LPS对鹅等级卵泡基质层TLR家族基因表达的影响
2017-04-07应诗家戴子淳郭佳佳施振旦
应诗家,戴子淳,郭佳佳,施振旦
(江苏省农业科学院畜牧研究所动物品种改良和繁育重点实验室,南京 210014)
LPS对鹅等级卵泡基质层TLR家族基因表达的影响
应诗家,戴子淳,郭佳佳,施振旦
(江苏省农业科学院畜牧研究所动物品种改良和繁育重点实验室,南京 210014)
【目的】研究鹅卵泡基质层TLR家族基因表达谱及其对LPS不同处理时间后的反应性。【方法】在大群散养条件下,实时观察鹅产蛋过程,分别在产蛋后8和2 h以及产前4、16和28 h注射LPS,并在产蛋后8h屠宰,即LPS作用0、6、12、24和36 h,每个时间点各5只鹅。屠宰时,取卵巢,观察卵泡外观形态,并分离F1 -F5级卵泡基质层。试验鹅自由饮水、自由采食、自然光照。LPS处理0、24和36 h的单个F1-F5级卵泡基质层或卵泡用于基因表达分析,而其他LPS处理的每只鹅等级卵泡基质层RNA等质量混合后用于基因表达分析。采用普通PCR方法检测10种鸟类TLR家族基因的在鹅等级卵泡基质层的表达谱,其中以脾脏组织为阳性对照,以不加cDNA模板为阴性对照。根据RT-PCR的表达谱结果,采用Real-time PCR检测不同等级卵泡基质层TLRs表达水平以及不同LPS处理时间对基质层TLRs表达水平的影响。对不同等级卵泡和不同LPS处理时间对基质层TLRs表达的数据,采用单因子方差分析,Duncan法多重比较;对变性卵泡与对照组基质层TLRs表达水平的数据,采用 T检验分析。【结果】(1)已公开的10种鸟类TLR家族基因,如TLRs 1A、1B、2A、2B、3、4、5、7、15和21基因均在等级卵泡基质层组织中表达。(2)随等级卵泡生长,TLRs 2A和15表达水平逐渐升高;F1级卵泡基质层TLR2A表达水平显著高于F3、F4和F5级卵泡,TLR15表达水平显著高于F5级卵泡;其他TLR家族基因,如TLRs 1A、1B、2B、3、4、5、7和21在不同等级卵泡基质层中的表达水平差异不显著。(3)在LPS处理0、6和12 h时,等级卵泡外观形态无明显变化,但在LPS处理24 h时,3只鹅的等级卵泡呈深黄色胶冻状变性;在LPS处理36 h时,全部试验鹅等级卵泡呈深黄色胶冻状变性。(4)与对照组(LPS处理0 h)相比,TLRs 2A、4和5表达水平在LPS处理12和24 h时显著升高,TLR2B表达水平在LPS处理24 h时显著升高,TLRs 7和15表达水平在LPS处理6 - 24 h期间均显著升高,而TLRs 1A、1B、3和21表达水平在LPS处理6 - 24 h期间差异不显著。(5)与等级卵泡基质层相比,在LPS处理24和36 h的变性卵泡中,TLRs 1A、2A、2B、4、5、7和15表达水平显著升高,TLR3表达水平显著降低,而在LPS处理36 h的变性卵泡中,TLRs 1B和21表达水平显著升高。【结论】已发现的10种鸟类TLR家族基因均在种鹅等级卵泡基质层表达,且随LPS作用时间延长,等级卵泡形态结构发生变化,但TLRs表达水平逐渐升高。
种鹅;等级卵泡;内毒素脂多糖(LPS);TLRs
0 引言
【研究意义】由于种鹅的喜水习性和水面交配行为,养殖水体中病原微生物及其释放的内毒素(lipopolysaccharide, LPS)易影响水禽繁殖性能[1-2]。禽类等级卵泡基质层富含血管组织,对卵泡细胞营养供应、卵黄沉积和内分泌调节具有关键性作用,但也是病原微生物侵染卵泡的优先部位。TLR家族基因是一类病原模式识别受体基因,其通过识别病原相关分子模式,激活胞内信号级联反应,诱导炎性因子生成,启动非特性免疫反应,清除病原微生物[3-5]。因此,开展鹅等级卵泡基质层 TLR家族基因表达及其对LPS的反应性研究,对探究禽类卵泡抵御病原微生物侵染的分子调控机制、维持水禽卵泡功能和繁殖性能稳定具有重要的作用。【前人研究进展】目前为止,鸟类共发现10种TLR家族基因,如TLRs 1A, 1B, 2A, 2B, 3, 4, 5, 7, 15和21,其识别广泛的病原微生物及其代谢产物,包括细菌、病毒和真菌等[6-10]。SUBEDI等报道鸡卵泡颗粒层表达TLRs 2, 4, 5和7,膜层表达TLRs 4和5[11];除TLRs 1A和2B,其他TLRs家族基因均在鸡卵巢中表达[12]。此外,9种TLRs家族基因在鸡阴道[13]、精子[14]、附睾[15]和睾丸[14]组织中表达。体内外试验均表明细菌LPS促进TLRs 2和4表达[6,11,16-17],并且,LPS促进鸡睾丸间质细胞TLRs 1A、2B、4、7、15和 21基因表达。【本研究切入点】目前,鹅部分 TLR家族成员,如 TLR21[18]、TLR7[19-20]、TLR5[21]和 TLR2A[22]全长和组织表达已见报道,但其他TLRs信息甚少,而且也不清楚其在鹅等级卵泡基质层中的表达情况。此外,不同LPS处理时间对鹅等级卵泡生长和 TLR家族基因表达的影响研究更少。【拟解决的关键问题】本试验研究 10种鸟类 TLR家族基因在鹅等级卵泡基质层中的表达谱及其对LPS不同作用时间的反应性,为病原微生物抑制水禽产蛋性能和等级卵泡免疫保护的分子调控机制提供参考。
1 材料与方法
动物饲养和屠宰试验于2015年4—5月在扬州朝天歌农牧科技有限公司鹅场进行;分子相关试验在江苏省农业科学院动物品种改良和繁育重点实验室进行。
1.1 试验试剂
大肠杆菌源LPS(055:B5)购自西格玛奥德里奇(上海)贸易有限公司;RNA抽提试剂盒购自天根生化科技(北京)有限公司;Taq PCR Master Mix购自上海翊圣生物科技有限公司;50 bp Maker和PrimeScript RT Master Mix购自宝生物工程(大连)有限公司;FastStart Universal SYBR Green Master购自罗氏诊断产品(上海)有限公司。
1.2 试验设计
种鹅处于产蛋高峰期时(产蛋率 > 45 %),在2 000只约540日龄种鹅的大群散养条件下,在舍内放置设有实时监控系统的产蛋箱,通过网络远程监测种鹅产蛋过程。产蛋时标记种鹅,产蛋后8 和2 h采用1mL注射器翅静脉注射LPS(1.5 mg·kg-1BW);由于种鹅产蛋间隔约48 h[23],因此,产蛋后44、32和20 h分别作为下一个产蛋前4、16和28 h的LPS注射时间点,每个时间点各 5只鹅。所有试验鹅均在产蛋后8 h屠宰,从而建立LPS作用0、6、12、24和36 h的试验模型。屠宰时,取等级卵泡,并观察外观形态。
1.3 饲养管理
采用“室内地面+室外运动场+人工水池”方式养殖种鹅。舍内放有料盘,舍外设有饮水器。试验鹅自由饮水、自由采食、自然光照。
1.4 等级卵泡处理
屠宰5 min内,取卵巢,用眼科剪分离F1 - F5级卵泡,并放入冰预冷的D-PBS中,参考文献[24]报道的方法,用眼科镊去除血管后分离卵泡基质层,液氮保存。对于胶冻状变性的卵泡,不能分离基质层,直接液氮保存。
1.5 总RNA提取和反转录
采用天根试剂盒说明书提取等级卵泡基质层或变性卵泡总RNA。RNA质量检测合格后,参照TakaRa反转录试剂盒说明,合成cDNA第一链,-20 ℃保存备用。其中,LPS处理0 h的单个F1—F5级卵泡基质层进行反转录;LPS处理6和12 h的等级卵泡基质层RNA等质量混合后再反转录;LPS处理24和36 h时试验鹅等级卵泡呈胶冻状变性,直接单独提取 RNA后反转录,未变性的单个卵泡基质层提取 RNA后再反转录。RNA反转录体系为10 μL:2 μL 5×Prime Script Buffer, 8 μL RNA(< 500 ng)。反转录程序:37 ℃ 15 min, 85 ℃ 5 s, 4 ℃ 保存。
1.6 RT-PCR反应和测序
根据GenBank登录号,利用Primer Premier 5.0软件设计兼并引物,引物信息见表 1,由上海英骏生物技术有限公司合成。由于目前鸟类10种TLR家族基因是否都在鹅等级卵泡基质层组织表达不清楚,因此,采用RT-PCR的方法检测了10种鸟类TLR家族基因的在基质层的表达谱,其中以脾脏组织为阳性对照[11],以不加cDNA模板为阴性对照。
PCR反应体系为20 μL:2 × Taq PCR Master Mix 10 μL, ddH2O 8 μL, 上游和下游引物各0.5 μL, RT产物1μL。反应程序:95 ℃预变性5 min;95 ℃变性30s,57/60 ℃退火30s,72 ℃延伸30s,35个循环;72 ℃延伸7 min,4 ℃保存。PCR产物用2.5 %琼脂糖凝胶电泳初步鉴定,由上海生工生物工程股份有限公司测序。
1.7 qRT-PCR
根据RT-PCR的表达谱结果,采用Real-time PCR检测不同等级卵泡基质层 TLRs表达水平以及不同LPS处理时间对基质层 TLRs表达的影响。Real-time PCR反应体系为20 μL:1 μL RT产物,上游和下游引物各0.6 μL,7.8 μL ddH2O,10 μL FastStart Universal SYBR Green Master(ROX)。反应程序:50 ℃ 2 min,95 ℃预变性10 min;PCR循环:95 ℃变性15 s,57/ 60 ℃退火30 s,72 ℃ 延伸30 s,读板,共35个循环。以管家基因β-actin为内参,每个样品重复3次,取平均Ct值进行计算,所得试验数据按2-△△Ct结果统计分析。
1.8 数据分析
采用SPSS 13.0软件进行统计分析,数据结果以“平均值 ± 标准误”(Mean ± SE)表示。不同等级卵泡基质层和不同LPS处理时间对基质层TLRs表达的数据采用单因子方差分析,Duncan法多重比较。对变性卵泡与LPS处理0和24 h基质层TLRs表达水平的数据,采用T检验分析。
2 结果
2.1 等级卵泡基质层TLR家族基因表达谱
等级卵泡基质层TLR家族基因表达谱见图1。以
脾脏为阳性对照,扩增等级卵泡基质层TLRs 1A、1B、2A、2B、3、4、5、7、15和21基因,与欲扩增目的片段大小一致。通过测序,并与GenBank已登录(表1)的序列比对,同源性为97%以上,表明PCR扩增片段为目的基因片段。并且,结果显示,已公开的10种鸟类 TLR家族基因均在等级卵泡基质层组织中表达。
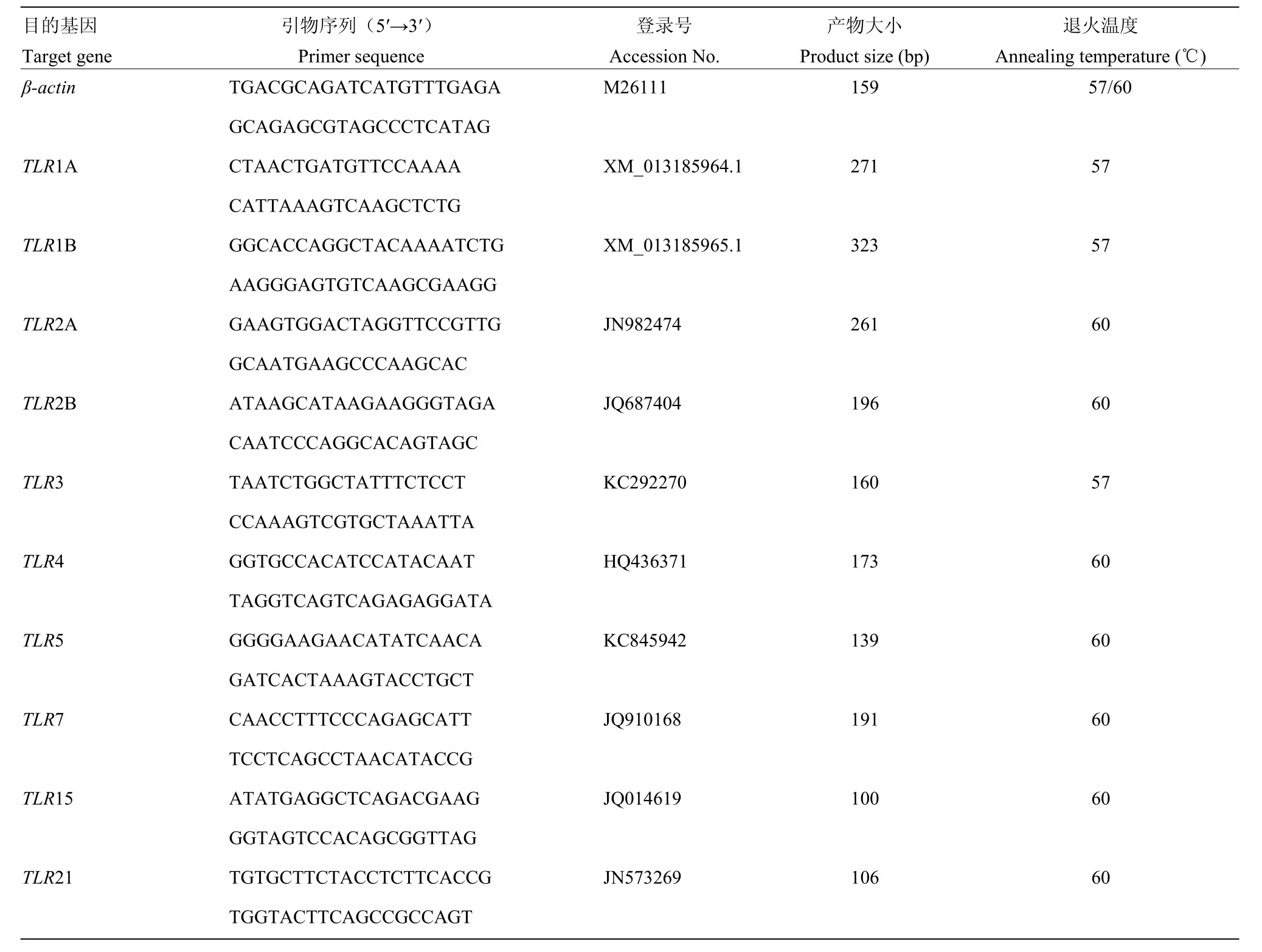
表1 RT-PCR和qRT-PCR引物Table 1 Primer pairs used for RT-PCR and qRT-PCR

图1 等级卵泡基质层TLR家族基因表达谱Fig. 1 Expressions of TLRs in stroma of hierarchical follicles
2.2 不同等级卵泡基质层TLR家族基因表达
不同等级卵泡基质层 TLR家族基因表达水平见图2。随等级卵泡生长,TLRs 2A表达水平逐渐升高。F1级卵泡基质层TLR2A表达水平显著高于F3、F4和F5级卵泡,TLR15表达水平显著高于F5级卵泡。其他TLR家族基因,如TLRs 1A、1B、2B、3、4、5、7和21的表达水平在不同等级卵泡基质层中差异不显著。

图2 不同等级卵泡基质层TLR家族基因表达Fig. 2 Changes in the expression of TLRs in follicular stroma during follicle growth
2.3 不同 LPS处理时间对鹅等级卵泡形态结构的影响
不同LPS处理时间对鹅等级卵泡形态结构的影响见图3。在LPS处理0、6和12 h时,等级卵泡形态无明显变化,但在LPS处理24 h时,3只鹅等级卵泡呈深黄色胶冻状变性,在LPS处理36 h时,全部试验鹅等级卵泡呈深黄色胶冻状变性。
2.4 不同LPS处理时间对鹅等级卵泡基质层TLRs表达的影响
不同LPS处理时间对鹅等级卵泡基质层TLRs表达的影响见图4。在10种TLR家族基因中,6种基因在LPS处理后显著升高。与对照组(LPS处理0h)相比,TLRs 2A、4和5基因表达水平在LPS处理12和24 h时显著升高;TLR2B在LPS处理24 h时显著升高;TLRs 7和15在LPS处理6—24 h期间均显著升高;而TLRs 1A、1B、3和21在LPS处理6—24 h期间差异不显著。
2.5 等级卵泡基质层与变性卵泡组织 TLR家族基因差异表达
等级卵泡基质层与变性卵泡组织TLR家族基因差异表达见图5。与等级卵泡基质层相比,在LPS处理24和36 h的变性卵泡中,TLRs 1A、2A、2B、4、5、7和15基因表达水平显著升高,TLR3表达水平显著降低;在LPS处理36 h的变性卵泡中,TLRs 1B和21表达水平显著升高。
3 讨论
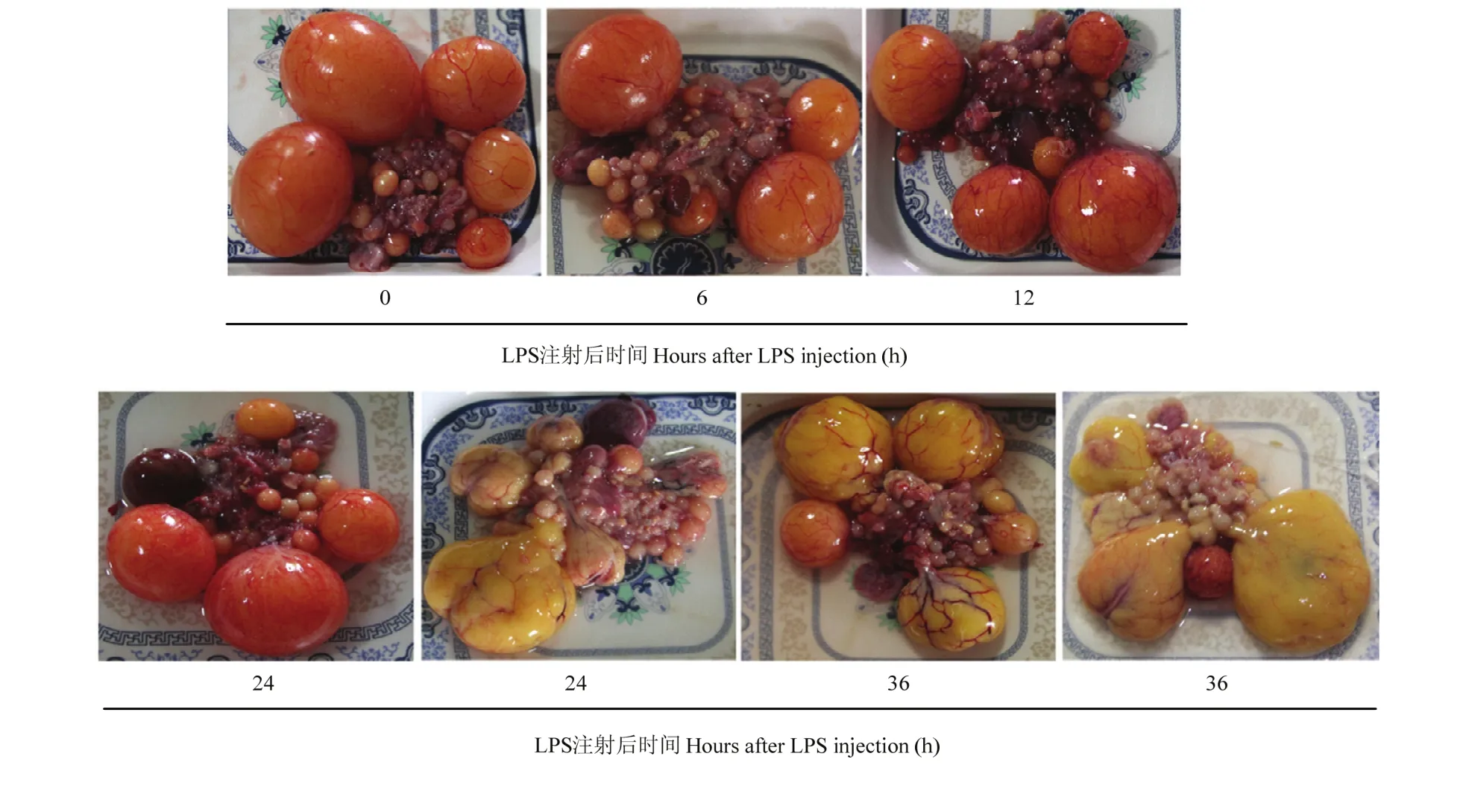
图 3 LPS处理不同时间对鹅等级卵泡形态结构的影响Fig. 3 The time course effect of LPS treatment on follicle morphology

图4 LPS处理不同时间对鹅等级卵泡基质层和变性卵泡TLRs表达的影响Fig. 4 Time course effect of LPS on TLRs mRNA expression in follicular stroma
禽类卵泡是病原微生物偏好侵染组织,因而易造成产蛋性能下降和蛋源食品安全风险[25]。TLRs能识别广泛的病原微生物,诱导炎性因子生成,启动宿主免疫反应,抵御病原微生物侵害[3-5]。TLR家族包含10个成员,识别相应的病原相关分子模式。TLRs 2A, 3, 4, 5、7和21分别识别革兰氏阳性菌肽聚糖、双链RNA、革兰氏阴性菌脂多糖、细菌鞭毛蛋白、单链RNA和细菌DNA CpG岛序列[7-10],而TLR15是禽类特异的识别病原相关分子模式的受体[12]。本试验中,10种TLR家族基因均在鹅等级卵泡基质层中表达,表明虽然由于种鹅的喜水习性造成病原微生物侵染卵巢的风险,但等级卵泡能有效的识别各种病原微生物,进而清除病原微生物,维持卵泡功能。

图5 等级卵泡基质层与变性卵泡组织TLRs差异表达Fig. 5 Differential expression of TLRs between follicular stroma and denatured hierarchical follicle
此外,在鸡卵巢中,TLRs 2、4、5和7基因在卵泡膜层表达,而TLRs 4和5基因在卵泡颗粒层表达[11];TLRs 1B、2、3、4、5和7基因均在输卵管伞部、峡部、膨大部、子宫部和阴道部表达[26]。在 10种TLRs中,8种TLRs家族基因在未成熟卵泡组织中表达[12],9种TLRs基因在鸡阴道、睾丸、附睾和精子中表达[13-15]。笔者先前的研究发现10种TLR家族基因均在种鹅输卵管各功能部位表达[27]。由于面临病原微生物侵染的威胁不同,因此,本试验的结果进一步表明TLR家族基因表达依赖于禽类生殖道不同的细胞和组织类型[6, 11-14, 28]。
卵泡基质层富含血管组织,且卵黄沉积能力大,易使血液病原微生物进入卵泡细胞。随着禽类卵泡生长,等级卵泡基质层血流量逐渐增加,造成大卵泡积聚异源分子数量多于小卵泡,大卵泡面临病原微生物侵染的风险更高。本试验中,随卵泡生长,基质层TLRs 2A和15基因表达逐渐升高。TLRs 2A和15基因是禽类识别病原微生物重要的病原相关识别受体[7-10]。因此,本试验的结果表明大卵泡对病原的识别能力更敏感,具有更强的抵御病原侵染的能力。此外,大量的研究表明TLRs信号通路可能参与动物卵泡内分泌功能[11,29-31],而且这种作用机制是由于TLR介导生成的炎性因子直接作用于卵泡。在禽类等级卵泡中,随卵泡生长,P4合成能力增强、E2合成能力减弱[24,32-33]。因此,TLRs 2A和15可能与种鹅卵泡生长密切相关或者其表达受卵泡类固醇激素的调控,但该推论需要进一步试验验证。
由于种鹅的亲水习性,国内普通采用依赖水面的养殖模式,该模式造成粪便及其中的肠杆菌直接进入养殖水体。随着养殖密度增加和养殖时间延长,养殖水体中的细菌利用粪便中的氮磷等营养物质不断增殖并在死亡后释放LPS[1-2]。细菌和LPS通过种鹅交配或饮水进入种鹅卵巢器官,轻则影响卵巢功能、降低产蛋性能,重则造成卵泡闭锁,甚至造成大肠杆菌和沙门氏菌性卵黄腹膜炎。本试验中,在LPS处理24 h,3只试验鹅等级卵泡出现不规则深黄色变性状态,而在LPS处理36 h后,全部试验鹅出现该变性状态,表明随LPS作用时间延长,等级卵泡形态结构逐渐改变,卵泡生长和排卵受到抑制。本试验建立一种LPS体外试验模型,有助于进一步研究LPS影响种鹅卵泡发育的分子机制。
TLRs 2和4是细菌LPS的病原识别模式受体,外源性LPS促进禽类生殖道,如输卵管[13,16,26]、卵巢[11,34]和睾丸[14,17]等组织TLRs 2和4基因表达。与此研究结果一致,本试验结果显示 LPS促进种鹅卵泡基质层TLRs 2和4基因表达。虽然TLRs 5、7和15基因识别各自相应的病原相关分子模式,但本试验结果发现LPS促进TLRs 5、7和15基因表达水平。由于TLRs结合相应配体后能诱导炎性因子生成,进而启动非特异性免疫反应,抵御病原微生物侵染。因此,本试验结果表明,LPS不仅通过其受体TLRs 2和4启动等级卵泡基质层免疫反应,而且能通过促进其他TLRs,如TLRs 5、7和15基因表达增强宿主免疫反应,进而加速清除病原微生物。
本试验中,TLRs 2A、4和 5基因表达水平在LPS处理12 h时开始显著提高,TLR2B基因表达水平在LPS处理24 h时显著升高,TLRs 7和15基因表达水平在LPS处理6 h时显著升高,而TLRs 1A、1B、3和21基因表达水平在LPS处理0 – 24 h期间差异不显著。因此,种鹅等级卵泡基质层TLR家族对外源LPS的敏感性由强到弱依次为TLRs 7和15;TLRs 2A、4和5;TLR2B;TLRs 1A、1B、3和21。TLR7基因特异性识别病毒[7-10],而TLR15是禽类特异的识别病原的受体[12],因此,在复杂的机体环境下,进入血液的LPS,可能降低了机体病原抵抗力,增加了病毒分子或其它异源分子进入等级卵泡的机率,造成其识别模式受体TLRs 7和15的表达上升,而且,TLRs 7和15对其病原相关分子模式的敏感性可能高于TLRs 2和4对LPS的敏感性。
在LPS或沙门氏菌侵染后,鸡等级卵泡颗粒细胞比等级前卵泡颗粒细胞具有更高的免疫反应性[34]。TLRs识别病原相关分子模式后诱导炎性反应,但过度炎症反应造成组织损伤[35-37]。炎性因子和趋化因子在禽类卵泡组织中表达,而且在LPS侵染后其表达水平显著提高[17,38-39]。本试验中,在LPS处理24 h后,种鹅等级卵泡发生深黄色不规则的胶冻状变性,然而,其TLRs表达水平显著高于未经LPS处理的基质层组织,说明随LPS处理时间延长,等级卵泡细胞炎性反应能力增强,但过度增强的炎性反应可能影响的卵泡形态结构。TLR3主要识别双链RNA 病毒[40]。与卵巢[12-13]和睾丸[28]组织对 LPS的反应性类似,本试验中变性的等级卵泡 TLR3表达显著低于未经LPS处理的基质层组织,说明等级卵泡在LPS过度刺激后,其识别双链RNA病毒的能力降低。
4 结论
已发现的10种鸡TLR家族基因均在种鹅等级卵泡基质层表达;随卵泡生长,TLRs 2A和15基因表达水平显著升高;随LPS处理时间延长,等级卵泡基质层TLRs 2A、2B、4、5、7和15基因表达显著升高;在内毒素处理24和36h时,等级卵泡形态结构发生改变,但其TLR家族基因表达水平显著升高。
[1] YANG X W, LIU L, JANG D L, WANG C L, SUN A D, SHI Z D. Improving geese production performance in “Goose-Fish” production system by competitive reduction of pathogenic bacteria in pond water. Journal of Integrative Agriculture, 2012, 11(6): 993-1001.
[2] JIANG D L, LIU L, WANG C L, CHEN F, SUN A D, SHI Z D. Raising on water stocking density reduces geese reproductive performances via water bacteria and lipopolysaccharide contaminations in "Geese-Fish" production system. Journal of Integrative Agriculture, 2011, 10(9): 1459-1466.
[3] ROEDER A, KIRSCHNING C J, RUPEC R A, SCHALLER M, WEINDL G, KORTING H C. Toll-like receptors as key mediators in innate antifungal immunity. Medical Mycology, 2004, 42(6): 485-498.
[4] AKIRA S, TAKEDA K, KAISHO T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nature Immunology, 2001, 2(8): 675-680.
[5] KAISHO T, AKIRA S. Toll-like receptor function and signaling. Journal of Allergy and Clinical Immunology, 2006, 117(5): 979-987.
[6] MICHAILIDIS G, ANASTASIADOU M, GUIBERT E, FROMENT P. Activation of innate immune system in response to lipopolysaccharide in chicken Sertoli cells. Reproduction, 2014, 148(3): 259-270.
[7] IQBAL M, PHILBIN V J, SMITH A L. Expression patterns of chicken Toll-like receptor mRNA in tissues, immune cell subsets and cell lines. Veterinary Immunology and Immunopathology, 2005, 104(1-2): 117-127.
[8] HONSTETTRE A, GHIGO E, MOYNAULT A, CAPO C, TOMAN R, AKIRA S, TAKEUCHI O, LEPIDI H, RAOULT D, MEGE J L. Lipopolysaccharide from Coxiella burnetii is involved in bacterial phagocytosis, filamentous actin reorganization, and inflammatory responses through Toll-like receptor 4. Journal of Immunology, 2004, 172(6): 3695-3703.
[9] BROWNLIE R, ALLAN B. Avian toll-like receptors. Cell and Tissue Research, 2011, 343(1): 121-130.
[10] KEESTRA A M, DE ZOETE M R, BOUWMAN L I, VAN PUTTEN J P. Chicken TLR21 is an innate CpG DNA receptor distinct from mammalian TLR9. Journal of Immunology, 2010, 185(1): 460-467.
[11] SUBEDI K, ISOBE N, NISHIBORI M, YOSHIMURA Y. Changes in the expression of toll-like receptor mRNAs during follicular growth and in response to lipopolysaccharide in the ovarian follicles of laying hens. Journal of Reproduction and Development, 2007, 53(6): 1227-1235.
[12] MICHAILIDIS G, THEODORIDIS A, AVDI M. Transcriptional profiling of Toll-like receptors in chicken embryos and in the ovary during sexual maturation and in response to Salmonella enteritidis infection. Animal Reproduction Science, 2010, 122(3-4): 294-302.
[13] MICHAILIDIS G, THEODORIDIS A, AVDI M. Effects of sexual maturation and Salmonella infection on the expression of Toll-like receptors in the chicken vagina. Animal Reproduction Science, 2011, 123(3-4): 234-241.
[14] DAS S C, ISOBE N, YOSHIMURA Y. Expression of Toll-like receptors and avian beta-defensins and their changes in response to bacterial components in chicken sperm. Poultry Science, 2011, 90(2): 417-425.
[15] ANASTASIADOU M, AVDI M, MICHAILIDIS G. Expression of avian beta-defensins and Toll-like receptor genes in the rooster epididymis during growth and Salmonella infection. Animal Reproduction Science, 2013, 140(3-4): 224-231.
[16] ARIYADI B, ISOBE N, YOSHIMURA Y. Toll-like receptor signaling for the induction of mucin expression by lipopolysaccharide in the hen vagina. Poultry Science, 2014, 93(3): 673-679.
[17] ZHANG M, NII T, ISOBE N, YOSHIMURA Y. Expression of Toll-like receptors and effects of lipopolysaccharide on the expression of proinflammatory cytokines and chemokine in the testis and epididymis of roosters. Poultry Science, 2012, 91(8): 1997-2003.
[18] QI Y, YAN B, CHEN S, CHEN H, WANG M, JIA R, ZHU D, LIU M, LIU F, YANG Q, SUN K, WU Y, CHEN X, JING B, CHENG A. CpG oligodeoxynucleotide-specific goose TLR21 initiates an anti-viral immune response against NGVEV but not AIV strain H9N2 infection. Immunobiology, 2016, 221(3): 454-461.
[19] QI Y, CHEN S, ZHAO Q, WANG M, JIA R, ZHU D, LIU M, LIU F, CHEN X, CHENG A. Molecular cloning, tissue distribution, and immune function of goose TLR7. Immunology Letters, 2015, 163(2): 135-142.
[20] WEI L, JIAO P, YUAN R, SONG Y, CUI P, GUO X, ZHENG B, JIA W, QI W, REN T, LIAO M. Goose Toll-like receptor 7 (TLR7), myeloid differentiation factor 88 (MyD88) and antiviral molecules involved in anti-H5N1 highly pathogenic avian influenza virus response. Veterinary Immunology and Immunopathology, 2013, 153(1-2): 99-106.
[21] FANG Q, PAN Z, GENG S, KANG X, HUANG J, SUN X, LI Q, CAI Y, JIAO X. Molecular cloning, characterization and expression of goose Toll-like receptor 5. Molecular Immunology, 2012, 52(3-4): 117-124.
[22] YONG Y, LIU S, HUA G, JIA R, ZHAO Y, SUN X, LIAO M, JU X. Identification and functional characterization of Toll-like receptor 2-1 in geese. BMC Veterinary Research, 2015, 11: 108.
[23] QIN Q, SUN A, GUO R, LEI M, YING S, SHI Z. The characteristics of oviposition and hormonal and gene regulation of ovarian follicle development in Magang geese. Reproductive Biology and Endocrinology, 2013, 11(1): 65.
[24] SECHMAN A, ANTOS P, KATARZYNSKA D, GRZEGORZEWSKA A, WOJTYSIAK D, HRABIA A. Effects of 2,3,7,8-tetrachlorodibenzop-dioxin on secretion of steroids and STAR, HSD3B and CYP19A1 mRNA expression in chicken ovarian follicles. Toxicology Letters, 2014, 225(2): 264-274.
[25] OLSEN R H, BISGAARD M, CHRISTENSEN J P, KABELL S, CHRISTENSEN H. Pathology and molecular characterization of Escherichia Coli associated with the avian Salpingitis-Peritonitis Disease Syndrome. Avian Diseases, 2016, 60(1): 1-7.
[26] OZOE A, ISOBE N, YOSHIMURA Y. Expression of Toll-like receptors (TLRs) and TLR4 response to lipopolysaccharide in hen oviduct. Veterinary Immunology and Immunopathology, 2009, 127(3-4): 259-268.
[27] 应诗家, 戴子淳, 郭佳佳, 李辉, 雷明明, 施振旦, 沈明君. 鹅输卵管 TLR家族基因差异表达的研究. 江苏农业学报, 2015, 31(06): 1395-1399.
YING S J, DAI Z C, GUO J J, LI H, LEI M M, SHI Z D, SHEN M J. Differential expression of Toll-like receptors in geese different segments of the oviduct. Jiangsu Journal of Agricultural Sciences, 2015, 31(06): 1395-1399. (in Chinese)
[28] ANASTASIADOU M, THEODORIDIS A, AVDI M, MICHAILIDIS G. Changes in the expression of Toll-like receptors in the chicken testis during sexual maturation and Salmonella infection. Animal Reproduction Science, 2011, 128(1-4): 93-99.
[29] LUTTGENAU J, HERZOG K, STRUVE K, LATTER S, BOOS A, BRUCKMAIER R M, BOLLWEIN H, KOWALEWSKI M P. LPS-mediated effects and spatio-temporal expression of TLR2 andTLR4 in the bovine corpus luteum. Reproduction, 2016, 151: 391-399.
[30] PRICE J C, SHELDON I M. Granulosa cells from emerged antral follicles of the bovine ovary initiate inflammation in response to bacterial pathogen-associated molecular patterns via Toll-like receptor pathways. Biology of Reproduction, 2013, 89(5): 119.
[31] PRICE J C, BROMFIELD J J, SHELDON I M. Pathogen-associated molecular patterns initiate inflammation and perturb the endocrine function of bovine granulosa cells from ovarian dominant follicles via TLR2 and TLR4 pathways. Endocrinology, 2013, 154(9): 3377-3386.
[32] JOHNSON A L. Ovarian follicle selection and granulosa cell differentiation. Poultry Science, 2015, 94(4): 781-785.
[33] JOHNSON A L, WOODS D C. Dynamics of avian ovarian follicle development: cellular mechanisms of granulosa cell differentiation. General and Comparative Endocrinology, 2009, 163(1-2): 12-17.
[34] WOODS D C, SCHOREY J S, Johnson A L. Toll-like receptor signaling in hen ovarian granulosa cells is dependent on stage of follicle maturation. Reproduction, 2009, 137(6): 987-996.
[35] SHI J, ZHAO Y, WANG Y, GAO W, DING J, LI P, HU L, SHAO F. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature, 2014, 514(7521): 187-192.
[36] OGUEJIOFOR C F, CHENG Z, ABUDUREYIMU A, FOULADINASHTA A A, WATHES D C. Global transcriptomic profiling of bovine endometrial immune response in vitro. I. Effect of lipopolysaccharide on innate immunity. Biology of Reproduction, 2015, 93(4): 9.
[37] OGUEJIOFOR C F, CHENG Z, ABUDUREYIMU A, ANSTAETT O L, BROWNLIE J, FOULADI-NASHTA A A, WATHES D C. Global transcriptomic profiling of bovine endometrial immune response in vitro. II. Effect of bovine viral diarrhea virus on the endometrial response to lipopolysaccharide. Biology of Reproduction, 2015, 93(4): 9.
[38] NII T, SONODA Y, ISOBE N, YOSHIMURA Y. Effects of lipopolysaccharide on the expression of proinflammatory cytokines and chemokines and the subsequent recruitment of immunocompetent cells in the oviduct of laying and molting hens. Poultry Science, 2011, 90(10): 2332-2341.
[39] ABDELSALAM M, ISOBE N, YOSHIMURA Y. Effects of lipopolysaccharide on the expression of proinflammatory cytokines and chemokines and influx of leukocytes in the hen ovary. Poultry Science, 2011, 90(9): 2054-2062.
[40] SCHWARZ H, SCHNEIDER K, OHNEMUS A, LAVRIC M, KOTHLOW S, BAUER S, KASPERS B, STAEHELI P. Chicken toll-like receptor 3 recognizes its cognate ligand when ectopically expressed in human cells. Journal of Interferon & Cytokine Research, 2007, 27(2): 97-101.
(责任编辑 林鉴非)
Time Course Effect of Lipopolysaccharide on Toll-Like Receptors Expression in the Goose Follicular Stroma
YING ShiJia, DAI ZiChun, GUO JiaJia, SHI ZhenDan
(Institute of Animal Science, Laboratory of Animal Improvement and Reproduction, Jiangsu Academy of Agricultural Science, Nanjing 210014)
【Objective】 This study aims to investigate the transcriptional profiling of Toll-like receptors (TLRs) and their responses to lipopolysaccharide with different treatment times in the goose follicular stroma. 【Method】 The laying process wasmonitored in a flock of Yangzhou geese. The geese were injected intravenously with LPS (1.5mg/kg body weight) 8 and 2 h after oviposition, and 4, 16 and 28 h before oviposition. All experimental geese were slaughtered 8 h after oviposition. Therefore, time course of LPS was achieved, namely at 0, 6, 12, 24 and 36 h after injection of LPS. Five geese at each time point were selected. After slaughtering, the ovaries were collected, the follicle morphology was observed, and the stroma of the first largest follicles (F1), F2, F3, F4 and F5 was isolated. The animals were provided with feed and water ad libitum, and maintained under natural photoperiod. For the geese 0, 24 and 36 h of after LPS treatment, all samples were used for gene expression analysis. For the other geese, RNA samples of follicular stroma of each goose were mixed with equal concentrations for gene expression analysis. RT-PCR was performed to examine the transcriptional profiling of 10 types of avian TLRs in follicular stroma. The spleen tissue was used as the positive control, and the sample without cDNA sample was used as the negative control. The expression levels of TLRs in follicular stroma among different hierarchical follicles and among different time points were tested using Real-time PCR. For data on the time course of LPS and different hierarchical follicles, the significance of differences was analyzed using the one-way ANOVA followed by Duncan’s multiple range tests. For data between stroma in control group and DF, statistical analysis was carried out using independent-samples T test. 【Result】 All 10 reported TLRs in poultry, namely TLRs 1A, 1B, 2A, 2B, 3, 4, 5, 7, 15 and 21 were expressed in goose stroma of hierarchical follicles. The expression level of TLR2A exhibited a tendency to increase with follicular growth. The TLR2A expression in F1 was higher than in F3, F4 and F5, and the TLR15 expression in F1 was higher than in F5. There were no significant effects of follicle sizes on the expression of TLRs 1A, 1B, 2B, 3, 4, 5, 7 and 21 in stroma. The morphology and colour of ovarian follicles were not changed at 0, 6 and 12 h after administration of LPS. However, the hierarchical follicles of three birds after 24 h and all birds after 36 h became an irregular ellipse or circle in shape and deep yellow in colour. Compared with the control (LPS treatment 0 h), the expression of TLRs 2A, 4 and 5 was significantly increased at 12 and 24 h after LPS treatment, the expression of TLR2B was significantly increased at 24 h, and the expression of TLRs 7 and 15 was significantly increased during the 6 to 24 h period. LPS stimulation did not significantly affect the expression of TLRs 1A, 1B, 3 and 21 during the 6 to 24 h period. Compared with the control, the expression of TLRs 1A, 2A, 2B, 4, 5, 7 and 15 was significantly increased in the denatured hierarchical follicles at 24 and 36 h, while the expression of TLRs 1B and 21 was significantly increased in the denatured hierarchical follicles at 36 h. 【Conclusion】 All the 10 members of avian TLR families are expressed in goose follicular stroma. Furthermore, with prolonged LPS treatment, the morphology of hierarchical follicles is changed, but the TLRs expression levels are still increased.
breeding goose; hierarchical follicle; lipopolysaccharide (LPS); TLRs
2016-07-25;接受日期:2017-01-22
江苏省自然科学基金(BK20130718), 国家水禽产业技术体系(CARS-43-16)
联系方式:应诗家,E-mail:ysj@jaas.ac.cn。通信作者:施振旦,E-mail::zdshi@jaas.ac.cn
