肝硬化和肝癌患者血清CA125、CA199、AFP和CEA水平变化
2017-04-26代伟伟刘正新徐宝宏
代伟伟,刘正新,徐宝宏
·肝癌·
肝硬化和肝癌患者血清CA125、CA199、AFP和CEA水平变化
代伟伟,刘正新,徐宝宏
目的 研究肝硬化和肝癌患者血清癌抗原(CA)125、CA199、甲胎蛋白(AFP)和癌胚抗原(CEA)的变化。方法采用ELISA法检测223例肝硬化患者、97例肝癌患者和120例健康人血清CA125、CA199、AFP和CEA水平。结果肝硬化和肝癌患者血清CA125分别为(261.64±32.47)U/ml和(265.80±30.44)U/ml,CA199分别为(25.73±3.39)U/ml和(30.54±3.29)U/ml,CEA分别为(4.03±0.36)ng/ml和(3.87±0.21)ng/ml,均显著高于健康人[(21.25±7.66)U/ml、(18.57±8.11)U/ml和(3.08±1.05)ng/ml,P<0.05];肝癌患者AFP(【20000.00±453.07)ng/ml】显著高于肝硬化患者[(7.52±2.01)ng/ml,P<0.05];肝硬化Child-Pugh C级患者CA125【(474.52±59.80)U/ml】、CA199【(27.80±5.94)U/ml】和CEA(【5.80±0.63)ng/ml】均显著高于Child-Pugh A级患者[分别为(55.65± 8.82)U/ml、(18.81±0.46)U/ml和(3.20±0.10)ng/ml,P<0.05];腹水患者CA125为(385.16±36.09)U/ml、CA199为(26.55±2.87)U/ml、AFP为(13.63±1.82)ng/ml和CEA为(4.85±0.39)ng/ml,均显著高于无腹水患者[分别为(62.75±15.45)U/ml、(19.58±0.75)U/ml、(9.39±1.26)ng/ml和(3.54±0.16)ng/ml,P<0.05];酒精性肝硬化患者血清CA125【(318.48±48.80)U/ml】和CA199【(26.63±3.22)U/ml】显著高于病毒性[(215.77±26.26)U/ml和(19.06±0.64)U/ml,P<0.05]或原发性胆汁性肝硬化患者[(129.73±28.55)U/ml和(18.00±0.00)U/ml,P<0.05];病毒性肝炎肝硬化患者血清AFP(【56.41±26.75)ng/ml】显著高于酒精性或胆汁性肝硬化患者[分别为(5.44±0.30)ng/ml和(7.35±1.47)ng/ml,均为P<0.05],血清CEA【(3.53±0.17)ng/ml】则显著低于酒精性或胆汁性肝硬化患者[分别为(5.19±0.35)n g/ml和(5.73±0.98)ng/ml,均为P<0.05]。结论 肝硬化和肝癌患者CA125、CA199、AFP和CEA水平存在差异,肝硬化患者CA125、CA199、AFP、CEA水平与Child-Pugh分级、腹水和病因有关。
肝癌;肝硬化;糖类抗原125;糖类抗原199;甲胎蛋白;癌胚抗原
检测血清肿瘤标志物水平可反映肿瘤细胞在恶性转化过程中各个阶段细胞表型及基因型的内在特性[1~3],从而直接反映患者病情进展状况[4,5]。癌抗原125(cancer antigen 125,CA125)是一种糖链抗原,为卵巢癌等肿瘤的标记物,同时在临床观察中,肝硬化尤其是腹水患者存在一定程度的血清CA125升高[6]。癌抗原199(CA199)是胰腺癌的肿瘤标记物,甲胎蛋白(alpha fetoprotein,AFP)为肝癌的肿瘤标记物,癌胚抗原(carcino-embryonic antigen,CEA)为存在于结肠癌、正常胚胎肠道、胰腺的一种蛋白多糖复合物[7,8]。上述四种肿瘤标记物的检测水平对肿瘤的早期诊断、治疗效果、预后判断等有重要的临床意义[9]。本研究选择肝硬化、肝癌患者和健康人进行上述四种肿瘤标记物的检测并进行其水平差异比较,旨在为肝脏疾病临床分析和判断病情提供一定的依据。
1 资料与方法
1.1 一般资料 2011年1月~2015年12月首都医科大学附属北京朝阳医院和附属北京潞河医院住院的肝硬化患者223例,男性136例,女性87例;平均年龄53.0±3.4岁。肝癌患者97例,男性59例,女性38例;平均年龄56.0±2.6岁。肝硬化和肝癌均符合第七版《内科学》[10]诊断标准,剔除合并有结核性腹膜炎、肾功能不全的患者,并排除胃肠道肿瘤、胆胰、生殖系统及其他系统恶性肿瘤患者。肝硬化患者按Child-Pugh分级法A级≤6分,B级7~9分,C级≥10分[11],其中A级 96例,B级91例,C级36例。另外,肝硬化合并腹水115例;乙型肝炎肝硬化117例,酒精性肝硬化79例,原发性胆汁性肝硬化27例。另选取健康人120例作为对照组,均无心肺疾病及其他肿瘤病史。
1.2 检测方法 采用ELISA法检测血清肿瘤标记物(南京新瑞生物技术公司)。四种肿瘤标记物参考值范围分别为 CA125:0~35 U/ml,CA199:0~37 U/ml,AFP:0~8.75 ng/ml,CEA:0~5 ng/dl,以高出临界值上限为阳性。
1.3 统计分析方法 应用SPSS 18.0统计软件进行分析,肿瘤标记物水平经对数转换后符合正态分布,以(±s)表示,多组间比较采用方差分析,组间两两比较采用t检验。采取双侧检验,P<0.05为差异具有统计学意义。
2 结果
2.1 三组人群血清四种肿瘤标记物水平比较 肝硬化患者血清CA125、CA199和CEA水平与肝癌患者无显著性差异,而肝癌患者血清AFP水平显著高于肝硬化患者,差异具有统计学意义(t=5.74,P<0.05);组间两两比较提示,肝硬化组血清CA125、CA199和CEA水平显著高于健康人(t=7.07,t=2.68,t=4.401,P<0.05),肝癌组肿瘤标记物水平也显著高于健康人(t=8.94,t=3.89,t=4.17,3.36,P<0.05,表1)。
2.2 不同肝功能分级肝硬化患者血清四种肿瘤标志物水平比较 肝硬化Child-Pugh B级和C级患者血清四种肿瘤标记物水平显著高于A级患者(P<0.05),Child-Pugh C级肝硬化患者CA199水平显著高于B级患者,且具有统计学差异(P<0.05,表2)。
表1 三组人群血清肿瘤标记物水平(±s)比较
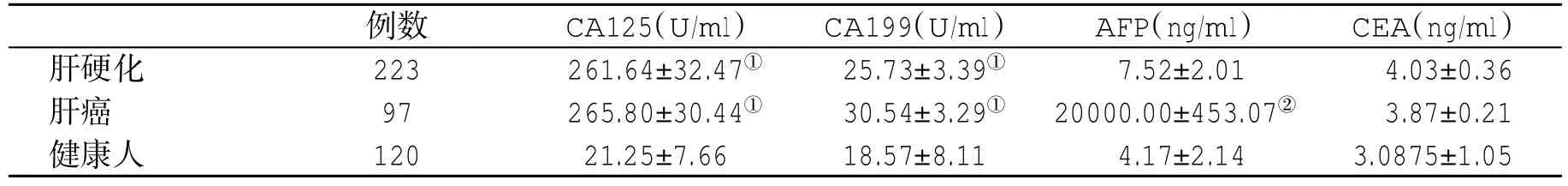
表1 三组人群血清肿瘤标记物水平(±s)比较
与健康人比,①P<0.05;与肝硬化患者比,②P<0.05
例数 CA125(U/ml) CA199(U/ml) AFP(ng/ml) CEA(ng/ml)肝硬化 223 261.64±32.47① 25.73±3.39① 7.52±2.01 4.03±0.36肝癌 97 265.80±30.44① 30.54±3.29① 20000.00±453.07② 3.87±0.21健康人 120 21.25±7.66 18.57±8.11 4.17±2.14 3.0875±1.05
表2 不同肝功能分级肝硬化患者血清肿瘤标记物水平(±s)比较
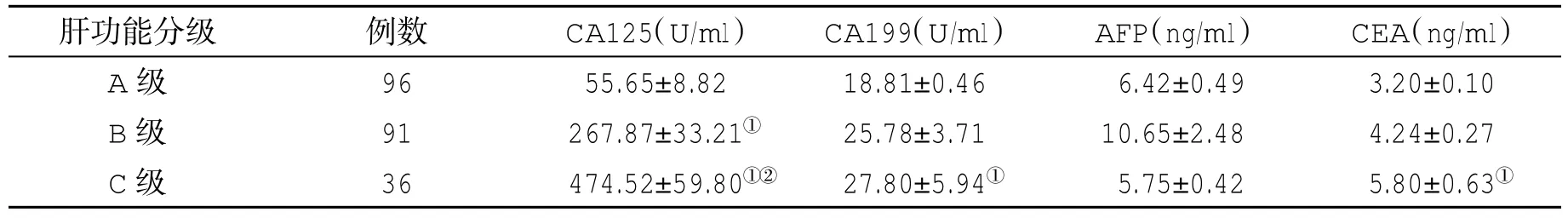
表2 不同肝功能分级肝硬化患者血清肿瘤标记物水平(±s)比较
与A级比,①P<0.05;与B级比,②P<0.05
肝功能分级 例数 CA125(U/ml) CA199(U/ml) AFP(ng/ml) CEA(ng/ml)A级 96 55.65±8.82 18.81±0.46 6.42±0.49 3.20±0.10 B级 91 267.87±33.21① 25.78±3.71 10.65±2.48 4.24±0.27 C级 36 474.52±59.80①② 27.80±5.94① 5.75±0.42 5.80±0.63①
2.3 有腹水与无腹水肝硬化患者血清肿瘤标志物水平比较 有腹水的肝硬化患者血清CA125、CA199、AFP和CEA水平显著高于无腹水的肝硬化患者,差异具有统计学意义(P<0.05,表3)。
2.4 不同病因肝硬化患者血清四种肿瘤标志物水平比较 酒精性肝硬化患者血清CA125和CA199水平显著高于病毒性或原发性胆汁性肝硬化患者,差异有统计学意义(P<0.05),病毒性肝炎肝硬化患者血清AFP水平显著高于,而血清CEA水平则显著低于酒精性或胆汁性肝硬化患者(P<0.05,表4)。
表3 腹水与无腹水肝硬化患者血清肿瘤标记物水平(±s)比较
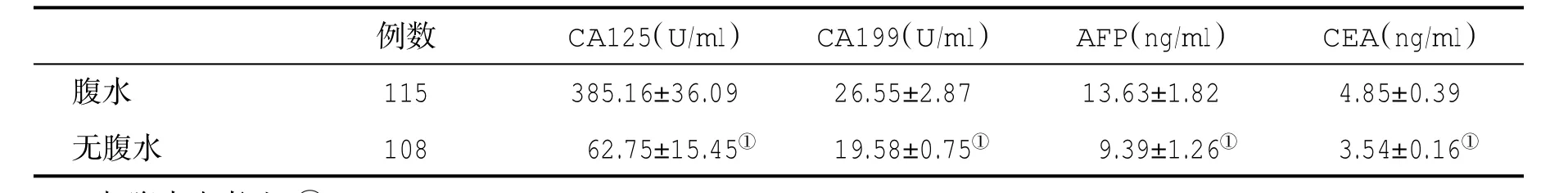
表3 腹水与无腹水肝硬化患者血清肿瘤标记物水平(±s)比较
与腹水患者比,①P<0.05
例数 CA125(U/ml) CA199(U/ml) AFP(ng/ml) CEA(ng/ml)腹水 115 385.16±36.09 26.55±2.87 13.63±1.82 4.85±0.39无腹水 108 62.75±15.45① 19.58±0.75① 9.39±1.26① 3.54±0.16①
表4 不同病因肝硬化患者血清肿瘤标记物水平(±s)比较
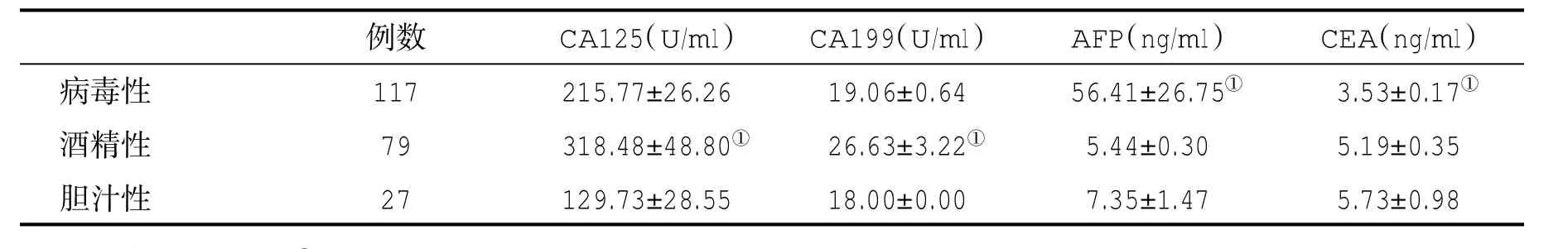
表4 不同病因肝硬化患者血清肿瘤标记物水平(±s)比较
与其他两组比,①P<0.05
例数 CA125(U/ml) CA199(U/ml) AFP(ng/ml) CEA(ng/ml)病毒性 117 215.77±26.26 19.06±0.64 56.41±26.75① 3.53±0.17①酒精性 79 318.48±48.80① 26.63±3.22① 5.44±0.30 5.19±0.35胆汁性 27 129.73±28.55 18.00±0.00 7.35±1.47 5.73±0.98
3 讨论
CA125是1983年由Bat et al从卵巢癌上皮细胞中检测出的一种可被单克隆抗体OC 125结合的糖蛋白,来源于胚胎发育期体胚上皮,位于染色体19p13.2区域,为含有5797个碱基对的跨膜糖蛋白,属于IgG1[12]。由于CA125的氨基酸序列具有一些黏蛋白分子的特性,故将其命名为CA125,基因为MUC16,相对分子质量为20万~100万。最常见于上皮性卵巢肿瘤患者血清中,健康成人CA125水平小于35 U/mL[13]。CA125不仅是卵巢癌的特异性肿瘤标记物,在输卵管腺癌、子宫内膜癌、宫颈癌、胰腺癌、直肠癌、结肠癌、肺癌患者血清CA125水平也会升高[14]。在非恶性肿瘤,如子宫内膜异位症、盆腔炎、卵巢囊肿、胰腺炎、病毒性肝炎、肝硬化等虽有不同程度的升高,但其水平不是非常高。另外,CA125是存在腹水患者的常用指标,对早期诊断腹水具有重要的临床价值。在本组肝硬化患者,尤其在存在腹水患者,血清CA125水平明显升高。
CA199在正常胰、胆管细胞、胃、结肠和唾液腺上皮细胞均可表达,是由单克隆抗体116NS19-9识别的抗原成分,是目前临床上最有诊断价值也是应用最多的一种肿瘤相关抗原[15,16],其血清正常值<37 U/ml。血清CA199明显升高时,首先应考虑为胰腺恶性肿瘤,其升高还见于肝胆系癌、胃癌、结直肠癌、慢性胰腺炎、胆石症、肝硬化、肾功能不全、糖尿病等[17]。本组部分肝硬化患者血清CA199也有升高。AFP主要在胎儿肝中合成,其分子量为6.9万,30周胎龄达最高峰,以后逐渐下降,至周岁时接近成人水平(低于30 μg/L)。在肝细胞再生时期,血清AFP水平轻度升高,在急、慢性肝炎、肝硬化时会有肝细胞再生,因而血清AFP水平也可升高[18,19]。在肝细胞受损以及肝细胞增生过程中AFP分泌均会增加,并可作为诊断肝癌的主要指标。上海瑞金医院报告,在急性黄疸型肝炎、急性无黄疸型肝炎、慢性肝炎和肝硬化患者各60例,测得AFP水平在25~40 ng/ml者分别占 13.3%、11.7%、3.5%和41.7%。甲胎蛋白偏高可能见于急、慢性肝炎、肝衰竭恢复期、肝硬化、先天性胆管闭塞和畸形胎儿等疾病[20,21]。本组肝癌患者血清AFP水平明显升高,部分肝硬化患者亦有升高。
CEA最初被发现于结肠癌和胎儿肠组织中,故名癌胚抗原。CEA升高常见于结肠癌、胰腺癌、胃癌、乳腺癌、甲状腺髓样癌等。但在良性肿瘤、炎症和退行性疾病,如结肠息肉、溃疡性结肠炎、胰腺炎和酒精性肝硬化患者血清CEA水平也有部分升高,但远远低于恶性肿瘤,一般小于20 ng/ml。CEA超过20 ng/ml时往往提示有消化道肿瘤。
综上所述,上述四种肿瘤标志物在不同病因肝硬化和不同肝功能分级患者、合并腹水的患者中存在显著升高,提示预后差,肝癌患者血清AFP水平明显升高。临床上,在病毒性肝炎或肝硬化患者,当血清AFP升高时,应及时进行影像学检查,以期早期发现肿瘤,以免延误治疗。
[1]Chowdhury MA,Xiubin Z,Wei H,et al.Cancer antigen-125 and ICAM-1 are together responsible for ascites in liver cirrhosis.Clin Lab,2014,60(4):653-658.
[2]Dai H,Liu J,Liang Y,et al.Increased lung cancer risk in patientswith interstitiallung disease and elevated CEA and CA125 serum tumour markers. Respirology,2014,19(5):707-713.
[3]Kayadibi H,Sertoglu E,Uyanik M.Evaluation of relationship between CA-125 levels and ascites in patients with liver cirrhosis.J Coll Physicians Surg Pak,2014,24(11):873.
[4]Chang K,Lu SN.Incidence and associated risk factors of hepatocellularcarcinoma in a duralhepatitisB and C virus endemirarea;a surveillance study.Kaohsiung J Med Sci,2011,27(3):85-90.
[5]Pissaia AJ,Bernard D,Scatton O,et al.Significance of serum tumor markers carcinoembryonic antigen,CA 19-9,CA 125,and CA 15-3 in pre-orthotopic livertransplantation evaluation. Transplant Proc,2013,41(2):682-684.
[6]Trevisani FD,Intino PE,Morselli-Labate AM,et al.Serum alpha2 fetoprotein for diagnosis of hepatocellular carcinoma in patients with chronic liver disease:influence of HBsAg and anti HCV status.J Hepatol,2012,34(4):570-575.
[7]Sorbye H,Dahl O.Carcinoembryonic antigen surge in metastatic colorectal cancer patients respongding to oxaliplatin combination chemotherapy:implications for tumor marker monitoring and guidelines.J Clin Oncol,2013,21(23):4466-4467.
[8]Gazelle GS,Hunink MG,Kuntz KM,et al.Cost-effectiveness of hepaticmetastasectomyin patients with metastatic colorectal carcinoma:a state-transition Monte Carlo decision analysis.Ann Surg,2013,237(4):544-555.
[9]Davies JR,Kirkham S,Svitacheva N,et al.MUC16 is produced in tracheal surface epithelium and submucosal glands and is present in secretions from normal human airway and cultured bronchial epithelial cells.Int J Biochem Cell Biol,2014,39:1943-1954.
[10]陆再英,钟南山.内科学.1版,北京:人民卫生出版社,2008:437-453.
[11]Wiesner R,Edwards E,Freeman R,et al.Model for end-stage liver disease(MELD)and allocation of donor livers.Gastroenterology,2013,124(1):91-96.
[12]Liu X,Chi X,Gong Q,et al.Association of serum level of growth differentiation factor 15 with liver cirrhosis and hepatocellular carcinoma.PLoS One,2015,10(5):e0127518.
[13]Yonemori K,Ando M,Shibata T,et al.Tumor marker analysis and verification of prognostic models in patients with cancer of unknown primary,receiving platinum based combination chemotherapy. J Cancer Res Clin Oncol,2016,132(10):635-642.
[14]Smith RA,Cokkinides V,von Eschenbach AC,et al.American cancer society guidelines for the early detection of cancer.CA Cancer J Clin,2012,52(1):8-22.
[15]Liu F,Kong X,Dou Q,et al.Evaluation of tumor markers for the differential diagnosis of benign and malignant ascites.Ann Hepatol,2014,13(3):357-363.
[16]刘丹,朱清静,万青松,等.清降钙素原联合C-反应蛋白检测诊断肝硬化并发自发性细菌性腹膜炎价值探讨.实用肝脏病杂志,2015,18(1):80-81.
[17]Qureshi MO,Dar FS,Khokhar N.Cancer antigen-125 as a markerofascites in patients with livercirrhosis.J Coll Physicians Surg Pak,2014,24(4):232-235.
[18]李佳红,付娜,牛学敏,等.573例原发性肝癌病因及临床特点分析.实用肝脏病杂志,2015,18(4):399-402.
[19]Wang M,Devarajan K,Singal AG,et al.The Doylestown algorithm:A test to improve the performance of AFP in the detection of hepatocellular carcinoma.Cancer Prev Res(Phila),2016,9(2):172-179.
[20]Conti F,Dall'Agata M,Gramenzi A,et al.Biomarkers for the early diagnosis of bacterial infection and the surveillance of hepatocellularcarcinoma in cirrhosis.Biomark Med,2015,9(12):1343-1351.
[21]阮林松,许成新,陈世勇.肝硬化患者血清及腹腔积液 CA199、CA125检测的意义.放射免疫学杂志,2011,24(1):107-108.
(收稿:2016-07-27)
(本文编辑:陈从新)
Serum CA125,CA199,AFP,CEA in patients with cirrhosis and primary liver cancer
Dai Weiwei1,Liu Zhengxin2,Xu Baohong1
(1.Department of Gastroenterology,Affiliated Beijing Luhe Hospital,Capital Medical University,Beijing 101100,China 2.Department of Gastroenterology,Affiliated Beijing Chaoyang Hospital,Capital Medical University,Beijing 100020,China)
Objective To investigate the changes of serum carbohydrate antigen(CA)125,CA199,alpha-fetoprotein (AFP)and carcinoembryonic antigen (CEA)in patients with live cirrhosis and primary liver cancer(PLC).Methods Serum levels of CA125,CA199,AFP and CEA in 440 individuals were detected by ELISA,including 223 patients with liver cirrhosis,97 patients with PLC and 120 healthy persons.Results The levels of CA125 in patients with liver cirrhosis and PLC were(261.64±32.47)U/ml and(265.80±30.44)U/ml,CA199 were(25.73±3.39)U/ml and(30.54±3.29)U/ml,CEA were(4.03±0.36)ng/ml and(3.87±0.21)ng/ml,much higher than those[(21.25±7.66)U/ml,(18.57±8.11)U/ml and (3.08±1.05)ng/ml,P<0.05]in healthy persons;serum AFP levels in patients with PLC were(20000.00±453.07)ng/ml,much higher than[(7.52± 2.01)ng/ml,P<0.05]in patients with liver cirrhosis;The levels of CA125(474.52±59.80)U/ml],CA199[(27.80± 5.94)U/ml]and CEA [(5.80±0.63)ng/ml]in patients with liver cirrhosis of class C were significantly higher than those of class A[(55.65±8.82)U/ml,(18.81±0.46)U/ml and(3.20±0.10)ng/ml,respectively,P<0.05];The levels of CA125[(385.16±36.09)U/ml],CA199[(26.55±2.87)U/ml],AFP[(13.63±1.82)ng/ml]and CEA [(4.85±0.39)ng/ml]in patients with cirrhotic ascites were higher than those without ascites[(62.75±15.45)U/ml,(19.58±0.75) U/ml,(9.39±1.26)ng/ml and(3.54±0.16) ng/ml,P<0.05];The levels ofCA125 [(318.48±48.80)U/ml]and CA199 [(26.63±3.22)U/ml]in patients with alcoholic liver cirrhosis were higher than those in patients with viralcirrhosis[(215.77±26.26)U/ml and(19.06±0.64)U/ml,P<0.05]or in patients with primary biliary cirrhosis[(129.73±28.55)U/ml and(18.00±0.00)U/ml,P<0.05];The level of AFP[(56.41±26.75)ng/ml]in patients with viral cirrhosis was higher than[(5.44±0.30)ng/ml or(7.35±1.47)ng/ml,respectively,P<0.05],while the CEA level[(3.53±0.17)ng/ml]was lower than[(5.19±0.35)ng/ml or(5.73±0.98)ng/ml,P<0.05]in patients with alcoholic liver cirrhosis or primary biliary cirrhosis.Conclusion The serum levels of CA125,CA199,AFP and CEA in patients with liver cirrhosis and PLC were different.The serum levels of CA125,CA199,AFP and CEA in patients with liver cirrhosis were associated with Child-Pugh scores,ascites and etiology.
Primary livercancer;Livercirrhosis;Carbohydrate antigen 125;CA199;Alpha-fetoprotein;Carcinoembryonic antigen
10.3969/j.issn.1672-5069.2017.01.021
101100北京市 首都医科大学附属北京潞河医院消化内科(代伟伟,徐宝宏);首都医科大学附属北京朝阳医院消化内科(刘正新)
代伟伟,女,30岁,医学硕士,住院医师。主要研究方向:胃肠道肿瘤防治
刘正新,E-mail:liuzhengx2003@vip.sina.com
