Synthesis and property of a layer vanadium oxide with transition metal coordination complexes
2017-07-06TIANShufangLIUYunLIJie
TIAN Shufang, LIU Yun, LI Jie
(College of Chemistry and Chemical Engineering, Henan University, Kaifeng 475004, Henan, China))
Synthesis and property of a layer vanadium oxide with transition metal coordination complexes
TIAN Shufang, LIU Yun, LI Jie*
(CollegeofChemistryandChemicalEngineering,HenanUniversity,Kaifeng475004,Henan,China))
The hydrothermal reaction of ammonium vanadate as starting materials with divalent first-row transition metal cations in the presence of nitrogen donor chelating ligands at 160 ℃ yielded the brown block solid of [Co(dien)2]2[V6O17]·3H2O (1) (dien = diethylenetriamine), which was structurally characterized by elemental analysis, IR and single-crystal X-ray diffraction analysis. Results indicate that the product 1 belongs to monoclinic system of space groupP2(1) /c, and its crystal data are determined to bea= 1.613 4(4) nm,b= 0.866 8(2) nm,c= 1.398 1(4) nm,β= 103.070(4)°,V= 1.904 7(9) nm3,ρ= 2.016 g/cm3,Z= 2. The crystal structure is composed of vanadium oxide layers and [Co(dien)2]2+fragments. These vanadium oxide layers are composed of a {VO}nhelical chain.
polyoxometalate; hydrothermal; crystal structure; hexavanadates
Although polyoxovanadates constitute an important subclass of metal oxide cluster with an exceptional capacity to form mixed-valence compounds that exhibit rich electronic and magnetic properties and have relevance to catalysis, medicine, and materials science[1], it is only recently that attention has been drawn to the synthesis and structure of such new compounds templated by organic amines[2-8]. Until now the examples of polyoxovanadates templated or coordinated by transition metal complexes are still few. They include one-dimensional metavanadate chain compounds[9-10], in which the co-ordination groups are attached to the V-O chains. Some new vanadium oxide layers have also been observed in [Ni(en)2][V6O14][11], (L2M)y[VOx] (L = bidentate amine, M = Cu or Zn, 0.16 ≤y≤ 0.33 and 2.335 ≤x≤ 12.8)[12], [Cd(enMe)2][V8O20][13], Ni(dien)V2O6[14], and K{V12IVV6VO42Cl[Ni(en)2]3}[15]. KHAN et al. have also reported some three-dimensional frameworks which consist of spherical [V18O42(XO4)] cages linked by bridging [M(H2O)4] groups (X = V, S; M = Fe, Co, Mn, Zn)[16]. Vanadium adopts so many co-ordination geometries and multifarious oxidation states, therefore the metal ions as well as the organic ligands also influence on the structures. Recently we have always devoted ourselves to the systemative investigation of polyoxovanadates in order to find the effective pathways to design and synthesize the metal oxide clusters with the neoteric and intriguing structures. In this paper, we report on the hydrothermal synthese, structure and thermal property of a layered vanadium oxide with interlayer metal coordination complex: [Co(dien)2]2[V6O17]·3H2O (1) (dien = diethylenetriamine).
1 Experiment
1.1 Materials and physical measurements
All reagents were purchased commercially and used without further purification. C, H, and N elemental analyses were performed on a Perkin-Elmer 240C elemental analyzer. Inductively coupled plasma (ICP) analysis was performed on a Perkin-Elmer Optima 2000 ICP-OES spectrometer. Infrared spectra were recorded on a Bruker VERTEX 70 IR spectrometer using KBr pellets from 400 to 4 000 cm-1.
1.2 Synthesis of compound 1
Raw materials used in the synthesis were NH4VO3, Co(NO3)2· 6H2O, dien, 1, 4-Bis(imidazole-l-ylmethyl)benze, and distilled water. In a typical synthesis of compound 1, 0.34 g of NH4VO3was added to 20 mL of distilled water, to which 0.58 g of Co(NO3)2· 6H2O, 0.25 mL of dien and 0.12 g of 1, 4-bis(imidazole-l-ylmethyl)benze were added with stirring. The reaction mixture was sealed in a 30 mL Teflon-lined autoclave and heated under autogenous pressure at 160 ℃ for 3 d. The crystals were separated mechanically, washed with water and dried in air at ambient temperature. The yield of compound 1 was ca. 12% based on vanadium. Anal. Calc. for C16H52Co2N12O20V6: C, 16.62; H, 4.53; N, 14.54. Found: C, 16.45; H, 4.67; N, 14.46.
1.3 Single-crystal X-ray diffraction (XRD)
The crystal structure of 1 was determined by single crystal X-ray diffraction methods. The data on a crystal with dimensions 0.31 mm × 0.27 mm × 0.23 mm were collected on a Bruker Smart Apex-II CCD diffractometer with graphite monochromated Mo Kαradiation (λ= 0.071 073 nm). Cell constants and an orientation matrix for data collection were obtained from least-squares refinements of the setting angles in the range of 1.30° ≤θ≤ 25.09°. Routine Lorentz polarization and an empirical absorption correction were applied to intensity data. On the basis of systematic absences and statistics of intensity, the space groups wereP2(1)/c. Their structures were determined and the heavy atoms were found by direct methods using the SHELXTL-97 program package[17]. The remaining atoms were found from successive full-matrix least-squares refinements onF2and Fourier syntheses. No hydrogens associated with water molecules were located from the difference Fourier map. Positions of the hydrogens attached to carbon and nitrogen were geometrically placed. All hydrogens were refined isotropically as a riding mode using the default SHELXTL parameters. A total of 9 425 reflections were collected, of which 3 393 unique reflections (Rint= 0.022 0) were used for structural elucidation. A summary of the crystallographic data is given in Table 1. CCDC reference number is 1542235.
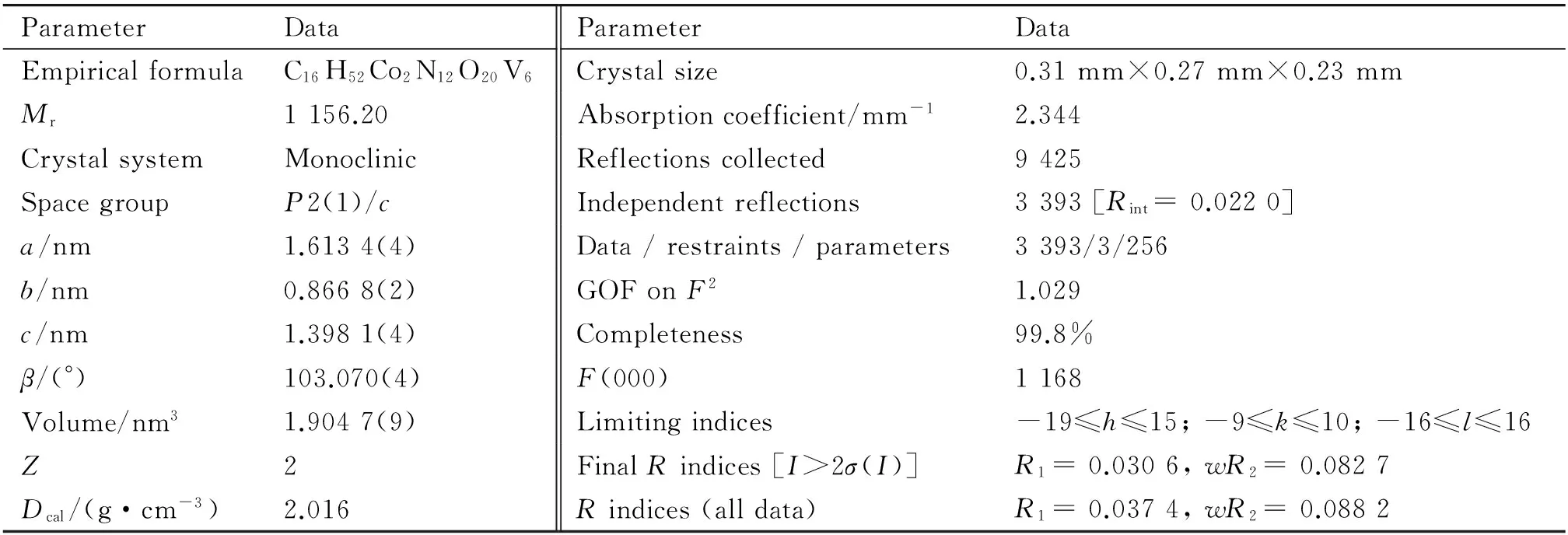
Table 1 Crystal data and structural refinements for compound 1
2 Results and discussion
2.1 Synthesis
The simple hydrothermal route is an effective way to synthesize a great variety of inorganic solid-state materials, which contain amine-ligated transition metal cations situated between vanadium oxide layers. The vanadium oxide [Co(dien)2]2[V6O17]·3H2O (1) is prepared in the hydrothermal method by the reaction of the transition metal element, the mixed ligands, and NH4VO3in aqueous solution at 160 ℃, and is isolated as thin brown block crystalline.
2.2 Structure determination
Compound 1 shares similar structural features of vanadium oxide layers as well as six-coordinate interlayer cations with [Zn(bipy)2]2[V6O17][12]. Fig. 1 shows the coordination environments around the Co and V atoms in 1. The Co atoms reside in a six-coordinate and very regular CoN6octahedral environment with the Co-N bond lengths [0.194 9(3)-0.196 6(3) nm], which are similar to those of the previously reported compounds[18-19]. The coordination atoms of Co atom in 1 are different from that of the Zn atoms in [Zn(bipy)2]2[V6O17], which are four N atoms from two bidentate amine ligands and two O atoms from the VO layer. The layers are built up in all cases from VO4te-trahedra or VO5square pyramids, connected by edge- or corner-sharing interactions. The three vanadium atoms V1, V2 and V3 of 1 are crystallographically distinct. They adopt distorted tetrahedral environments and are corner-shared by O1, O4 and O7 atoms to form VO4helical chains instead of V4O12tetramer rings as in the case of Cu(dien)V2O6· H2O[14]. The bond distances of the bridging oxygen and the vanadium atoms (V-μ2-O) are the V-O1 [mean: 0.180 3(2) nm], V-O4 [mean: 0.180 5(3) nm] and V-O7 [mean: 0.178 9(3) nm], which are close to each other, however these values are very much smaller than those in [Ni(en)2][V6O14] [mean: 0.213 7(2) nm][11]. The remaining terminal oxygen atoms have V=O distances between 0.160 7(3) and 0.164 0(3) nm, which are bigger than those of [Ni(en)2][V6O14] [1.576(6)-1.603(8) nm]. The the O-V-O angles vary from 105.15(13)° to 111.53(13)°, which are comparable to the reported vanadates data.
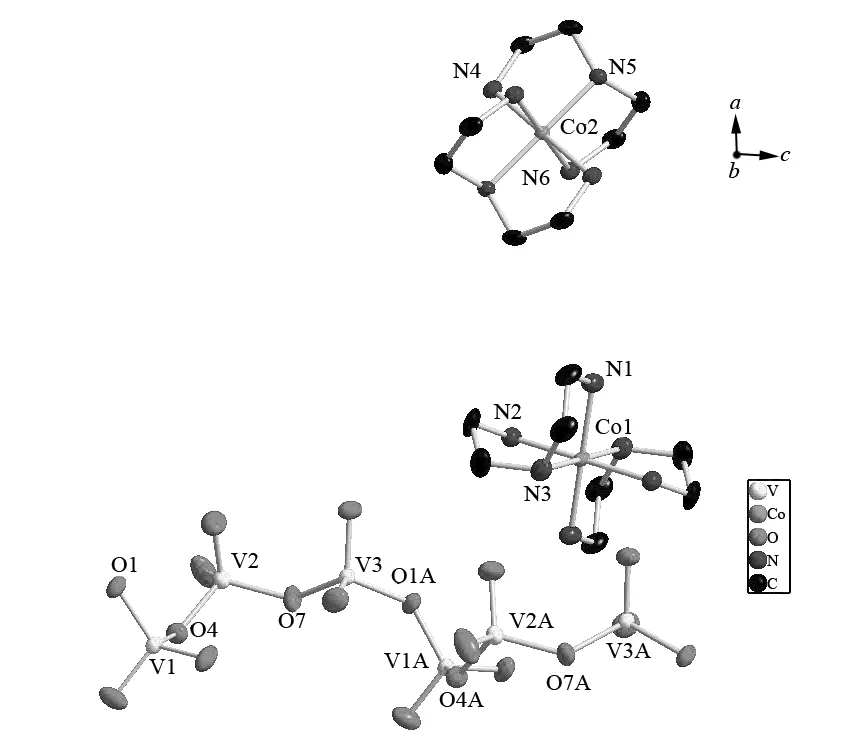
Fig.1 Co-ordinations around the Co and V atoms with the atomic labeling scheme in compound 1 with H atoms omitted for clarity
Fig.2 displays the framework structure of compound 1. The inorganic layers formulated by [V6O17]4-are composed solely of VO4tetrahedra. All of VO4te-trahedra have two vanadyl oxygen groups (V=O) toward opposite sides of the layer and share two corners with two neighboring VO4units to form a ‘V’ shape V3O10building group. The V3O10groups are connected through sharing corners in the alternating “up” and “down” orientations, which leads to the information of a helical chain. This unique characteristic of the polyhedral arrangement has not been reported for any other single-layer vanadium oxide, and it has even been suggested that this arrangement of two corner-shared vanadium tetrahedra oriented in the same direction is not possible[20]. The screw pitch of the inorganic framework helical chain is about 0.727 nm.
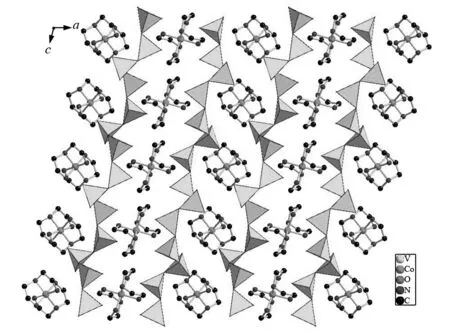
Fig.2 Projection of compound 1 along the b axis, showing the vanadium oxide layers with [Co(dien)2]2+ complexes located in the interlayer positions. The hydrogen atoms are omitted for clarity
On the basis of valence sum (∑s) calculations[21], the oxidation states of the two Co atoms are +2 (∑s= 2.15-2.19) and the six V atoms are +5 (∑s= 5.22-5.24) in 1. The oxidation states of the Co and V atoms are consistent with the formula of compound 1.
2.3 IR spectrum
In the IR spectrum of compound 1, strong bands at 1 012 and 983 cm-1are assigned to the terminal V=O stretching in VO4tetrahedra. Features at 883 and 761 cm-1are related to the symmetric and antisymme-tric stretching of V-O-V (Fig. 3). In addition, stretches of -OH, -NH2, and -CH2are observed at 3 437, 3 302 and 3 210, 2 938 and 2 880 cm-1for 1. Bands at 1 629, 1 570, and 1 451 cm-1for 1 are assigned to bending vibration of -OH, -NH2, and -CH2, respectively[22]. The vibration pattern ofν(C-N) is at 1 390 cm-1for 1. The occurrence of these resonance signals confirms the presence of organic amine groups, which are consistent with the single-crystal structural analyses.
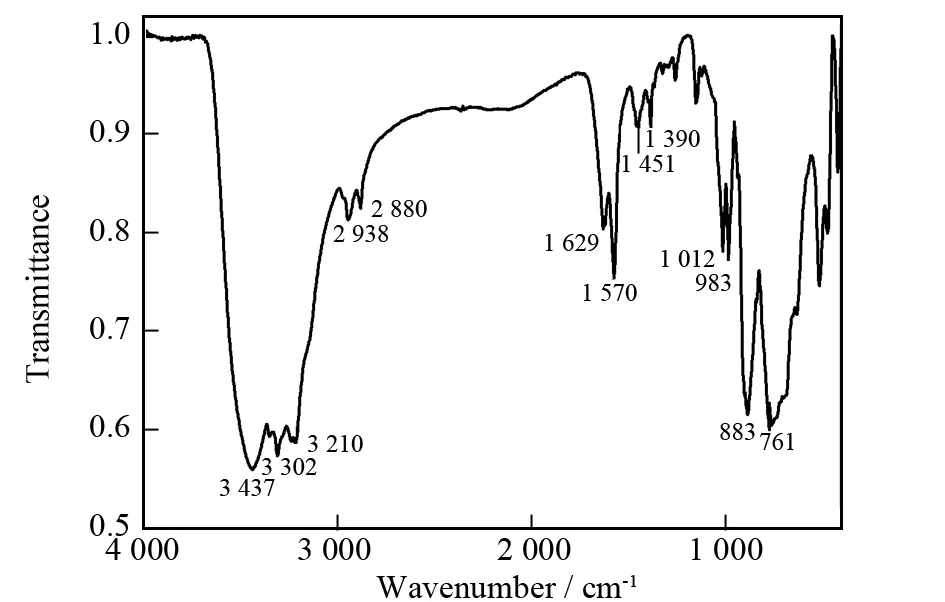
Fig.3 IR spectrum of 1
3 Conclusion
In conclusion, this work describes the structure of a new vanadium oxide with transition metal coordination complexes. The result demonstrates that compound 1 bears such a kind of structure type with vanadium oxide layers. These vanadium oxide layers are composed of a {VO}nhelical chain. The V-O-V linkages play a critical role in constructing the layer structure.
[1] (a) POPE M T, MÜLLER A. Polyoxometalate chemistry: An old field with new dimensions in several disciplines [J]. Angewandte Chemie International Edition in English, 1991, 30: 34-48; (b) MÜLLER A, RETERS F, POPE M T, et al. Polyoxometalates: very large clusters nanoscale magnets [J]. Chemical Reviews, 1998, 98(1): 239-272; (c) MÜLLER A. Induced molecule self-organization [J]. Nature, 1991, 352(6331): 115-116; (d) POPE M T. Heteropoly and isopoly oxometalates [M]. Berlin: Springer, 1983: 1-426.
[2] CHIRAYIL T G, BOYLAN E A M, MAMAK P Y, et al. NMe4V3O7: critical role of pH in hydrothermal synthesis of vanadium oxides [J]. Chemical Communications, 1997(1): 33-34.
[3] NAZAR L F, KOENE B E, BRITTEN J F. Hydrothermal synthesis and crystal structure of a novel layered vanadate with 1,4-diazabicyclo[2.2.2]octane as the structure-directing agent: (C6H14N2)V6O14· H2O [J]. Chemical Material, 1996, 8: 327-329.
[4] ZHANG Y, HAUSHALTER R C, CLEARFIELD A. [HN(C2H4)3NH][V6O14]·H2O: a mixed-valence layered vanadium oxide with interlamellar organic cations [J]. Chemical Communications, 1996(9): 1055-1056.
[5] ZHANG Y, HAUSHALTER R C, CLEARFIELD A. Hydrothermal syntheses and structural characterization of layered vanadiumoxides incorporating organic cations:α-β-(H3N(CH2)2NH3)[V4O10] andα-β-(H2N(C2H4)2NH2)[V4O10] [J]. Inorganic Chemistry, 1996, 35(17): 4950-4956.
[6] ZHANG Y, O’CONNOR C J, CLEARFIELD A, et al. An organically templated layered vanadium oxide: hydrothermal synthesis, single-crystal structure, and magnetic properties of (H3N(CH2)3NH3)[V4O10] [J]. Chemical Material, 1996, 8(3): 595-597.
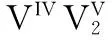
[8] HUAN G, JOHNSON J W, JACOBSON A J, et al. Hydrothermal synthesis and single-crystal structural characterization of VO(VO3)6(VO(C10H8N2)2)2[J]. Journal of Solid State Chemistry, 1991, 91(2): 385-389.
[9] ASCHWANDEN S, SCHMALLE H W, RELLER A, et al. Preparation, crystal structure, thermal and catalytical behaviour of copper diammine divanadate [J]. Materials Research Bulletin, 1993, 28(1): 45-48.
[10] DEBORD J R D, ZHANG Y, HAUSHALTER R C, et al. One-dimensional vanadium oxide chains containing covalently bound copper coordination complexes: hydrothermal synthesis and characterization of Cu(H2N(CH2)2NH2)[V2O6], Cu(C10H8N2)[V2O6], and Cu(C10H8N2)2[V2O6] [J]. Journal of Solid State Chemistry, 1996, 122(2): 251-258.
[11] LIN B Z, LIU S X. Hydrothermal synthesis, structure and characterization of a novel layered vanadium oxide with metal coordination complexes: [Ni(en)2][V6O14] [J]. Journal of Chemistry Society, Dalton Transaction, 2002: 865-869.
[12] ZHANG Y, DEBORD J R D, O’CONNOR C J, et al. Solid-state coordination chemistry: hydrothermal synthesis of layered vanadium oxides with interlayer metal coordination complexes [J]. Angewandte Chemie International Edition in English, 1996, 35(9): 989-991.
[13] ZHANG L, SHI Z, YANG G, et al. Hydrothermal synthesis and crystal structure of a layered vanadium oxide with an interlayer metal co-ordination complex: Cd[C3N2H11]2[V8O20] [J]. Journal of Chemistry Society, Dalton Transaction, 2000, 3: 275-278.
[14] ZHENG L M, ZHAO J S, LI K H, et al. One- and two-dimensional materials containing vanadium oxide: structures and magnetic properties of Cu(dien)V2O6·H2O and Ni(dien)V2O6(dien = diethylenetriamine) [J]. Journal of Chemistry Society, Dalton Transaction, 1999, 6: 939-943.
[15] PAN C L, XU J Q, LI G H, et al. A two-dimensional framework of novel vanadium clusters bridged by [Ni(en)2]2: K{V12IVV6VO42Cl[Ni(en)2]3}·8H2O [J]. Dalton Transaction, 2003, 4: 517-518.
[16] KHAN M I. Novel extended solids composed of transition metal oxide clusters [J]. Journal of Solid State Chemistry, 2000, 152(1): 105-112.
[17] SHELDRICK G M. SHELXL 97: Program for refinement crystal structure [CP]. Göttingen: University of Göttingen, 1997.
[18] KHAN M I, YOHANNES E, NOME R C, et al. Inorganic-organic hybrid materials containing porous frameworks: synthesis, characterization, and magnetic properties of the open framework solids [{Co(4,4′-bipy)}V2O6] and [{Co2(4,4′-bipy)3(H2O)2}V4O12]·2H2O [J]. Chemical Material, 2004, 16: 5273-5279.
[20] ENJALBERT R, GALY J. A refinement of the structure of V2O5[J]. Acta Crystallographica, 1986, C42: 1467-1469.
[21] BRESE N E, O'KEEFFE M. Bond-valence parameters for solids [J]. Acta Crystallographica, 1991, B47: 192-197.
[22] LIU Y B, CUI X B, XU J Q, et al. Hydrothermal synthesis and characterization of three one-dimensional chain materials formed by reduced tetra-capped Keggin polyoxoanions and [M(en)2]2+(M = Cu, Co and Ni) cations [J]. Journal of Molecular Structure, 2006, 825: 45-52.
[责任编辑:吴文鹏]
含有过渡金属配合物的层状钒氧化物的合成和性质
田淑芳,刘 云,李 杰*
(河南大学 化学化工学院,河南 开封 475004)
以钒酸铵为起始原料,在螯合配体存在下,利用水热合成法将其与二价第一行过渡金属阳离子在160 ℃反应得到了一个棕色块状化合物[Co(dien)2]2[V6O17]·3H2O (1) (dien = 二乙烯三胺),并用元素分析、红外光谱和X 射线单晶衍射对之进行了定性分析. 结果表明,化合物1 属于单斜晶系,P2(1) /c空间群;其晶格参数为:a= 1.613 4(4) nm,b= 0.866 8(2) nm,c= 1.398 1(4) nm;β= 103.070(4)°;V= 1.904 7(9) nm3;ρ= 2.016 g/cm3;Z= 2. 化合物1是由钒氧层和[Co(dien)2]2+构成. 这些钒氧层是由{VO}n螺旋链组成.
多金属氧酸盐;水热合成;晶体结构;六钒酸盐
The Foundation of Education Department of Henan Province(15A150037).
, E-mail:lllijie2007@henu.edu.cn.
O614.5 Document code: A Article ID: 1008-1011(2017)03-0321-05
Received date: 2007-04-13.
Biography: TIAN Shufang (1982-),female,lecturer,research field: polyoxomolybdate chemistry.*
