干旱胁迫对转JERF36银中杨苗木叶片解剖结构及光合特性的影响*
2017-06-23张伟溪丁昌俊苏晓华黄秦军
黄 绢 陈 存 张伟溪 丁昌俊 苏晓华 黄秦军
(林木遗传育种国家重点实验室 国家林业局林木培育重点实验室 中国林业科学研究院林业研究所 北京100091)
干旱胁迫对转JERF36银中杨苗木叶片解剖结构及光合特性的影响*
黄 绢 陈 存 张伟溪 丁昌俊 苏晓华 黄秦军
(林木遗传育种国家重点实验室 国家林业局林木培育重点实验室 中国林业科学研究院林业研究所 北京100091)
【目的】 以转JERF36银中杨(ABJ01)和非转基因银中杨(9#)为试验材料,开展干旱胁迫对2个株系苗高生长、叶片形态解剖结构、光合特性的影响研究,以期为转基因杨树的抗旱性评价提供参考,并为转基因杨树的推广应用提供科学依据。【方法】 于2015年6月底,苗高约45 cm时,选取生长一致的苗木进行土壤干旱胁迫试验。胁迫程度分为3个梯度: 正常供水、中度胁迫、重度胁迫,土壤含水量分别控制在田间持水量的60%~80%,40%~60%,20%~40%,胁迫时间为30天。【结果】 干旱胁迫下,2个株系的苗高生长均受到抑制,随着胁迫程度的加剧,受抑制程度增大,但ABJ01受抑制程度较低,重度胁迫下其苗高显著高于9#。叶片形态数据显示,与正常供水相比,干旱胁迫处理后,2个株系的单叶面积显著降低,说明干旱胁迫抑制杨树叶片生长; 中度、重度胁迫下9#的单叶面积均显著低于ABJ01,表明ABJ01叶片生长受抑制程度低。叶片解剖结构数据显示,ABJ01和9#的叶表皮细胞生长和叶肉细胞生长均受干旱胁迫的抑制,但ABJ01受抑制程度较低。中度胁迫下,ABJ01的叶片上、下表皮厚度分别比9#显著高出5.55%和4.70%,栅栏组织厚度比9#显著高出6.17%,海绵组织厚度和叶片组织疏松度(SR)则分别比9#显著降低12.35%和12.38%; 重度胁迫下ABJ01的叶片上、下表皮厚度分别比9#显著高出16.27%和10.58%,海绵组织厚度和SR则分别比9#显著降低11.71%和11.58%。ABJ01具有更发达的栅栏组织和相对少的海绵组织,这有助于CO2向光合场所的传导,维持叶片较高的净光合速率(Pn),以适应干旱胁迫条件。光合生理数据显示,干旱胁迫下,ABJ01的Pn显著高于非转基因株系9#(10.50%~18.97%),说明干旱胁迫下ABJ01具有更强的光合能力。正常供水下,2个株系的气孔导度(Gs)、最大光化学效率(Fv/Fm)差异不显著,干旱胁迫下转基因株系ABJ01的Gs、Fv/Fm下降幅度比非转基因株系9#小,说明ABJ01受干旱胁迫影响程度较低; ABJ01的蒸腾速率(Tr)小于9#,表明ABJ01在干旱胁迫下具有更强的保水能力。ABJ01的叶片叶绿素a、叶绿素b和总叶绿素含量较9#高,Fv/Fm值较9#高,说明转基因株系维持叶绿素含量稳定的能力较强且光系统受损伤程度小。【结论】 外源基因JERF36可能通过影响叶片结构发育提高转基因银中杨在干旱胁迫下的气体交换能力和保水能力,从而增强转基因银中杨的抗旱能力。
干旱胁迫; 转基因杨树; 叶片解剖结构; 光合特性
各种各样恶劣的环境条件影响着植物的生长、代谢、生产力等,其中,干旱是限制植物生长和产量的主要因素之一(Arausetal., 2002; Reddyetal., 2004),它对农作物和林木造成的损失仅次于病虫害类生物胁迫,在所有的非生物限制因子中占首要地位(马耀光等, 2003)。叶片是植物进行光合作用的主要器官,也是植物对干旱胁迫最敏感的部位之一(李欢等, 2013; 燕玲等,2002),在干旱环境中生长的植物,往往会形成多种抗旱性的形态结构,其中叶片结构最能反映植物对干旱生境的适应性(Zhaoetal., 1981; 李芳兰等, 2005)。此外,植物叶片抗旱性生理可塑性强,因此叶片形态结构和生理变化可用来反映植物对干旱的适应性强弱(Rubio de Casasetal., 2007)。
银中杨(Populusalba×P.berolinensis)是以银白杨(Populusalba)为母本、中东杨(Populusberolinensis)为父本,经人工杂交选育而成,具有速生、树形优美等特点,但耐盐能力较差,影响了其推广应用。隶属于ERF类的转录因子JERFs来源于番茄(Lycopersiconesculentum),它能专一结合GCC-box,激活植物下游抗逆相关基因的表达,提高植物耐盐、抗旱、抗寒性(李文正等, 2006; Zhangetal., 2010; Wuetal., 2008),在烟草(Nicotianatabacum)中表达的JERF3还能激活光合碳同化相关基因(Wuetal., 2008)。中国林业科学研究院林业研究所于2000年通过农杆菌介导法将JERF36基因导入银中杨(苏晓华等, 2009)。经分子生物学检测、温室盐胁迫和大田盐碱地试验,证明目的基因成功导入并稳定表达,转JERF36银中杨的耐盐性得到显著提高(李义良, 2008; Lietal., 2009),然而,前期研究并未对转基因银中杨的抗旱性进行相关评价。本研究以转JERF36银中杨(ABJ01)和非转基因银中杨(9#)为试验材料,从叶片形态、解剖结构和光合生理特性角度探索转JERF36基因银中杨对干旱胁迫的响应,研究JERF36基因的导入对银中杨抗旱性的影响,以期为转基因杨树的抗旱性评价提供参考,并为转基因杨树的推广应用提供科学依据。
1 材料与方法
1.1 试验材料
试验材料为中国林业科学研究院林业研究所2000年通过农杆菌介导法成功转化获得的转JERF36基因银中杨(ABJ01)和非转基因银中杨(9#)。2015年4月10日将ABJ01和9#的1年生植株,截成15 cm左右的硬枝插穗,扦插于塑料小盆中(10 cm × 10 cm),基质为草炭土和珍珠岩,每盆1株,在温室正常供水条件下培养。2015年5月20日将生长良好且一致的植株移栽至高度21 cm、直径19 cm的塑料大盆中,基质由黄土、沙土和草炭土按10∶2∶1的比例混合配制而成,每盆基质质量5.5 kg,正常供水条件下培养。
1.2 干旱处理方法
待苗高达到45 cm左右时(6月底),每个株系选择大小一致、生长健壮的苗木18株(盆),进行干旱胁迫处理。试验采用完全随机区组设计,共3个水分处理: 正常供水、中度胁迫、重度胁迫,土壤含水量分别控制在田间持水量的60~80%,40~60%,20~40%,每个处理重复3次。每天17: 00时称取各盆质量,补充水分,使各盆土壤含水量保持在试验控制范围之内。干旱胁迫处理30天后(7月底)测定各项指标。
在中国林业科学研究院温室内进行试验,温室内6—9月平均温度控制在25~30 ℃,平均相对湿度为55%; 温室透光性良好,晴天9:00—11:00室内光合有效辐射PAR约为800 μmol·m-2s-1,室内CO2浓度约为380 μmol·mol-1。
1.3 指标测定方法
1.3.1 苗高及叶片数量测定 苗高指从苗木土痕处到苗木最高点; 叶片数量是指从顶端第1片完全展开叶到苗木下端最后1片存在生理活性的叶片总数。
1.3.2 叶面积测量 采用CI-203激光叶面积仪活体测量植株叶面积,测量叶片均为植株顶端向下第5,6,7片功能叶,每个处理重复3次。
1.3.3 叶片解剖结构观察 在苗木中部偏上选取干旱胁迫后新形成的、大小基本一致的叶片,靠近其中脉位置处切取0.3 cm × 0.3 cm的小片,立即放入FAA溶液中固定24 h以上。采用半薄切片技术,经脱水和透明,置换和浸透,Spurr树脂包埋和聚合,制成包埋样品; 使用半薄切片机(Leica EMUC7切片机, Germany)进行切片,薄切片厚度为3 μm,制片后采用0.5%的甲苯胺蓝(toluidine blue O,TBO)染色,随后在显微镜下观察照相(Zeiss Axioskop2 plus; AxioVisionRel.4.4),并用软件(AxioVisionRel.4.4)测量各指标值: 叶片厚度、上表皮厚度、下表皮厚度、栅栏组织厚度、海绵组织厚度、叶片组织紧密度(CTR)、叶片组织疏松度(SR)。每个处理重复3次,每个株系8~10个切片,每个切片每个指标记录3个数据。CTR和SR的计算公式如下(潘存娥, 2011):
CTR(%)=栅栏组织厚度/叶片厚度 × 100,
SR(%)=海绵组织厚度/叶片厚度 × 100。
1.3.4 气体交换参数测定 采用美国Li-COR公司生产的LI-6400XT便携式光合测定仪,于9:00—11:00进行Pn、Gs、Ci、Tr等气体交换参数测定。LI-6400XT的光合有效辐射(PAR)设定为800 μmol·m-2s-1,CO2浓度控制为400 μmol·mol-1。测量叶片均为植株顶端向下第5,6,7片功能叶,每片叶重复测量3次,每个处理3次重复。
1.3.5 PSⅡ最大光化学效率(Fv/Fm)测定 采用德国WALZ公司的Mini-PAM超便携式调制叶绿素荧光仪,于23:00—24:00时测定暗适应后的各株系中部叶片最大荧光产量(Fm)和可变荧光(Fv),计算Fv/Fm。每个处理重复3次,每个株系记录5片叶数据。
1.3.6 叶绿素含量测定 叶绿素含量测定参考《植物生理学实验指导》(高俊凤, 2006)。称取植物新鲜叶片0.1 g于25 mL容量瓶中,加入0.5 mL纯丙酮和15 mL 80%丙酮,盖上瓶盖室温下暗处浸提过夜(期间摇动3~4次),直至样品全部变白为止,用80%丙酮定容浸提液至25 mL。5 000 r·min-1下离心5 min,以80%丙酮为空白对照,用分光光度计在645,663 nm波长下测定浸提液的吸光度,计算组织中叶绿素含量。测定的叶片为从植株顶端向下第5,6,7片功能叶,每个处理重复3次。
1.4 数据处理
采用Excel 2013进行数据处理,并通过SPSS17.0统计软件进行多重比较(采用Duncan新复极差法)。
2 结果与分析
2.1 干旱胁迫下植株生长、叶片形态及解剖结构变化
2.1.1 干旱胁迫下植株生长及叶片形态变化 正常供水条件下,ABJ01和9#的苗高、叶片数量及单叶面积差异均不显著。干旱胁迫下,ABJ01和9#的苗高生长均受到一定程度的抑制,随着胁迫程度的加剧,受抑制程度增大,但ABJ01受抑制程度较低,在中度和重度干旱胁迫下苗高比9#分别高5.39%和9.38%,重度干旱胁迫下二者差异达到显著水平。与正常供水相比,2个株系的叶片数量在干旱胁迫下均显著减少,但ABJ01叶片数量略高于9#。干旱胁迫下,2个株系的叶面积生长均受到显著抑制,ABJ01和9#分别比正常供水低3.50%~27.39%和15.19%~32.86%。中度和重度干旱胁迫下,9#的叶面积比ABJ01分别显著低10.82%和13.79%,说明ABJ01叶片生长优于9#。以上结果说明,转基因银中杨在干旱胁迫下具有更强的生长能力,其抗旱能力可能得到增强。

表1 干旱胁迫下苗木生长及叶片形态差异Tab.1 Differences of seedling growth and leaf phenotype under drought stress①
小写字母表示相同系号不同干旱处理间差异显著,大写字母表示相同干旱处理条件下不同株系间差异显著,显著性水平为0.05。下同。Lowercase letters represent significant differences of the same poplar lines at different treatment, capital letters represent significant difference test of different poplar lines at the same treatment. Significant difference test level is at 0.05. The same below.
2.1.2 干旱胁迫下植株叶片解剖结构组成和变化 研究表明,干旱胁迫下,2个株系的叶片厚度、上表皮厚度、海绵组织厚度、下表皮厚度与正常供水条件相比显著降低。9#栅栏组织厚度干旱胁迫时比正常供水显著低5.96%~8.32%,而ABJ01则干旱胁迫时比正常供水显著高7.36%~9.55%。中度胁迫下,ABJ01的叶片上、下表皮厚度比9#分别显著高5.55%和4.70%,栅栏组织厚度比9#显著高6.17%,表明ABJ01具有更发达的栅栏组织,但海绵组织厚度和SR则分别比9#显著低12.35%和12.38%; 重度胁迫下, ABJ01的叶片上、下表皮厚度分别比9#显著高16.27%和10.05%,海绵组织厚度和SR则分别比9#显著低11.71%和11.58%。以上结果说明,ABJ01叶片结构可能更有利于提高其在干旱胁迫时的适应能力。

图1 叶片解剖结构组成Fig.1 Leaf anatomical structures图中大写字母A、B、C分别表示9#在正常供水、中度胁迫、重度胁迫下的叶片结构组成; D、E、F分别表示ABJ01在正常供水、中度胁迫、重度胁迫下的叶片结构组成。Capital letters A, B, C respectively represent leaf anatomical structures of 9# at control, moderate drought stress and severe drought stress; D, E, F respectively represent leaf anatomical structures of ABJ01 at control, moderate drought stress and severe drought stress.
2.2 干旱胁迫下植株光合特性变化
2.2.1 干旱胁迫下植株气体交换参数变化 正常供水下,ABJ01与9#的净光合速率(Pn)差异不显著,随着干旱胁迫程度的加剧,2个株系的Pn均逐渐降低。中度和重度胁迫下,ABJ01的Pn分别比9#降低10.50%和18.97%,重度胁迫时二者达显著水平(图2A)。结果说明ABJ01在干旱胁迫下具有更强的光合能力,这可能与ABJ01具有发达的栅栏组织有关(表2)。
正常供水下,ABJ01与9#的气孔导度(Gs)、胞间CO2浓度(Ci)和蒸腾速率(Tr)差异均不显著,随着干旱胁迫程度的加剧,2个株系的Gs、Ci和Tr均逐渐降低(图2B,C,D),变化趋势与Pn一致。与正常供水相比,重度胁迫下2个株系的Gs显著降低,且在重度胁迫时ABJ01的Gs比9#高17.80%(图2B),说明ABJ01可能具有更强的气体交换能力。中度干旱胁迫下,ABJ01的Ci比9#显著低1.67%(图2C),这与其具有较高的Pn有关。 重度胁迫时ABJ01的Tr比9#显著低8.29%(图2D),说明干旱胁迫下,ABJ01的保水能力较强。

表2 干旱胁迫下各株系叶片解剖结构变化Tab.2 Changes of leaf anatomical structure in different poplar lines under drought stress
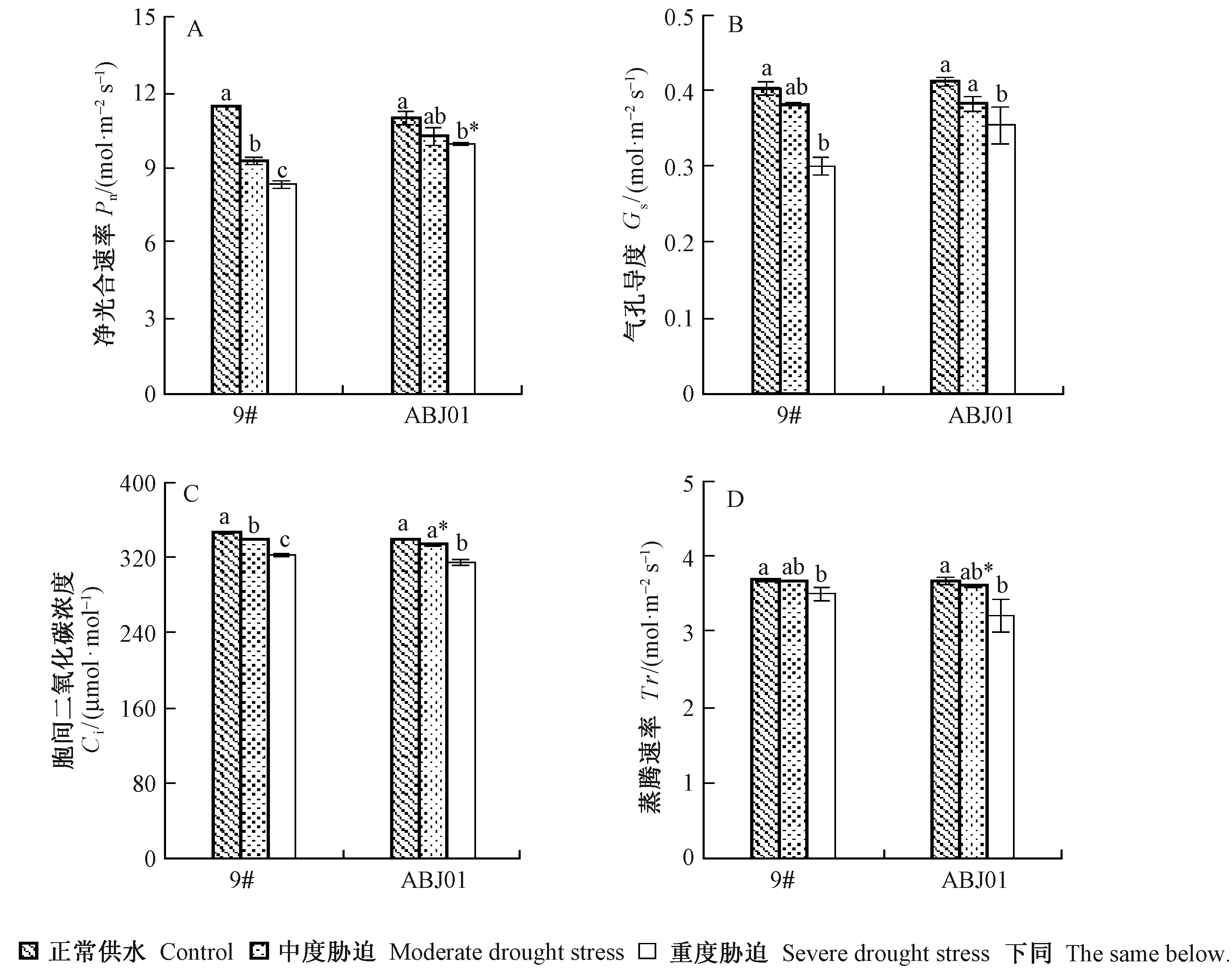
图2 干旱胁迫下杨树气体交换参数变化Fig.2 Changes of gas exchange parameters in poplar under drought stresses小写字母表示相同株系不同干旱处理间差异显著,*表示相同处理下不同系号间差异显著,显著性水平均为0.05。Lowercase letters represent significant differences result of same poplar lines at different treatments; * represent significant difference test result of different poplar lines at same treatment;Significant level is at 0.05.
2.2.2 干旱胁迫下植株Fv/Fm变化 干旱胁迫下,2个株系的PSⅡ最大光化学效率(Fv/Fm)变化如图3。正常供水下,2个株系的Fv/Fm差异不显著。干旱胁迫下2个株系的Fv/Fm显著降低,中度胁迫下,9#和ABJ01分别比正常供水降低2.82%和1.35%,重度胁迫下,则达到5.70%和5.20%。另外,在干旱胁迫时ABJ01的Fv/Fm高于9#,说明干旱胁迫处理后,ABJ01的光系统受损程度较9#小,这与其具有较高的Pn有关(图2A),ABJ01的抗旱能力可能得到增强。

图3 干旱胁迫下转基因银中杨Fv/Fm变化Fig.3 Changes of Fv/Fm in transgenic poplar under drought stress
2.2.3 干旱胁迫下植株叶片叶绿素含量变化 干旱胁迫下,2个株系的叶绿素含量变化如图4。正常供水下,ABJ01与9#的叶绿素a、叶绿素b和总叶绿素含量差异均不显著。干旱胁迫下,2个株系的叶绿素a、叶绿素b及总叶绿素的含量较正常供水均呈降低趋势(ABJ01在重度胁迫下叶绿素b含量例外),但ABJ01的降低幅度较9#小。重度干旱胁迫下,ABJ01的叶绿素a、叶绿素b和总叶绿素含量分别比9#显著高18.50%,29.13%和21.28%。说明干旱胁迫下转基因株系维持叶绿素含量稳定的能力较非转基因株系强。
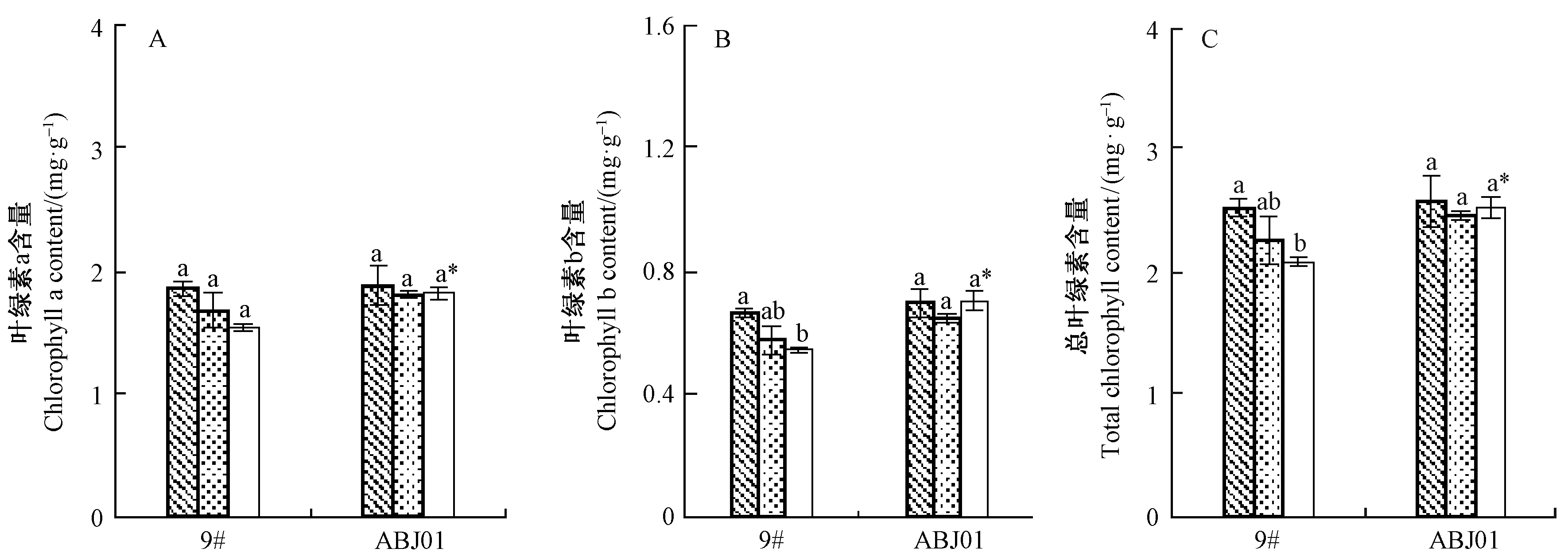
图4 干旱胁迫下杨树叶片叶绿素含量差异比较Fig.4 Differences of leaf chlorophyll content in poplar under drought stress
3 讨论
植物生长量的降低是对干旱胁迫的一种响应(Boughallebetal., 2011),树木一般通过减小叶面积、降低蒸腾来适应干旱胁迫(叶龙华等, 2014)。本研究表明,干旱胁迫下,2个株系的苗高生长均受到一定程度的抑制,但ABJ01苗高比9#分别高5.39%和9.38%,其中重度胁迫下二者差异达到显著水平; 2个株系的单叶面积均显著降低,但9#的单叶面积显著低于ABJ01,表明ABJ01的苗高和单叶面积生长受干旱胁迫程度较9#小(表1)。干旱胁迫下,转基因株系具有更强的生长能力,其抗旱能力可能得到提高。
干旱胁迫通常会引起植物形态、叶片水势、光合碳同化等生理生化进程发生改变(Wangetal., 2003),进而影响植物的生长发育。植物叶片形态解剖学特征和光合生理特征等指标比较容易观察和测量,可用来反映植物对干旱胁迫的适应性(Boughallebetal., 2011; Ennajehetal., 2010; 陈昕等, 2012; 潘存娥等, 2011; 李欢等, 2013)。本研究表明,干旱胁迫下,2个株系的叶片表皮细胞厚度和海绵组织厚度显著降低,ABJ01的栅栏组织厚度呈增加趋势,9#的栅栏组织厚度呈减少趋势(表2),这与Chartzoulakis等 (2002)研究干旱胁迫对鳄梨(Perseaamericana)叶片解剖结构影响的研究结果类似。与9#相比,在干旱胁迫下,ABJ01的叶片表皮厚度较大,具有发达的栅栏组织,而海绵组织厚度和叶片组织疏松度则较小(表2),表现出较强的干旱适应能力。较厚的表皮细胞和发达的栅栏组织有利于植物保持水分以及更好地保护内部组织,从而提高植物的存活率并有助于植物生长(Bacelaretal., 2004)。此外,叶肉栅栏组织发达、海绵组织厚度相对减少及叶片组织紧密度增大等特征,有助于CO2从孔下室传导至光合作用场所,在气孔导度较低的情况下维持植物较高的光合速率,提高植物的保水能力和抗旱能力(Chartzoulakisetal., 2002; Evansetal., 2000)。本研究显示,中度和重度干旱胁迫下,ABJ01的Pn分别比9#高10.50%和18.97%,重度干旱胁迫下达显著水平(图2A),在重度胁迫时ABJ01的Tr比9#显著低8.29%(图2D); 重度干旱胁迫下,ABJ01的Gs较9#高17.80%(图2B),说明干旱胁迫下转基因银中杨的叶片保水能力和CO2交换能力更强,能在较低的Gs下维持较高的Pn。研究表明,干旱胁迫导致植物叶绿素a、叶绿素b和总叶绿素含量显著降低(王林龙等, 2015; 井大炜等, 2014),本研究结果与之相似,这可能是由于叶绿体膜受到破坏,造成叶肉细胞水分缺失所致(Anjumetal., 2011)。干旱胁迫导致Fv/Fm显著降低,表明植物光系统受损严重(井大炜等, 2013),本研究结果与之相似。与9#相比,重度干旱胁迫时ABJ01的叶片叶绿素a、叶绿素b、总叶绿素含量(图4)及Fv/Fm较9#高(图3),表明转基因银中杨叶肉细胞维持叶绿素含量稳定的能力较强,保水能力更强,且光系统受损程度更小,其抗旱能力得到提高。另外,有研究表明转录因子JERFs的导入可激活转基因烟草中与光合碳同化相关基因(Wuetal., 2008),维持植物在干旱胁迫时较高的光合速率。下一步可通过研究干旱胁迫下转基因银中杨中JERF36的表达与光合碳同化相关基因的关系,阐明转基因杨树在干旱胁迫下维持较高的Pn的机制,为进一步研究转基因银中杨的抗旱性机制奠定基础。
4 结论
属于ERF类的转录因子JERF36可能通过影响转基因银中杨叶片结构发育提高转基因银中杨在干旱胁迫下的气体交换能力和保水能力,增强转基因银中杨的抗旱能力。
陈 昕, 徐宜凤, 张振英. 2012. 干旱胁迫下石灰花楸幼苗叶片的解剖结构和光合生理响应. 西北植物学报, 32(1): 111-116.
(Chen X, Xu Y F, Zhang Z Y. 2012. Leaf anatomical, structure and photosynthetic physiology responses ofSorbusfolgneriseedlings under drought stress. Acta Bot Boreal-Occidentalia Sinica, 32(1): 111-116. [in Chinese])
高俊凤. 2006.植物生理学实验指导. 北京: 高等教育出版社.
(Gao J F. 2006. Guide of plant ohysiology experiment. Beijing: Higher Education Press. [in Chinese])
井大炜, 邢尚军, 马海林, 等. 2014. I-107 欧美杨对不同强度干旱胁迫的形态与生理响应. 东北林业大学学报, 42 (1): 10-13.
(Jing D W, Xing S J, Ma H L,etal. 2014. Morphological and physiological responses ofPopulus×euramericanacv. ‘Neva ’to water stress. Journal of Northeast Forestry University, 42 (1):10-13. [in Chinese])
井大炜, 邢尚军, 杜振宇, 等. 2013. 干旱胁迫对杨树幼苗生长、光合特性及活性氧代谢的影响. 应用生态学报, 24(7): 1809-1816.
(Jing D W, Xing S J, Du Z Y,etal. 2013. Effects of drought stress on the growth, photosynthetic characteristics,and active oxygen metabolism of poplar seedlings.Chinese Journal of Applied Ecology, 24(7): 1809-1816. [in Chinese])
李芳兰, 包维楷. 2005. 植物叶片形态解剖结构对环境变化的响应与适应. 植物学通报, 22(增刊): 118-127.
(Li F L, Bao W K. 2005. Responses of the morphological and anatomical structure of the plant leaf to environmental change. Chinese Bulletin of Botany, 22(S1): 118-127. [in Chinese])
李 欢, 樊军锋, 高建社, 等. 2013. 黑杨叶片旱生结构的比较. 西北林学院学报, 28(3): 113-118.
(Li H, Fan J F, Gao J S,etal. 2013. Comparison on drought resistance on anatomical structures of black poplar leaf. Journal of Northwest Forestry University, 28(3): 113-118. [in Chinese])
李义良. 2008.转基因杨树的分子检测及抗逆性评价. 北京: 北京林业大学博士学位论文.
(Li Y L. 2008. Molecular detection and evaluation of stress tolerance in transgenic poplars. Beijing: PhD thesis of Beijing Forestry University. [in Chinese])
李文正, 张海文, 王俊英, 等. 2006. ERF转录因子及其在烟草抗逆性改良中的应用. 生物技术通报, (4):30-34.
(Li W Z, Zhang H W, Wang J Y,etal. 2006. Ethylene responsive factors and their application in improving tobacco tolerance to biotic and abiotic stresses. Biotechnology Bulletin, (4):30-34. [in Chinese])
马兰青, 王丽雪, 王有年,等. 1996. 干旱对苹果叶片形态解剖结构的影响. 北京农学院学报, 11(2): 27-31.
(Ma L Q, Wang L X, Wang Y N,etal. 1996. Affection of aridity to anatomical structure on apple leaves. Journal of Beijing Agricultural College, 11(2): 27-31. [in Chinese])
马耀光,张保军,罗志成,等. 2003. 旱地农业节水技术. 北京: 化学工业出版社, 11.
(Ma Y G, Zhang B J, Luo Z C,etal. 2003. Dryland agriculture water-saving technology. Beijing: Chemical Industry Press, 11.
潘存娥, 田丽萍, 李贞贞, 等. 2011. 5种杨树无性系叶片解剖结构的抗旱性研究. 中国农学通报, 27(2):21-25.
(Pan C E, Tian L P, Li Z Z,etal. 2011. Studies on drought resistance on anatomical structure of leaves of 5 poplar clones. Chinese Agricultural Science Bulletin, 27(2):21-25. [in Chinese])
苏晓华, 张冰玉, 黄秦军. 2009.杨树基因工程育种. 北京: 科学出版社.
(Su X H, Zhang B Y, Huang Q J. 2009.Poplar genetic engineering breeding. Beijing: Science Press. [in Chinese])
王林龙, 李清河, 徐 军, 等. 2015. 不同种源油蒿形态与生理特征对干旱胁迫的响应. 林业科学, 51(2): 37-43.
(Wang L L, Li Q H, Xu J,etal. 2015. Morphology and physiology characteristic responses of different provenances ofArtemisiaordosicato drought stress. Scientia Silvae Sinicae, 51(2): 37-43. [in Chinese])
燕 玲, 李 红, 刘 艳. 2002. 13种锦鸡儿属植物叶的解剖生态学研究. 干旱区资源与环境, 16(1): 100-106.
(Yan L, Li H, Liu Y. 2002. The anatomical ecology studies on the leaf of 13 species inCaraganagenus. Journal of Arid Land Resources and Environment, 16(1): 100-106. [in Chinese])
叶龙华, 黄香兰, 薛 立. 2014. 干旱胁迫对树木叶片性状及抗旱生理的影响. 世界林业研究, 27(1): 29-34.
(Ye L H, Huang X L, Xue L. 2014. Effects of drought on leaf traits and drought-resistant physiology of trees. World Forestry Research, 27(1): 29-34. [in Chinese])
张计育, 王庆菊, 郭忠仁. 2012. 植物AP2/ERF类转录因子研究进展. 遗传, 34(7):835-847.
(Zhang J Y, Wang Q J, Guo Z R. 2012. Progresses on plant AP2/ERF transcription factors. Hereditas, 34(7): 835-847. [in Chinese])
Anjum S A, Xie X,Wang L C,etal. 2011. Morphological, physiological and biochemical responses of plants to drought stress. African Journal of Agricultural Research, 6(9): 2026-2032.
Araus J L, Slafer G A, Reynolds M P,etal. 2002. Plant breeding and drought in C3 cereals: What should we breed for? Ann Bot, 89(7): 925-940.
Bacelar E A, Correia C M, Moutinho-Pereira J M,etal. 2004. Sclerophylly and leaf anatomical traits of five field-grown olive cultivars growing under drought conditions. Tree Physiol, 24(2): 233-239.
Boughalleb F, Hajlaoui H. 2011. Physiological and anatomical changes induced by drought in two olive cultivars (cv Zalmati and Chemlali). Acta Physiol Plant, 33(1): 53-65.
Chartzoulakis K, Patskas A, Kofidis G,etal. 2002. Water stress affects leaf anatomy, gas exchange, water relations and growth of two avocado cultivars. Scientia Horticulturae, 95(1): 39-50.
Ennajeh M, Vadel A M, Cochard H,etal. 2010. Comparative impacts of water stress on the leaf anatomy of a drought-resistant and a drought-sensitive olive cultivar. J of Hortic Sci & Biotech, 85(4): 289-294.
Evans J R, Loreto F. 2000. Acquisition and diffusion of CO2in higher plant leaves∥Leegood R C, Sharkey T D, von Caemmerers.Photosynthesis:phystology and metabolism.Netherlands:Kluver Academic Publishers, 321-351.
Li Y L, Su X H, Zhang B Y,etal. 2009. Expression of jasmonic ethylene responsive factor gene in transgenic poplar tree leads to increased salt tolerance. Tree Physiology, 29(2): 273-279.
Reddy A R, Chiatanya K V, Vivekanandan M. 2004. Drought induced responses of photosynthesis and antioxidant metabolism in higher plants. J Plant Physiol, 161(11): 1189-1202.
Rubio De Casas R R, Vargas P, Pérez-Corona E,etal. 2007. Field patterns of leaf plasticity in adults of the long-lived evergreenQuercuscoccifera. Annals of Botany, 100(2): 325-334.
Wang W, Vincour B, Altman A. 2003. Plant responses to drought, salinity and extreme temperatures: towards genetic engineering for stress tolerance. Planta, 218(1): 1-14
Wu L J, Zhang Z J, Zhang H W,etal. 2008. Transcriptional modulation of ethylene response factor proteinJERF3 in the oxidative stress response enhances tolerance of tobacco seedlings to salt, drought, and freezing. Plant Physiology, 148(4): 1953-1963.
Zhang H W, Liu W, Wan L Y,etal. 2010. Functional analyses of ethylene response factorJERF3 with the aim of improving tolerance to drought and osmotic stress in transgenic rice. Transgenic Res, 19(5): 809-818.
Zhao C X, Huang Z S. 1981. A preliminary study of xeromorphism of some important xerophytes growing in Tungeli desert. Journal of Integrative Plant Biology, 23(4): 278-283.
(责任编辑 王艳娜 郭广荣)
Effects of Drought Stress on Anatomical Structure and Photosynthetic Characteristics of TransgenicJERF36Populusalba×P.berolinensisSeedling Leaves
Huang Juan Chen Cun Zhang Weixi Ding Changjun Su Xiaohua Huang Qinjun
(State Key Laboratory of Tree Genetics and Breeding Key Laboratory of Tree Breeding and Cultivation of State Forestry Administration Research Institute of Forestry, Chinese Academy of Forestry Beijing 100091 )
【Objective】 In this study, transgenicPopulusalba×P.berolinensisline (ABJ01) and non-transgenic line (9#) were used to test effects of drought stress. To provide a new reference for drought assessment and scientific basis for promotion and application of transgenic poplars, seedling height, morphological and anatomical structure of leaves, and photosynthetic characteristics of transgenic poplar and non-transgenic poplar under drought stress were measured. 【Method】 At the end of June 2015, a soil drought stress experiment was conducted with seedlings of transgenic poplar and non-transgenic poplar at the average height around 45 cm. The seedlings were subjected to three regimes of water (control, moderate stress, and severe stress), and the soil moisture was controlled at 60%-80%, 40%-60%, 20%-40% of the field water capacity, respectively for 30 days. 【Result】 Seedling height growth of the two lines was inhibited to a certain degree by drought stress, and the inhibition was increasing severe with the stress level increased. The seedling height of transgenic line ABJ01 was 9.38% higher than non-transgenic line 9# under severe drought stress. Single leaf area of the two lines was significantly reduced under drought stress, indicating that drought stress suppressed growth of poplar leaves. Single leaf area of 9# was significantly lower than ABJ01, accounting for 10.82% and 13.79% of the control, respectively, indicating limitation of leaf growth in ABJ01 was lower under drought stress. Anatomical structure of leaves showed that growth of leaf epidermal cells and mesophyll cells in ABJ01 and 9# were inhibited by drought stress, however the inhibited degree of ABJ01 was lower. Under moderate drought stress, leaf upper epidermal thickness and lower epidermal thickness of ABJ01 were 5.55% and 4.70% significantly greater than that of 9#, respectively. Thickness of palisade tissue of ABJ01 was 6.17% significant greater than 9#. In contrast, sponge tissue thickness and SR were 12.35% and 12.38% significantly lower than those of 9#, respectively. Under severe drought stress condition, leaf upper epidermal thickness and lower epidermal thickness of ABJ01 were 16.27% and 10.05% significantly higher than 9#, respectively, but sponge tissue thickness and SR were 11.71% and 11.58% significant lower than those of 9#, respectively. The more developed palisade tissue and relatively reduced spongy tissue may facilitate the conduction of CO2, and maintain the higherPnin leaf of ABJ01, which would contribute to adaptation to drought stress. Photosynthetic physiological data suggested thatPnof 9# was 2.8 times lower than that of ABJ01, and ABJ01Pnwas significantly higher than non-transgenic lines 9# (10.50%-18.97%), indicating ABJ01 had a greater photosynthetic capacity. Under control treatment, there were no significant differences inGs,Fv/Fmbetween the two lines. However under drought stress, the decreased trend in transgenic line was relatively smaller compared with non-transgenic line under drought stress, indicating that ABJ01 indexes was less affected by drought stress. In addition, ABJ01Trwas less than 9#, suggesting that ABJ01 had stronger capacity in water holding under drought conditions. The chlorophyll a, chlorophyll b and total chlorophyll content in ABJ01 were higher than those of 9#. ABJ01Fv/Fmwas higher than 9#, indicating that the ability of maintaining the stability of chlorophyll content was stronger and the damage of PSⅡ was less in transgenic line. 【Conclusion】 The study suggests that exogenous geneJERF36 may improve the gas exchange capacity and water-holding capacity of transgenicPopulusalba×P.berolinensisunder drought stress by impacting the leaf structural of transgenic poplar, finally enhance drought tolerance of transgenicPopulusalba×P.berolinensis.
drought stress; transgenic poplar; leaf anatomical structure; photosynthetic characteristics
10.11707/j.1001-7488.20170502
2016-03-01;
2016-06-13。
国家“863”计划课题(2013AA102703)。
S718.5
A
1001-7488(2017)05-0008-08
*黄秦军为通讯作者。
