsolateral cortical area, piriform cortex and the temporo-parieto-occipital area), subpallium (medial striatum, lateral striatum and the globus pallidus), diencephalon, mesencephalon (pretectum and tectum), and cerebellum. Generally, the telencephalic regions had a higher density of PCNA- and DCX-immunoreactive cells than other brain regions in both breeds of pigeons examined, while the lowest density of cells immunoreactive to both markers was observed in the cerebellum. In the majority of the telencephalic regions, the density of PCNA- and DCX-immunoreactive cells increased from rostral to caudal in both pigeon breeds, except in the mesopallium where the staining density of cells fi rst decreased from rostral to middle levels and then increased at caudal levels. DCX immunoreactivity was more intense in fi bres than in cell bodies. DCX-immunoreactive cells included small rounded cells, fusiform bipolar cells, and large round and/or polygonal multipolar cells in terms of cell shapes.
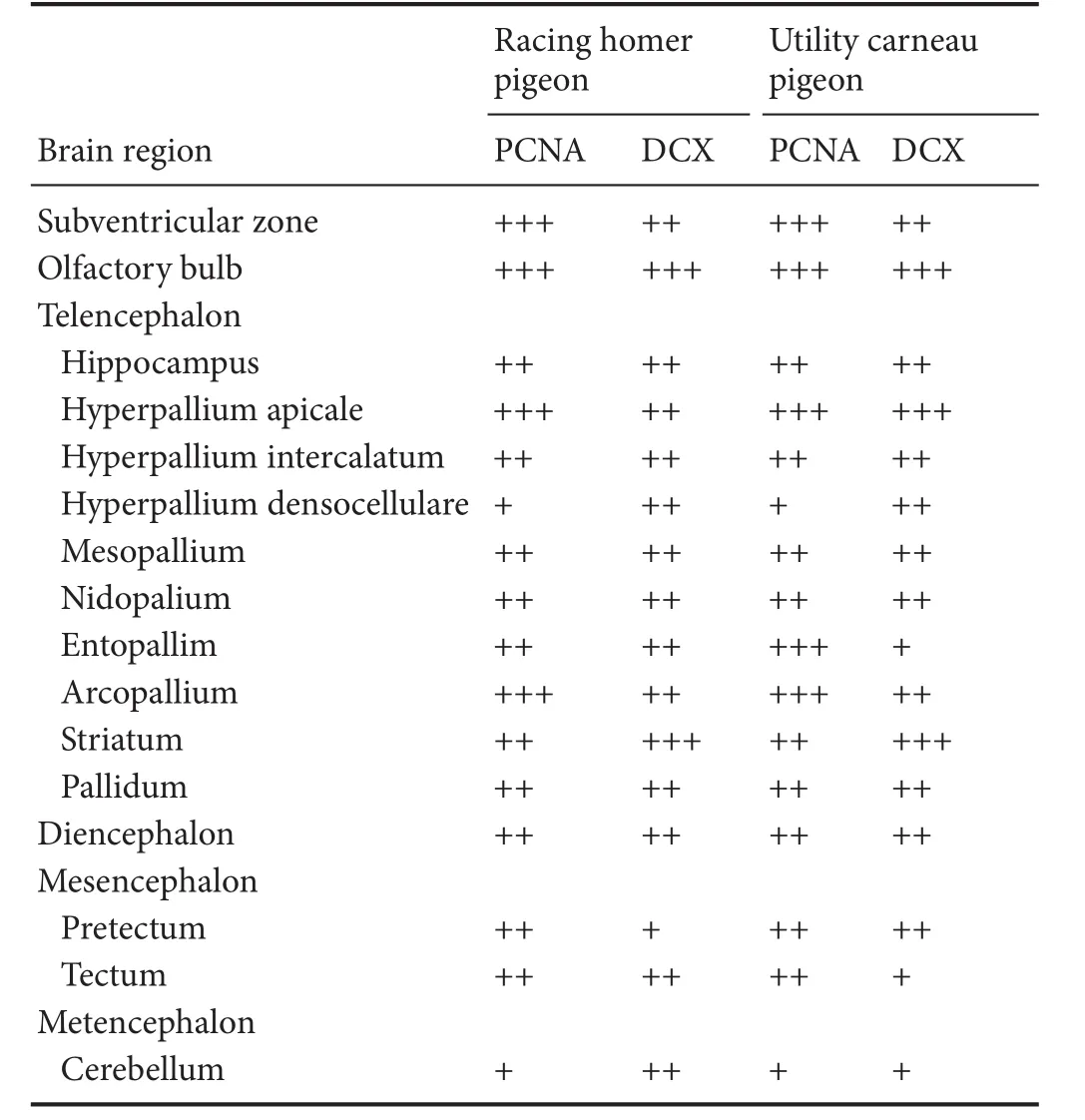
Table 1 Qualitative distribution and density of PCNA and DCX-immunoreactive structures in the brains of the racing homer and utility carneau pigeons
Distribution of PCNA-immunoreactive cells
Olfactory bulbs
The olfactory bulbs contained the highest density of PCNA-immunoreactive cells of all regions of the brain in both pigeon breeds. PCNA-immunoreactive cells were observed at high density in the internal granular layer, mitral cell layer and external plexiform layer, while they were found at a lower density in the glomerular layer and olfactory nerve layer (Figure 2A and C).
Subventricular zones of the lateral, third, fourth, and tectal ventricles
PCNA-immunoreactive cells at a high density were observed in the walls of the lateral, third, fourth and tectal ventricles, forming an uninterrupted layer, one to four cells thick, of cells with dark-stained nuclei in both pigeon breeds. Proliferative hotspots were observed in both dorsal and ventral poles of the lateral ventricle, mostly in rostral levels of the brain (Figure 3A and C). PCNA-immunoreactive cells at a high density was observed in the racing homer pigeon associated with the walls of the lateral ventricle abutting the medial striatum, nidopallium, nidopallium caudale and hyperpallium apicale when compared to the utility carneau pigeon.
Hyperpallium, mesopallium, nidopallium, and entopallium
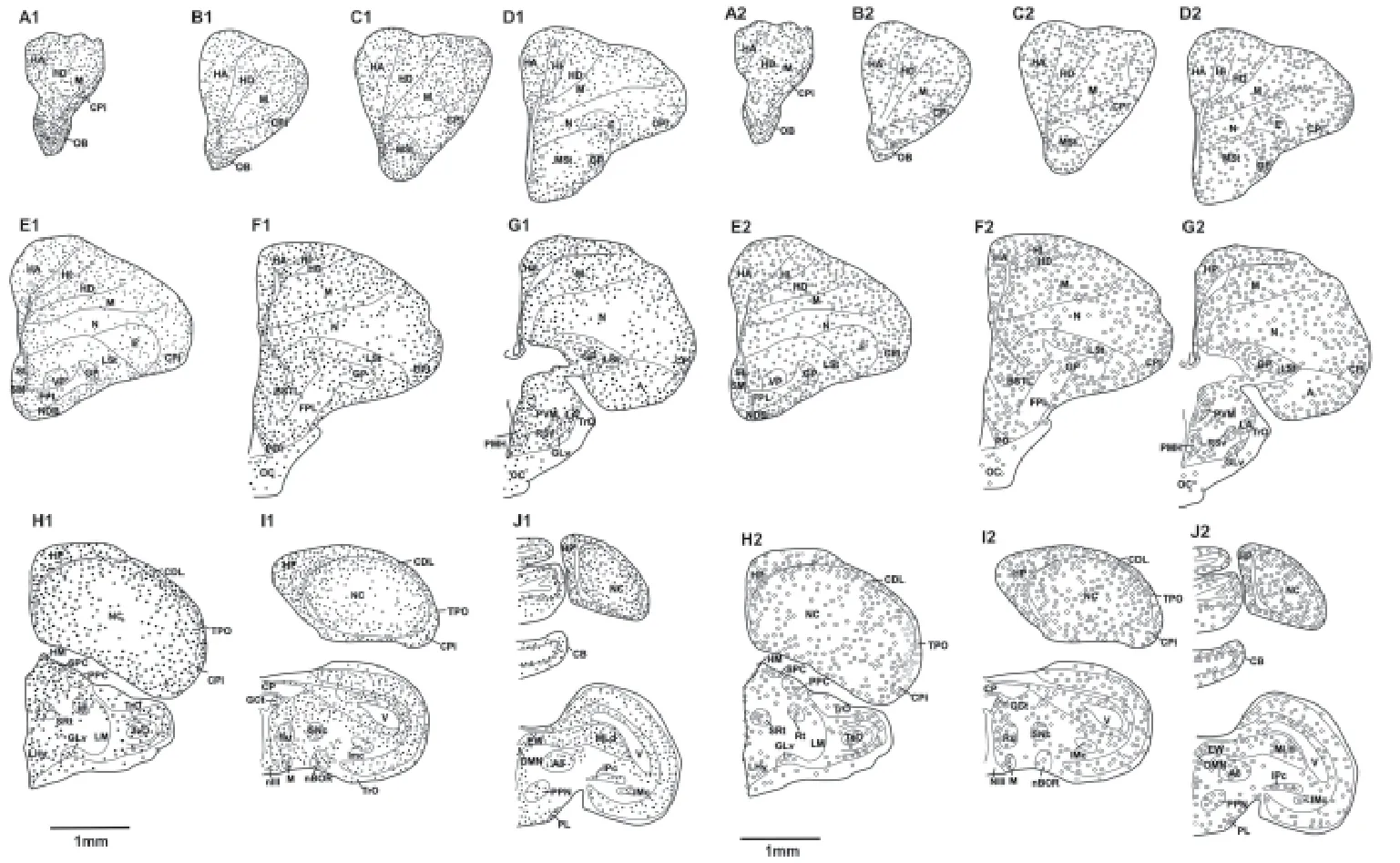
Figure 1 Reconstruction of coronal sections through one half of the brain of the racing homer pigeon showing areas of the detected PCNA-immunoreactive cells and DCX-immunoreactive immature and migrating neurons.
In the pallial regions (hyperpallium apicale, hyperpallium intercalatum, hyerpallium densocellulare, mesopallium and nidopallium), PCNA-immunoreactive cells were observed in high density along the cortical margins and regions adjacent to the lateral wall of the lateral ventricle, but this density was obviously reduced in the core regions of hyerpallium densocellulare, mesopallium and nidopallium in both pigeon breeds (Figure 1). At caudal levels, the nidopallium caudale exhibited an increased density of PCNA-immunoreactive cells at the regions adjacent to the walls of the lateral ventricle and also towards the lateral cortex. The core of the NC had scattered PCNA-immunoreactive cells. In the entopallium, there was a higher density of PCNA-immunoreactive cells rostrally, but the density gradually decreased towards the caudal levels of the telencephalon in all pigeons. In all these telencephalic regions, the density of PCNA-immunoreactive cells appeared greater in the racing homer pigeons than in the utility carneau pigeons.
Hippocampus, piriform cortex, and dorsolateral cortical area
Striatum, septum, and arcopallium
In the striatum of both pigeonbreeds, a moderate density of PCNA-immunoreactive cells was observed in the medial striatum adjacent to the ventrolateral wall of the lateral ventricle, while the lateral striatum exhibited a low density of PCNA-immunoreactive cells. Generally in the striatum, there was a rostro-caudal decline in the density of PCNA-immunoreactive cells. In the racing homer pigeon, the medial septum housed a medium density of PCNA-immunoreactive cells, the density of which lessened towards the most medial margin of this nucleus. The SM of the utility carneau pigeon had very densely packed PCNA-immunoreactive cells with large nuclei, which was prominent at the level of the anterior commissure (Figure 5A, C and D). In the lateral septum of both pigeonbreeds, a higher density of PCNA-immunoreactive cells wasobserved when compared to the medial septum, and within this nucleus there was a rostrocaudal decline in PCNA-immunoreactive cellular density. The arcopallium in both pigeonbreeds exhibited a moderate density of PCNA-immunoreactive cells along the cortical margins and towards the ventral parts of the nidopallium caudale, while the central areas had a very low to absent density of PCNA-immunoreactive cells.
Diencephalon, optic chiasm, and anterior commissure
The diencephalon exhibited a high density of PCNA-immunoreactive cells in the various nuclei compared to the surrounding tissues. This was observed in the paraventricular nuclei which include nucleus preopticus medialis, periventricular magnocellular nucleus and nucleus medialis hypothalamic posteriolis medially, the dorsal margin in the nucleus dorsomedialis anterior thalami, nucleus dorsolateralis anterior thalami, pars lateralis nuclei and the lateral margin in the nucleus rotundus and nucleus geniculatus lateralis, pars ventralis. Further laterally the optic tract housed a moderate density of PCNA-immunoreactive cells. In the core regions of the hypothalamus, PCNA-immunoreactive cells were either absent or in a low density.e optic chiasm of both pigeon breeds exhibited a cluster of PCNA-immunoreactive cells which increased slightly in density adjacent to the ventral pole of the subventricular zone of the third ventricle (Figure 6A and C).ere were few scattered PCNA-immunoreactive cells in the anterior commissure.
Pretectum, tectum, posterior commissure, and cerebellum
Distribution of DCX-immunoreactive cells
DCX-immunoreactive cells were identified throughout the brains of the two adult pigeon breeds and were generally small fusiform shaped, bipolar and round multipolar cells with slight variations in size in the di ff erent brain regions. Any size and/or shape variations are noted in relevant regions.
Olfactory bulb
Subventricular zone
The subventricular zone of the lateral and third ventricles evinced 3 to 4 layers of DCX-immunoreactive cells in both breeds, which intermittently formed cell clusters of intensely stained cells at high density.is arrangement was more apparent on the lateral wall of the lateral ventricle than on the medial wall. The DCX-immunoreactive fibres emanating from the subventricular zone were oriented either parallel or orthogonal to the ventricular wall (Figure 3B and D).e fourth and tectal ventricles of the racing homer pigeon exhibited a continuous layer of DCX-immunoreactive cells without cell clustering, whereas in the utility carneau pigeon, the tectal ventricles had more DCX-immunoreactive fi bres rather than cells.
Hyperpallium, mesopallium, nidopallium, and entopalliuma
Hippocampus, piriform cortex, and dorsolateral cortical areae medial region of the ventral hippocampus had sparsely distributed DCX-immunoreactive cells and fi bres, while the triangular and the lateral regions exhibited a medium density of DCX-immunoreactive cells and fi bres in both pigeon breeds (Figure 4B, C, E and F). In the dorsomedial region of the hippocampus, DCX-immunoreactive cells were observed at a higher density in the central area and towards the dorsomedial wall of the lateral ventricle.ere were low-density DCX-immunoreactive cells and fi bres in the dorsolateral region of the hippocampus, close to the border with dorsomedial region medially. The density of DCX-immunoreactive cells in the dorsolateral region gradually increased towards the dorsolateral cortical region.
In the dorsolateral cortical region, there were moderate-density DCX-immunoreactive cells and fibres. At the caudal level, the dorsolateral cortical region had higher density DCX-immunoreactive cells and fi bres in areas adjacent to the dorsolateral margin of the lateral ventricle.e piriform cortex had higher density intensely stained DCX-immunoreactive cells and fi bres in both pigeon breeds.
Striatum, septum, and arcopallium
In the striatum, the medial striatum exhibited a medium density of DCX-immunoreactive structures with a higher density of stained structures in areas abutting the ventral parts of the lateral wall of the lateral ventricle, while the density of immunoreactive structures was less in the core regions. Ventral to the striatum, there was a high density of DCX-immunoreactive cells in the olfactory tubercle and nucleus of the fascicurius diagonalis Brocae.e lateral striatum had moderate to low density of DCX structures in both pigeon breeds, but a high density of DCX-immunoreactive structures was observed in the globus pallidus and interpeduncular nucleus at levels caudal to the anterior commissure.
In the racing homer pigeon, the septal nuclear complex exhibited a low density of DCX-immunoreactive structures in the medial septum, whereas the lateral septum had a moderate density of DCX-immunoreactive structures, although this density was reduced at the caudal level. In the utility carneau pigeon, the medial septum exhibited a moderate density of DCX-immunoreactive cells and fibres (Figure 5B).ere was a moderate density of DCX-immunoreactive cells and fi bres in the arcopallium, especially along the ventral margins in both pigeon breeds, but the core areas had less dense staining in the utility carneau pigeon.
Diencephalon, optic chiasm, and anterior commissure
Pretectum, tectum, posterior commissure, and cerebellum
In the mesencephalic pretectum, a moderate density of DCX-immunoreactive structures were observed in the tegmentum in the supraoptic decussation. In the tectal region of the mesencephalon, a moderate density of DCX-immunoreactive structures were observed medially in the nucleus ruber, griseum centralis, oculomotor nucleus and the Edinger-Westphal nucleus, dorsally in the nucleus harbenularis medialis (HM) and nucleus spiriformis lateralis, and ventrally in the nucleus tuberis, pedunculopontine tegmental nucleus and the nucleus of the basal optic root.e Edinger-Westphal nuclei and nucleus ruber contained large round DCX-immunoreactive cells. DCX-immunoreactive structures were scattered in the nucleus rotundus and in the layers of the optic tectum.e posterior commissure exhibited a few DCX-immunoreactive structures especially on margins adjacent to the walls of the cerebral aqueduct. In the cerebellum, the granule cell layer of the utility carneau pigeon had a moderate density of DCX-immunoreactive structures, while in both pigeon breeds, the Purkinje cell layer had a high density of DCX-immunoreactive structures.e dendrites of the DCX-immunoreactive cells projected into the molecular layer of the cerebellum (Figure 7B and D).
Discussion
General considerations regarding PCNA- and DCX-immunoreactivity in pigeons
In the current study, we observed potential adult neurogenesis in the brains of the two breeds of the adult pigeons, the racing homer and the utility carneau. Putative adult neurogenesis was demonstrated through the expression of the PCNA and DCX molecules, revealed using immunohistochemical techniques, throughout the brains of both pigeon breeds. The two antibodies were localized in similar brain regions of the pigeon breeds with putative adult neurogenesis including the olfactory bulb, telencephalic subdivisions (hyperpallium apicale, hyperpallium intercalatum, hyerpallium densocellulare, mesopallium, nidopallium, nidopalliun caudale, entopallium, medial striatum, lateral striatum, lateral septum, medial septum), the subventricular zone, the diencephalon, the mesencephalon and the cerebellum.
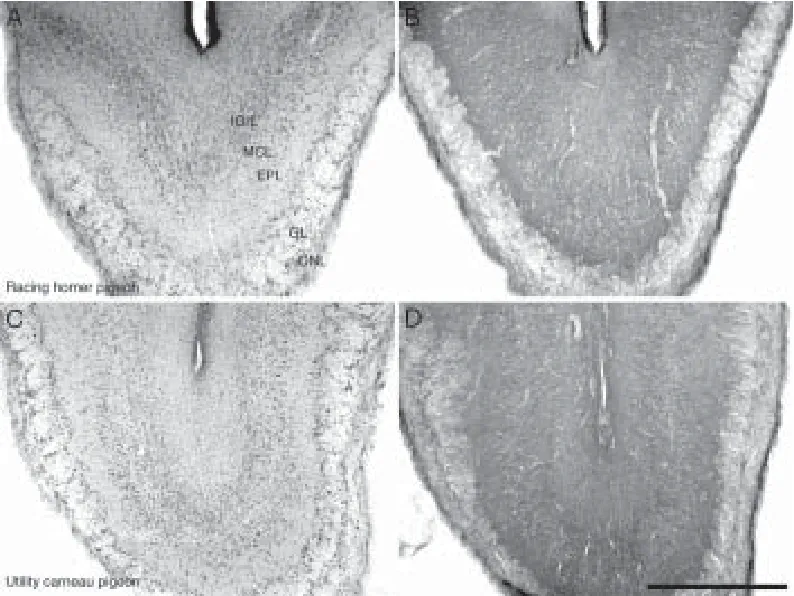
Figure 2 Photomicrographs showing the olfactory bulbs of the racing homer pigeon (A, B) and the utility carneau pigeon (C, D).
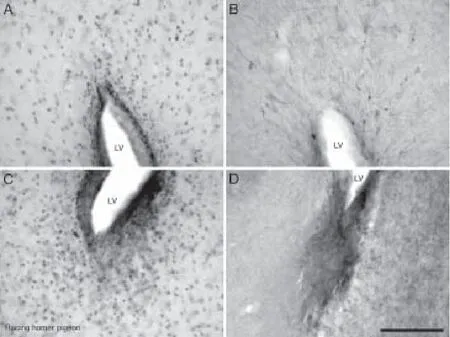
Figure 3 Photomicrographs showing the distribution of PCNA (A, C) and DCX (B, D) immunoreactivity in the dorsal and ventral reaches of the subventricular zone of the lateral ventricle (LV) in the racing homer pigeon.
PCNA is a marker for proliferating cells that is essential for DNA replication and repair. It is synthesized during the early G1 and S phases of the cell cycle, abundant during the S phase and declines during G2/M phase (Kurki et al., 1988; Hall et al., 1990). In the two pigeon breeds studied, the density of PCNA-immunoreactive cells was higher in the olfactory bulbs, subventricular zone, nidopalliun caudale and the piriform cortex, but was generally less dense in the potential adult neurogenic sites such as the diencephalon, mesencephalon and the cerebellum. This pattern of cellular staining is similar to that seen in other non-mammalian vertebrates including teleost fi sh (Zupanc et al., 2005), amphibians (Cerri et al., 2009), and chickens (Hannan et al., 1999). However, BrdU-positive cells in the subtelencephalic regions in canaries did not co-express neuron speci fi c marker Hu protein suggesting that the proliferating cells in this region are of glial cell lineage (Vellema et al., 2010). In the subventricular zone of the lateral ventricles, a high density of PCNA-immunoreactive cells was observed in the ventral and dorsal borders of the ventricles. In these regions, the putative proliferating cells formed intermittent clusters that were consistent with the proliferative ‘hotspots’ identi fi ed by Alvarez-Buylla et al. (1990b).

Figure 4 Photomicrographs showing the distribution of proliferating cell nuclear antigen (PCNA) and doublecortin (DCX) in the hippocampus of the racing homer and utility carneau pigeons.
Proliferating cells were also observed throughout the telencephalon and four possible reasons can be inferred for this: (1) these putative proliferating cells may have migrated from the subventricular zone throughout the parenchyma (Almli and Wilczynski, 2007); (2) resident progenitors may be proliferating (Alvarez-Buylla et al., 1990a, b; Doetsch et al., 1999; Zupanc et al., 2005); (3) putative proliferating cells may be resident glial cells (Cerri et al., 2009); and (4) putative proliferative cells may be both neuronal and glial cells undergoing DNA replication and repair (Hall et al., 1990).
DCX is a microtubule associated protein that plays a key role in the migration of neurons during development and in post-mitotic neurons undergoing migration, remodelling of their dendritic processes and synaptogenesis in adulthood (Gleeson et al., 1999; Capes-Davis et al., 2005; Couillard-Despres et al., 2005). In this study, we observed that DCX expression was widespread and heterogeneous in the brains of the two breeds of domestic pigeons (Columba livia domestica).e density of DCX-immunoreactive cells and fi bres was most intense in the olfactory bulbs, associated olfactory cortices, telencephalic pallial structures including the hippocampus, the striatum and the nidopalliun caudale, but less dense in subtelencephalic structures such as the diencephalon, mesencephalon and cerebellum.is widespread expression of DCXwas consistent with observations made in other non-song birds such as the developing domestic chick brain (Capes-Davis et al., 2005), in song birds such as adult canaries (Boseret et al., 2007; Vellema et al., 2014) and in subadult zebra fi nches (Kim et al., 2006). Moderate intensity DCX expression in the prosencephalon has been reported in numerous studies (Kim et al., 2006; Boseret et al., 2007; Vellema et al., 2010, 2014; Mezey et al., 2012; Melleu et al., 2013). The majority of pioneering studies on adult neurogenesis in birds reported that the process was only limited to the prosencephalon (Alvarez-Buylla and Nottebohm, 1988; Kirn et al., 1994). Few studies reported the presence of DCX expression in the subtelencephalic regions in pathologically normal adult species (Boseret et al., 2007; Balthazart et al., 2008; Vellema et al., 2014). However, increased levels of adult neurogenesis have been reported in the subtelencephalic regions under injury and pathological conditions (Cao et al., 2002; Chen et al., 2006).
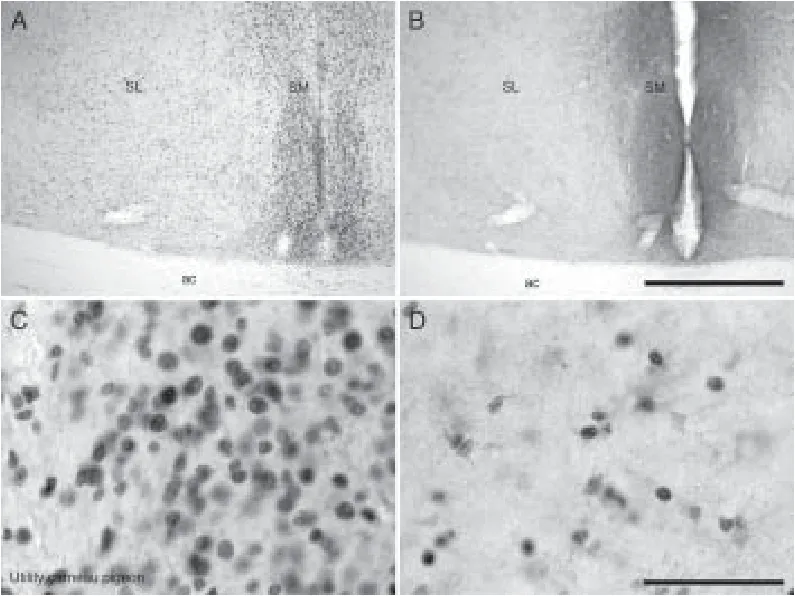
Figure 5 Photomicrographs showing distribution of proliferating cell nuclear antigen (PCNA)- and doublecortin (DCX)-immunoreactive cells in the septum of the utility carneau pigeon.
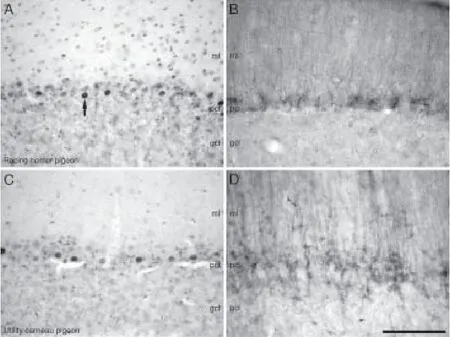
Figure 7 Photomicrographs of the cerebellar cortex of the racing homer (A, B) and utility carneau pigeons (C, D).

Figure 6 Photomicrographs of proliferating cell nuclear antigen (PCNA) (A, C, E) and doublecortin (DCX) (B, D, E) immunostained sections through the dorsal aspect of the optic chiasm (OC), at its border with the fl oor of the third ventricle (3V) in racing homer (A, B) and utility carneau (C, D, E, F) pigeons.
We also noted that that the areas with the densest expression of DCX-immunoreactive structures were adjacent to the walls of the ventricles in all brain regions, suggesting that new neurons were generated in the subventricular zone and migrated to their target regions. Migration of newly born neurons follows well de fi ned routes in mammalian species, where they migrate from the subgranular zone of the dentate gyrus to the granule cell layer, and the subventricular zone of the lateral ventricle to the olfactory bulbs through the rostral migratory stream (Lois and Alvarez-Buylla, 1994; Chawana et al., 2013; Patzke et al., 2013). In birds studied to date, including the current study, the migratory routes appear di ff use, with many regions of the brain appearing to incorporate new neurons. Despite this, as in mammals, a rostral migratory-like stream has been identi fi ed in rock pigeons, seen to stretch from the caudal nidopallium to the rostral mesopallium, hyperpallium apicale and densocellulare (Melleu et al., 2013). New neurons migrate from the subventricular zone into the brain parenchyma through radial glia scaffolding (Alvarez-Buylla and Nottebohm, 1988; Alvarez-Buylla et al., 1988).is phenomenon led to the identi fi cation of DCX-immunoreactive neuroblasts with the morphology typical of migrating cells (small round and fusiform bipolar cells) (Alvarez-Buyllaand Nottebohm, 1988; Alvarez-Buylla et al., 1988; Melleu et al., 2013) in the parenchyma away from the subventricular zone. However, DCX immunoreactivity was also observed in post-migratory cells of the Purkinje cell layer of the cerebellum in the current study (Capes-Davis et al., 2005) and in areas of the telencephalic structures where large round and polygonal multipolar cells with elaborate dendritic processes were also seen to express DCX. In the rock pigeons, these mature-like DCX-immunoreactive cells did not co-express neuron speci fi c marker NeuN (Melleu et al., 2013), suggesting that these cells were immature neurons establishing synaptic contacts in local circuits (Gleeson et al., 1999). Despite this, DCX-immunoreactive cells outside the subventricular zone in the mouse brain were found to express NeuN (Yang et al., 2004), indicating that DCX is also expressed in mature neurons that undergo dendritic arborization and axonal growth (Nacher et al., 2001; Brown et al., 2003).
DCX expression has been found to persist for more than 20 days in cells in the adult canary brain (Balthazart et al., 2008), explaining its presence in morphologically distinct neurons in the cores of the hippocampus and other pallial structures as observed in the current study, and in rock pigeons (Melleu et al., 2013).is suggests that DCX-immunoreactive cells in deeper areas of the telencephalic parenchyma represent earlier generations of new-born neurons that completed migration prior to the sacrifice of the animal. In addition, the concept also explains the abundance of DCX-immunoreactive cells observed in the telencephalon of avian species in previous studies (Kim et al., 2006; Melleu et al., 2013; Vellema et al., 2014).
Olfactory neurogenesis in pigeons
The olfactory bulbs are known to continuously incorporate new neurons in adulthood in various mammalian species (Kempermann, 2012). We found that the olfactory bulbs and associated brain areas such as the piriform cortex, olfactory tubercles and dorsolateral cortical areas exhibited high densities of PCNA- and DCX-immunoreactive structures. Similar fi ndings were reported in other bird species such as domestic chicks and rock pigeons (Mezey et al., 2012; Melleu et al., 2013). In contrast to olfactory neurogenesis in mammals where new neurons migrate long distances from the subventricular zone, the presence of PCNA- and DCX-immunoreactive cells in the walls of the olfactory ventricle and adjacent olfactory bulb layers suggests that new neurons are generated and incorporated in the olfactory bulbs locally rather than by migration. Melleu et al. (2013) also found conspicuous BrdU labelled cells in the walls of the olfactory ventricles. Retrograde tracing studies in pigeons have shown that olfactory bulb neuronal fibres project to the piriform cortex, medial septum (Reiner and Karten, 1985) and the hyperpallium densocellulare (Patzke et al., 2011). Similar to a report by Melleu et al. (2013) in rock pigeons, we also observed a stream of DCX-immunoreactive cells that appeared to be migrating from the olfactory bulbs to the hyperpallium densocellurare. Processing of the olfactory sense varies greatly in di ff erent orders of birds studied to date (Balthazart and Taziaux, 2009). In homing pigeons, olfactory processing is used during navigation from unfamiliar places to home los (Patzke et al., 2010) by following familiar airborne odours (Papi et al., 1972). In other species of the order columbiformes, olfactory cues were associated with parental behavior in ring doves (Bonadonna et al., 2003), social and reproductive behaviors in rock pigeons (Patzke et al., 2010), feeding behaviors in domestic chicken (Jones and Roper, 1997), and food localization in vultures (Graves, 1992) and sea birds (Grubb, 1972).us, process of adult neurogenesis and neural plasticity in birds might aid in replenishing neurons and renewing circuits to facilitate adaptation into natural environments using the olfactory sense.
Hippocampal neurogenesis
In birds, adult hippocampal neurogenesis has been associated with the integration of new experiences, such as new songs, and the clearance of old memories (Nottebohm, 1981; Wilbrecht and Kirn, 2004; Kempermann, 2008), thus, the process may serve to prevent interference between old and new memories, especially in food caching birds that need to recall both old and new food caches accurately (Barnea et al., 2006; Wiskott et al., 2006; Pravosudov and Smulders, 2010). Kempermann (2008) also suggested that AHN may contribute to a neurogenic reserve that can be incorporated only when there is a need for new learning. Generally, AHN decreases with advancing age in both mammals and non-mammalian vertebrates (Barnea and Nottebohm, 1994; Kempermann et al., 1997; Eriksson et al., 1998; Kim et al., 2006); however, in some reptiles and fish, AHN appears to contribute, in part, to the continuous growth of the nervous system (Zupanc and Horschke, 1995; Ngwenya et al., 2013). Barnea and Nottebohm (1994) found that AHN varies seasonally, with more neuronal accumulation occurring in the autumn in black capped chickadees.e hippocampal formation has also been associated with spatial memory dependent tasks related to learning and acquisition of a spatial map and its operation in homing pigeons (Bingman et al., 2005), migration and food caching (Bingman and Cheng, 2005; Hoshooley and Sherry, 2007).
Neurogenesis in other brain areas
Our observation of wide spread DCX immunoreactivity throughout the brains of domestic pigeons is similar to that reported for adult chicken, canaries and zebra finches (Capes-Davis et al., 2005; Kim et al., 2006; Boseret et al., 2007; Balthazart et al., 2008). In the pallial structures, PCNA and DCX-immunoreactive structures were apparent in the margins of the hyperpallium apicale, mesopallium, nidopallium and nidopalliun caudale rather than their core areas. In addition, we observed PCNA and DCX immunoreactivity inthe septum, striatum and the entopallium in both pigeons. Expression of the DCX antibody in the entopallium was comparatively denser in racing homer pigeon than in utility carneau pigeon. Mezey et al. (2012) reported similar fi ndings but mild DCX expression in the entopallium of chicken.e striatum complex and the entopalium participate in the processing of visual information which is vital during navigating long distances particularly by homing pigeons. Birds are heavily visual and auditory reliant during feeding, reproduction, socialisation and many other daily functions (Grubb, 1972; Papi et al., 1972; Graves, 1992; Bonadonna et al., 2003; Patzke et al., 2011), but the cores of the telencephalon that process visual and auditory information exhibited very low to absent densities of PCNA- and DCX-immunoreactive structures in the domestic pigeon breeds studied. In contrast, the diencephalic and mesencephalic regions associated with these senses exhibited moderate densities of PCNA-and DCX-immunoreactive structures for example the dorsal nuclei of the thalamus which connects with the medial striatum (MSt) and also the tectum which connects with the lateral striatum (Kuenzel et al., 2011; Shanahan et al., 2013). We also observed PCNA and DCX immunoreactivity in the optic chiasma, anterior and posterior commissures and suggested that glial cells and neuronal axons may be proliferating and remodelling respectively.
In conclusion, adult neurogenesis appears to be a conserved process in the pigeon brain and may help in continuous reinforcement and remodelling of neuronal circuits and behavior.
Author contributions:AOI and PRM designed the study and analyzed data. PM, AB and PN collected data and performed a preliminary analysis. PM wrote the paper. All authors read and approved the fi nal version of this paper.
Con fl icts of interest:None declared.
Research ethics:All animals were treated and used according to the guidelines of the University of the Witwatersrand Animal Ethics Committee (approval No. 2013/05/02B), which parallel those of the NIH Guide for the Care and Use of Animals in scienti fi c experimentation and “Consensus Author Guidelines on Animal Ethics and Welfare” produced by the International Association of Veterinary Editors (IAVE).e article was prepared in accordance with the “Animal Research: Reporting of In Vivo Experiments Guidelines” (ARRIVE Guidelines).
Open access statement:
Contributor agreement:A statement of “Publishing Agreement” has been signed by an authorized author on behalf of all authors prior to publication.
Plagiarism check:This paper has been checked twice with duplication-checking soware ienticate.
Peer review:A double-blind and stringent peer review process has been performed to ensure the integrity, quality and signi fi cance of this paper.
Almli LM, Wilczynski W (2007) Regional distribution and migration of proliferating cell populations in the adult brain of Hyla cinerea (Anura, Amphibia). Brain Res 1159:112-118.
Alvarez-Buylla A, García-Verdugo JM, Mateo AS, Merchant-Larios H (1998) Primary neural precursors and intermitotic nuclear migration in the ventricular zone of adult canaries. J Neurosci 18:1020-1037.
Alvarez-Buylla A, Kirn JR, Nottebohm F (1990a) Birth of projection neurons in adults avian brain may be related to perceptual or motor learning. Science 249:1444-1446.
Alvarez-Buylla A, Nottebohm F (1988) Migration of young neurons in adult avian brain. Nature 335:353-354.
Alvarez-Buylla A, Ling CY, Yu WS (1994) Contribution of neurons born during embryonic and adult life to the brain of adult canaries: Regional speci fi city and delayed birth of neurons in the song-control nuclei. J Comp Neurol 347:233-248.
Amrein I, Lipp H-P (2009) Adult hippocampal neurogenesis of mammals: evolution and life history. Biol Lett 5:141-144.
Atoji Y, Wild JM (2004) Fiber connections of the hippocampal formation and septum and subdivisions of the hippocampal formation in the pigeon as revealed by tract tracing and kainic acid lesions. J Comp Neurol 475:426-461.
Balthazart J, Boseret G, Konkle A, Hurley LL, Ball GF (2008) Doublecortin as a marker of adult neuroplasticity in the canary song control nucleus HVC. Eur J Neurosci 27:801-817.
Balthazart J, Charlier TD, Barker JM, Yamamura T, Ball GF (2010) Sex steroid-induced neuroplasticity and behavioral activation in birds. Eur J Neurosci 32:2116-2132.
Balthazart J, Taziaux M (2009)e underestimated role of olfaction in avian reproduction? Behav Brain Res 200:248-259.
Barnea A, Mishal A, Nottebohm F (2006) Social and spatial changes induce multiple survival regimes for new neurons in two regions of the adult brain: An anatomical representation of time? Behav Brain Res 167:63-74.
Barnea A, Nottebohm F (1994) Seasonal recruitment of hippocampal neurons in adult free-ranging black-capped chickadees. Proc Natl Acad Sci U S A 91:11217-11221.
Barnea A, Pravosudov V (2011) Birds as a model to study adult neurogenesis: Bridging evolutionary, comparative and neuroethological approaches. Eur J Neurosci 34:884-907.
Bartkowska K, Turlejski K, Grabiec M, Ghazaryan A, Yavruoyan E, Djavadian RL (2010) Adult neurogenesis in the hedgehog (erinaceus concolor) and mole (talpa europaea). Brain Behav Evol 76:128-143.
Bingman VP, Cheng K (2005) Mechanisms of animal global navigation: comparative perspectives and enduring challenges. Ethol Ecol Evol 17:295-318.
Bingman VP, Gagliardo A, Hough GE, Ioalé P, Kahn MC, Siegel JJ (2005)e avian hippocampus, homing in pigeons and the memory representation of large-scale space. Integr Comp Biol 45:555-564.
Bingman VP, Siegel J, Gagliardo A, Erichsen J (2006) Representing the richness of avian spatial cognition: properties of a lateralized homing pigeon hippocampus. Rev Neurosci 17:17-28.
Bonadonna F, Cunningham GB, Jouventin P, Hesters F, Nevitt GA (2003) Evidence for nest-odour recognition in two species of diving petrel. J Exp Biol 206:3719-3722.
Boseret Gr, Ball GF, Balthazart J (2007) The microtubule-associated protein doublecortin is broadly expressed in the telencephalon of adult canaries. J Chem Neuroanat 33:140-154.
Brown JP, Couillard-Després S, Cooper-Kuhn CM, Winkler J, Aigner L, Kuhn HG (2003) Transient expression of doublecortin during adult neurogenesis. J Comp Neurol 467:1-10.
Cao J, Wenberg K, Cheng M-F (2002) Lesion induced new neuron incorporation in the adult hypothalamus of the avian brain. Brain Res 943:80-92.
Capes-Davis A, Tolhurst O, Dunn JM, Je ff rey PL (2005) Expression of doublecortin (DCX) and doublecortin-like kinase (DCLK) within the developing chick brain. Dev Dyn 232:457-467.
Cerri S, Bottiroli G, Bottone MG, Barni S, Bernocchi G (2009) Cell proliferation and death in the brain of active and hibernating frogs. J Anat 215:124-131.
Chancellor LV, Roth TC, LaDage LD, Pravosudov VV (2011)e e ff ect of environmental harshness on neurogenesis: A large-scale comparison. Dev Neurobiol 71:246-252.
Charvet CJ, Striedter GF (2008) Developmental species di ff erences in brain cell cycle rates between northern bobwhite quail (Colinus virginianus) and parakeets (Melopsittacus undulatus): implications for mosaic brain evolution. Brain Behav Evol 72:295-306.
Chawana R, Patzke N, Kaswera C, Gilissen E, Ihunwo AO, Manger PR (2013) Adult neurogenesis in eight Megachiropteran species. Neuroscience 244:159-172.
Chen G, Bonder EM, Cheng MF (2006) Lesion-induced neurogenesis in the hypothalamus is involved in behavioral recovery in adult ring doves. J Neurobiol 66:537-551.
Couillard-Despres S, Winner B, Schaubeck S, Aigner R, Vroemen M, Weidner N, Bogdahn U, Winkler J, Kuhn HG, Aigner L (2005) Doublecortin expression levels in adult brain re fl ect neurogenesis. Euro J Neurosci 21:1-14.
Doetsch F, Caillé I, Lim DA, García-Verdugo JM, Alvarez-Buylla A (1999) Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell 97:703-716.
Eriksson PS, Per fi lieva E, Björk-Eriksson T, Alborn aM, Nordborg C, Peterson Da, Gage FH (1998) Neurogenesis in the adult human hippocampus. Nat Med 4:1313-1317.
Gleeson JG, Peter T L, Flanagan LA, Walsh CA (1999) Doublecortin is a microtubule-associated protein and is expressed widely by migrating neurons. Neuron 23:257-271.
Graves G (1992) Greater yellow-headed vulture (Cathartes-melambrotus) locates food by olfaction. vol. 26, pp 38-39. Raptor Research Foundation Inc., Hastings, MN, USA.
Grubb TC (1972) Smell and foraging in shearwaters and petrels. Nature 237:404-405.
Hall P, Levison D, Woods A, Yu CW, Kellock D, Watkins J, Barnes D, Gillett C, Camplejohn R, Dover R (1990) Proliferating cell nuclear antigen (PCNA) immunolocalization in para ffi n sections: An index of cell proliferation with evidence of deregulated expression in some, neoplasms. J Pathol 162:285-294.
Hannan AJ, Henke RC, Seeto GS, Capes-Davis A, Dunn J, Je ff rey PL (1999) Expression of doublecortin correlates with neuronal migration and pattern formation in diverse regions of the developing chick brain. J Neurosci Res 55:650-657.
Hoshooley JS, Sherry DF (2007) Greater hippocampal neuronal recruitment in food-storing than in non-food-storing birds. Dev Neurobiol 67:406-414.
Jarvis ED, Güntürkün O, Bruce L, Csillag A, Karten H, Kuenzel W, Medina L, Paxinos G, Perkel DJ, Shimizu T, Striedter G, Wild JM, Ball GF, Dugas-Ford J, Durand SE, Hough GE, Husband S, Kubikova L, Lee DW, Mello CV, et al. (2005) Avian brains and a new understanding of vertebrate brain evolution. Nat Rev Neurosci 6:151-159.
Jones RB, Roper TJ (1997) Olfaction in the domestic fowl: a critical review. Physiol Behav 62:1009-1018.
Karten HJ, Hodos W (1967) A Stereotaxic Atlas of the Brain of the Pigeon (Columba livia). Baltimore: Johns Hopkins Press.
Kempermann G (2012) New neurons for’survival of the fi ttest. Nat Rev Neurosci 13:727-736.
Kempermann G, Kuhn HG, Gage FH (1997) Genetic in fl uence on neurogenesis in the dentate gyrus of adult mice. Proc Natl Acad Sci U S A 94:10409-10414.
Kim YH, Peregrine J, Arnold AP (2006)e distribution of expression of doublecortin (DCX) mRNA and protein in the zebra fi nch brain. Brain Res 1106:189-196.
Kirn J, Nottebohm F (1993) Direct evidence for loss and replacement of projection neurons in adult canary brain. J Neurosci 13:1654-1663.
Kirn J, O’Loughlin B, Kasparian S, Nottebohm F (1994) Cell death and neuronal recruitment in the high vocal center of adult male canaries are temporally related to changes in song. Proc Natl Acad Sci U S A 91:7844-7848.
Kuenzel WJ, Medina L, Csillag A, Perkel DJ, Reiner A (2011)e avian subpallium: new insights into structural and functional subdivisions occupying the lateral subpallial wall and their embryological origins. Brain Res 1424:67-101.
Kurki P, Ogata K, Tan E (1988) Monoclonal antibodies to proliferating cell nuclear antigen (PCNA)/cyclin as probes for proliferating cells by immuno fl uorescence microscopy and fl ow cytometry. J Immunol Methods 109:49-59.
Ling C, Zuo M, Alvarez-Buylla A, Cheng MF (1997) Neurogenesis in juvenile and adult ring doves. J Comp Neurol 379:300-312.
Lipkind D, Nottebohm F, Rado R, Barnea A (2002) Social change affects the survival of new neurons in the forebrain of adult songbirds. Behav Brain Res 133:31-43.
Lois C, Alvarez-Buylla A (1994) Long-distance neuronal migration in the adult mammalian brain. Science 264:1145-1148.
Mehlhorn J, Rehkämper G (2009) Neurobiology of the homing pigeon - A review. Naturwissenschaen 96:1011-1025.
Melleu FF, Pinheiro MV, Lino-de-Oliveira C, Marino-Neto J (2016) Defensive behaviors and prosencephalic neurogenesis in pigeons (Columba livia) are a ff ected by environmental enrichment in adulthood. Brain Struct Funct 221:2287-2301.
Melleu FF, Santos TS, Lino-de-Oliveira C, Marino-Neto J (2013) Distribution and characterization of doublecortin-expressing cells and fi bers in the brain of the adult pigeon (Columba livia). J Chem Neuroanat 47:57-70.
Mezey S, Krivokuca D, Bálint E, Adorján A, Zachar G, Csillag A (2012) Postnatal changes in the distribution and density of neuronal nuclei and doublecortin antigens in domestic chicks (Gallus domesticus). J Comp Neurol 520:100-116.
Nacher J, Crespo C, McEwen BS (2001) Doublecortin expression in the adult rat telencephalon. Eur J Neurosci 14:629-644.
Ngwenya A, Patzke N, Spocter Ma, Kruger J-L, Dell L-A, Chawana R, Mazengenya P, Billings BK, Olaleye O, Herculano-Houzel S, Manger PR (2013)e Continuously Growing Central Nervous System of the Nile Crocodile (Crocodylus niloticus). Anat Rec (Hoboken) 296:1489-1500.
Nottebohm F (1981) A brain for all seasons: cyclical anatomical changes in song control nuclei of the canary brain. Science 214:1368-1370.
Nottebohm F (1985) Neuronal replacement in adulthood. Ann N Y Acad Sci 457:143-161.
Olaleye OO, Ihunwo AO (2014) Adult neurogenesis in the four-striped mice (Rhabdomys pumilio). Neural Regen Res 9:1907-1911.
Papi F, Fiore L, Fiaschi V, Benvenuti S (1972) Olfaction and homing in pigeons. Monit Zool Ital (NS) 6:85-95.
Paton JA, Nottebohm FN (1984) Neurons generated in the adult brain are recruited into functional circuits. Science 225:1046-1048.
Patzke N, Kaswera C, Gilissen E, Ihunwo A, Manger P (2013) Adult neurogenesis in a giant otter shrew (Potamogale velox). Neuroscience 238:270-279.
Patzke N, Manns M, Güntürkün O. (2011) Telencephalic organization of the olfactory system in homing pigeons (Columba livia). Neuroscience 194:53-61.
Patzke N, Manns M, Güntürkün O, Ioale P, Gagliardo A (2010) Navigation-induced ZENK expression in the olfactory system of pigeons (Columba livia). Eur J Neurosci 31:2062-2072.
Pravosudov VV, Smulders TV (2010) Integrating ecology, psychology and neurobiology within a food-hoarding paradigm. Philos Trans R Soc Lond B Biol Sci 365:859-867.
Rehkämper G, Frahm HD, Cnotka J (2008) Mosaic evolution and adaptive brain component alteration under domestication seen on the background of evolutionary theory. Brain Behav Evol 71:115-126.
Reiner A, Karten H (1985) Comparison of olfactory bulb projections in pigeons and turtles. Brain Behav Evol 27:11-27.
Reiner A, Perkel DJ, Bruce LL, Butler AB, Csillag A, Kuenzel W, Medina L, Paxinos G, Shimizu T, Striedter G, Wild M, Ball GF, Durand S, Güntürkün O, Lee DW, Mello CV, Powers A, White SA, Hough G, Kubikova L, et al. (2004) Revised nomenclature for avian telencephalon and some related brainstem nuclei. J Comp Neurol 473:377-414.
Shanahan M, Bingman VP, Shimizu T, Wild M, Güntürkün O (2013) Large-scale network organization in the avian forebrain: a connectivity matrix and theoretical analysis. Front Comput Neurosci 7:89.
Sherry DF, Hoshooley JS (2010) Seasonal hippocampal plasticity in food-storing birds. Philos Trans R Soc Lond B Biol Sci 365:933-943.
Stringham SA, Mulroy EE, Xing J, Record D, Guernsey MW, Aldenhoven JT, Osborne EJ, Shapiro MD (2012) Divergence, convergence, and the ancestry of feral populations in the domestic rock pigeon. Curr Biol 22:302-308.
Vellema M, Hertel M, Urbanus SL, Linden A, Gahr M (2014) Evaluating the predictive value of doublecortin as a marker for adult neurogenesis in canaries (Serinus canaria). J Comp Neurol 522:1299-1315.
Vellema M, Van der Linden A, Gahr M (2010) Area-speci fi c migration and recruitment of new neurons in the adult songbird brain. J Comp Neurol 518:1442-1459.
Wilbrecht L, Kirn JR (2004) Neuron addition and loss in the song system: regulation and function. Ann N Y Acad Sci 1016:659-683.
Wiskott L, Rasch MJ, Kempermann G (2006) A functional hypothesis for adult hippocampal neurogenesis: avoidance of catastrophic interference in the dentate gyrus. Hippocampus 16:329-343.
Yang HK, Sundholm-Peters NL, Goings GE, Walker AS, Hyland K, Szele FG (2004) Distribution of doublecortin expressing cells near the lateral ventricles in the adult mouse brain. J Neurosci Res 76:282-295.
Zupanc GK, Hinsch K, Gage FH (2005) Proliferation, migration, neuronal di ff erentiation, and long-term survival of new cells in the adult zebra fi sh brain. J Comp Neurol 488:290-319.
Zupanc GK, Horschke I (1995) Proliferation zones in the brain of adult gymnotiform fish: a quantitative mapping study. J Comp Neurol 353:213-233.
Copyedited by Li CH, Song LP, Zhao M
Amadi O. Ihunwo, Ph.D., Amadi.ihunwo@wits.ac.za.
10.4103/1673-5374.211187
*< class="emphasis_italic">Correspondence to: Amadi O. Ihunwo, Ph.D., Amadi.ihunwo@wits.ac.za.
orcid: 0000-0003-1097-7424 (Amadi O. Ihunwo)
杂志排行
中国神经再生研究(英文版)的其它文章
- SoxC transcription factors in retinal development and regeneration
- Umbilical cord: an unlimited source of cells di ff erentiable towards dopaminergic neurons
- Targeting 14-3-3 adaptor protein-protein interactions to stimulate central nervous system repair
- RACK1 regulates neural development
- Schwann cell development, maturation and regeneration: a focus on classic and emerging intracellular signaling pathways
- BDNF pro-peptide: a novel synaptic modulator generated as an N-terminal fragment from the BDNF precursor by proteolytic processing
