Neural stem cells over-expressing brain-derived neurotrophic factor promote neuronal survival and cytoskeletal protein expression in traumatic brain injury sites
2017-04-07,
,
1 Department of Neurosurgery, the First Affiliated Hospital of Dalian Medical University, Dalian, Liaoning Province, China
2 Department of Histology and Embryology, Dalian Medical University, Dalian, Liaoning Province, China
3 Department of Oncology, Dalian Central Hospital, Dalian, Liaoning Province, China
Neural stem cells over-expressing brain-derived neurotrophic factor promote neuronal survival and cytoskeletal protein expression in traumatic brain injury sites
Tao Chen1, Yan Yu2, Liu-jiu Tang2, Li Kong2, Cheng-hong Zhang2, Hai-ying Chu2, Liang-wei Yin3,*, Hai-ying Ma2,*
1 Department of Neurosurgery, the First Affiliated Hospital of Dalian Medical University, Dalian, Liaoning Province, China
2 Department of Histology and Embryology, Dalian Medical University, Dalian, Liaoning Province, China
3 Department of Oncology, Dalian Central Hospital, Dalian, Liaoning Province, China
How to cite this article:Chen T, Yu Y, Tang LJ, Kong L, Zhang CH, Chu HY, Yin LW, Ma HY (2017) Neural stem cells over-expressing brain-derived neurotrophic factor promote neuronal survival and cytoskeletal protein expression in traumatic brain injury sites. Neural Regen Res 12(3):433-439.
Open access statement:This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Funding:This research was supported by grants from the National Natural Science Foundation of China, No. 31300812 and No. 31371218.
Graphical Abstract
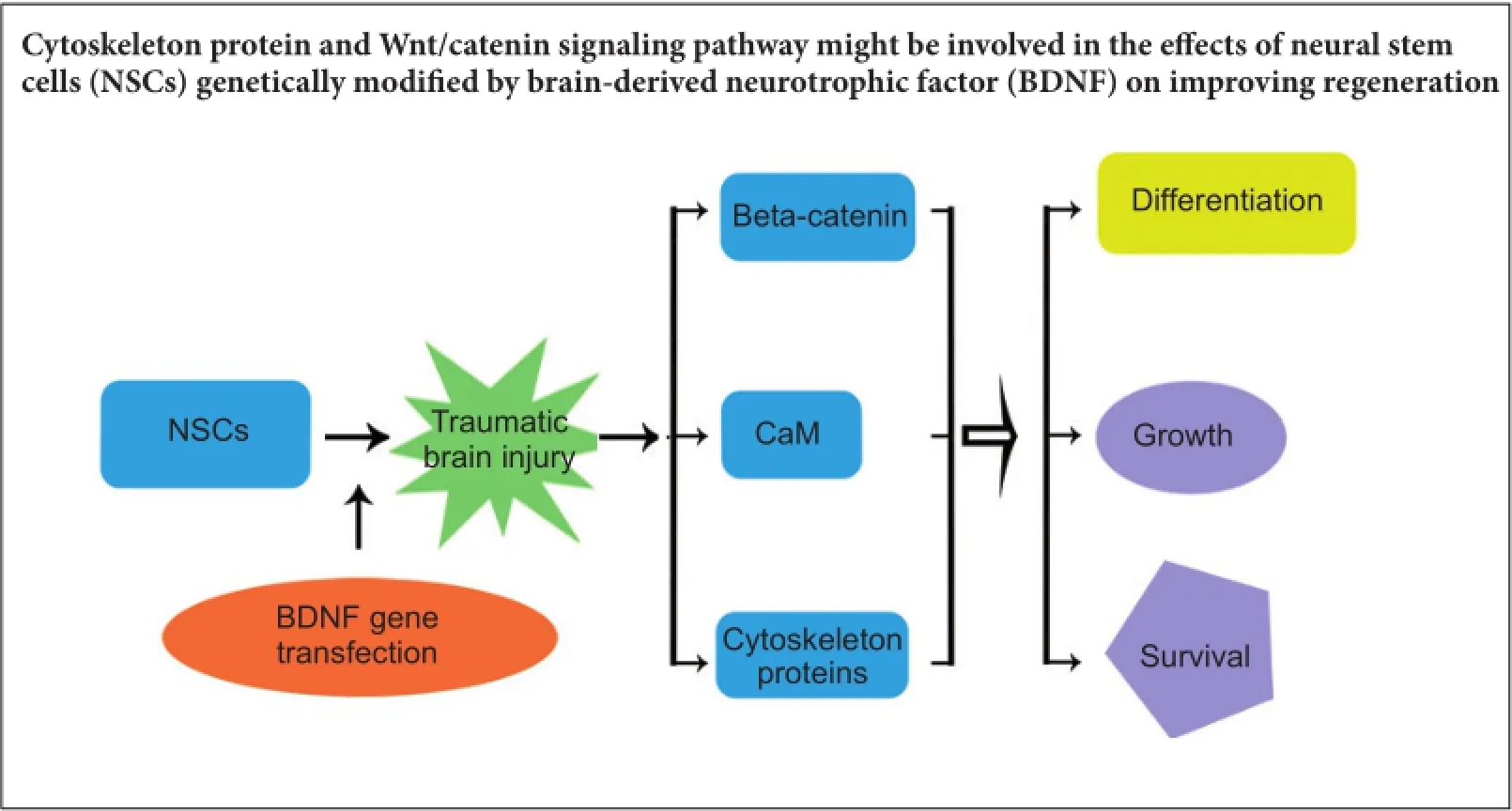
Cytoskeletal proteins are involved in neuronal survival. Brain-derived neurotrophic factor can increase expression of cytoskeletal proteins during regeneration after axonal injury. However, the effect of neural stem cells genetically modified by brain-derived neurotrophic factor transplantation on neuronal survival in the injury site still remains u7nclear. To examine this, we established a rat model of traumatic brain injury by controlled cortical impact. At 72 hours after injury, 2 × 10 cells/mL neural stem cells overexpressing brain-derived neurotrophic factor or naive neural stem cells (3 mL) were injected into the injured cortex. At 1-3 weeks after transplantation, expression of neurofilament 200, microtubule-associated protein 2, actin, calmodulin, and beta-catenin were remarkably increased in the injury sites. These findings confirm that brain-derived neurotrophic factor-transfected neural stem cells contribute to neuronal survival, growth, and differentiation in the injury sites. The underlying mechanisms may be associated with increased expression of cytoskeletal proteins and the Wnt/ β-catenin signaling pathway.
nerve regeneration; brain-derived neurotrophic factor; neural stem cells; transfect; differentiation; traumatic brain injury; cytoskeleton; neurofilament; microtubule-associated proteins; calmodulin; Wnt/β-catenin; neural regeneration
Introduction
Traumatic brain injury (TBI) is a major cause of death and disability globally, and is particularly associated with traffic accidents and disaster (Lin et al., 2016). Previous research has shown that the multi-directional differentiation potential of neural stem cells (NSCs) in the central and peripheral nervous systems plays an important role, and may also be involved in clinical treatment of nerve system injury and degeneration following NSC transplantation (McKay, 1997; Lee et al., 2015; Santamaria and Garica-Sanz, 2015; Lou et al., 2016; Yao et al., 2016). In different environments, embryonic NSCs from different areas of the central nervous system can be differentiated into neurons, astrocytes and oligodendrocytes (Kallur et al., 2006; Shetty and Hattiangady, 2013; Yuan et al., 2015; Ye et al., 2016). Survival, proliferation, and differentiation of transplanted NSCs are influenced by many factors including cytokines, endogenous gene regulation, and biochemical changes in the lesion microenvironment (Chodobski et al., 2011; Mendes Arent et al., 2014). An experimental study showed that NSCs transplantation recovered lost neurological function and cognitive disorder to a certain extent, as well as partly improving reconstruction of neural circuitry, and restoration and growth of cells in lesions of a rat model of TBI (Shear et al., 2011). Besides cell replacement therapy, the mechanisms might be associated with neurotrophic factor release and signal transduction (Castorina et al., 2015). However, the underlying mechanisms are still not clear. Further, the means by which lesions can be influenced to protect cells from loss has not been determined in TBI.
Brain-derived neurotrophic factor (BDNF) is a member of the neurotrophin family of growth factors that plays a critical role in improving differentiation, maturation, and survival of neurons, suppressing apoptosis in the central nervous system, and also exerting a neuroprotective effect under adverse conditions (Davies, 1994; Kang and Schuman, 1995; Wang et al., 2014; Lv et al., 2016). We previously found that NSCs over-expressing BDNF (BDNF/NSCs) significantly increased the number of surviving engrafted cells, proportion of engrafted cells with a neuronal phenotype, and expression of synaptic proteins and growth-associated protein 43 (Ma et al., 2012). Moreover, functional recovery following BDNF/ NSCs transplantation was significantly improved in a rat model of TBI (Ma et al., 2012). We also found that expression of calmodulin (CaM) and the cytoskeletal protein, actin, were strongly associated with neuronal growth and maturationin vitro(Kitamura et al., 1995; Levinson et al., 2004; Schaloske et al., 2005; Larsson, 2006; Difato et al., 2011; Yu et al., 2011). BDNF can increase cytoskeletal protein expression during regeneration after axonal injury (Difato et al., 2011). Thus, these proteins may promote survival of transfected NSCs in TBI sites. Consequently, in this study we sought to determine the effect of BDNF/NSCs transplantation on the cytoskeleton and neuronal survival and growth in a rat model of TBI.
Materials and Methods
NSCs culture
Primary NSCs were isolated from forebrain tissue of embryonic 14-day (E14) Wistar rats, and cultured as previously described (Ma et al., 2010). The plasmid recombinant pcDNA3.1-BDNF was constructed and BDNF/NSCs obtained (Ma et al., 2011, 2012). Cells were incubated at 37°C in a humid atmosphere with 5% CO2. Culture medium consisted of Dulbecco’s modified Eagle’s medium and F12 (1:1; Sigma-Aldrich, St. Louis, MO, USA) with L-glutamine, hydroxyethyl piperazine ethanesulfonic acid, NaHCO3, glucose, heparin (all from Sigma), N2 supplement (1%; Invitrogen, Carlsbad, CA, USA), basic fibroblast growth factor, and epidermal growth factor (Invitrogen, Thermo Fisher Scientific, Inc., Waltham, MA, USA). NSCs were cultured in suspension and dissociated to single cell suspensions using Accutase (Termo Fisher, Inc.) every 5-7 days.
TBI model establishment
Sixty specific-pathogen-free adult male Wister rats, weighing 200-220 g, were provided by the Experimental Animal Laboratory of Dalian Medical University, China, (license number SYXK (Liao) 2013-0006). Procedures were approved by the Institutional Animal Care and Use Committee of the Dalian Medical University, China.
A controlled cortical impact model of brain injury was performed, as previously described (Ma et al., 2012). Briefly, the skull was exposed between lambda and bregma. Next, a 3 mm craniotomy was performed over the left parietotemporal cortex. A controlled cortical impact was induced using a 2.0 mm diameter pneumatic impactor (Air-Power, Inc., High Point, NC, USA), which indented the exposed surface of the brain.
NSCs transplantation
Controlled cortical impact rats were randomly assigned to two groups for transplantation. At 72 hours after TBI, BDNF/NSCs or naive NSCs were harvested and dissociated to single cell suspensions with Accutase, washed again, and then resuspended in sterile phosphate-buffered saline (PBS) at a concentration of 2 × 107cells/mL. PKH-26 (Sigma-Aldrich) was used to label these cells immediately before transplantation, as previously described (Fauza et al., 2008; Ma et al., 2012). Labeled cells were resuspended at a density of 1 × 105cells/mL and maintained on ice for transplantation. TBI rats were anesthetized again. In the BDNF/NSCs group (n= 30), a Stoelting quintessential injector (Stoelting Co., Wood Dale, IL, USA) was used to provide a controlled injection of BDNF/NSCs suspension (3 mL per animal) to the cortex below the injury cavity on the ipsilateral hemisphere: anteroposterior, -3.0 mm from bregma; dorsoventral, 1.1 mm; and mediolateral, 1.0 mm. The injection rate was 1 μL/min for 3 minutes using a 10 μL Hamilton syringe (Hamilton Company, Reno, NV, USA). The NSCs group (n= 30) received an equivalent naive NSCs suspension.
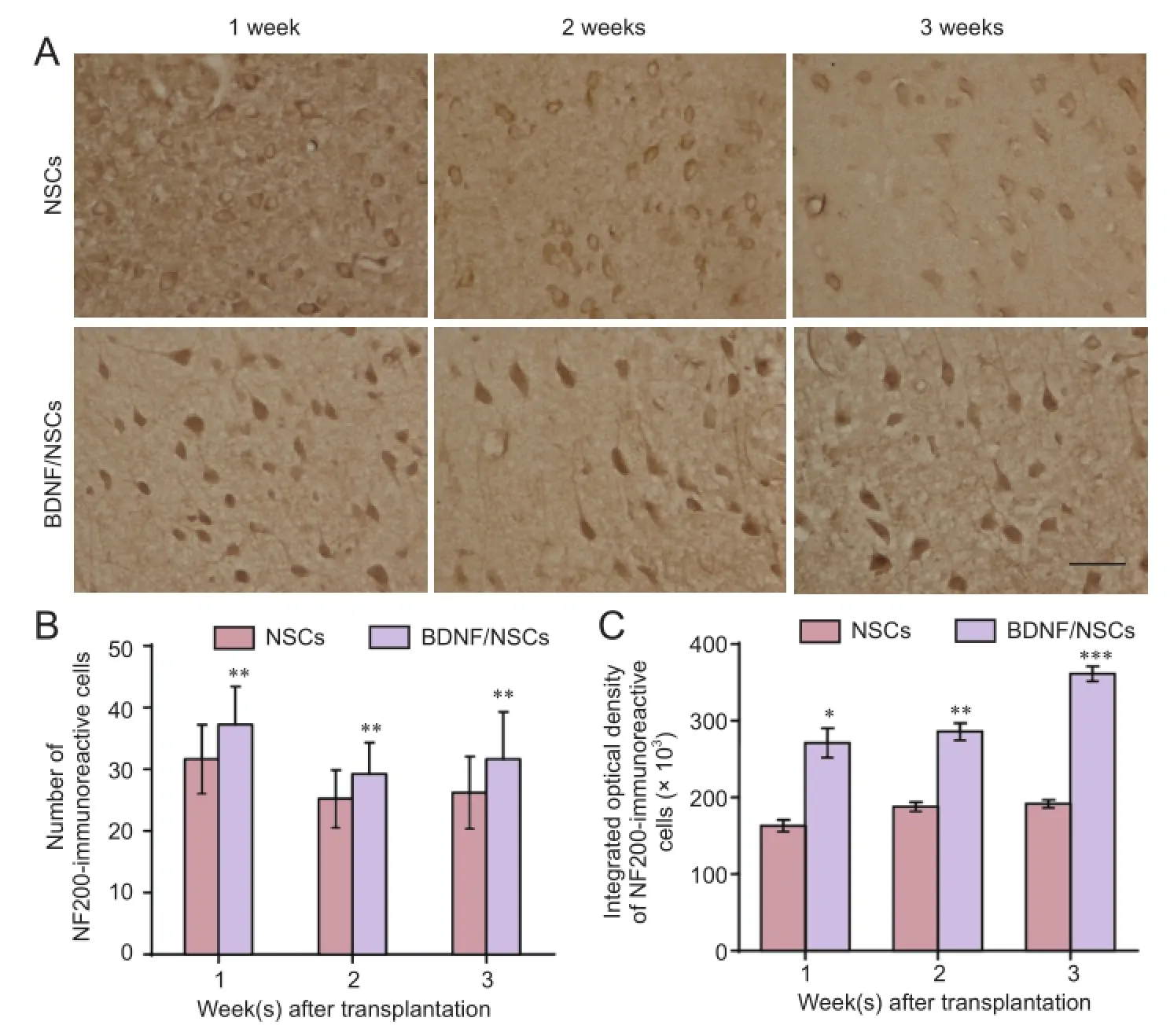
Figure 1 Effect of BDNF/NSCs on NF200 immunoreactivity and neuronal morphology in lesions following transplantation after traumatic brain injury.
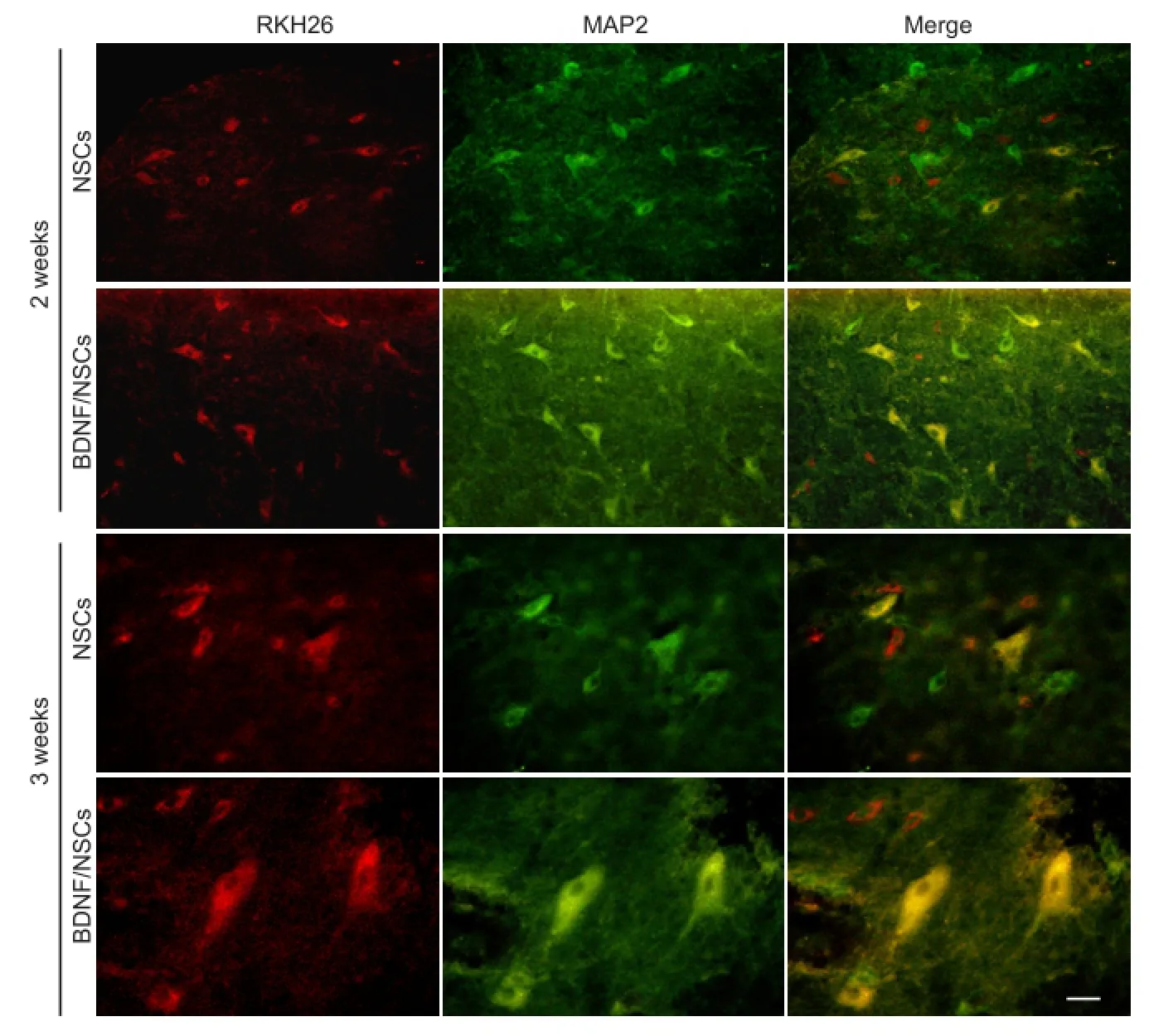
Figure 2 Effect of BDNF/NSCs on MAP2 expression in host cells and neuron-like cells with concomitant PKH26 at 2 and 3 weeks after BDNF/NSCs or NSCs transplantation (immunofluorescence staining).
Immunohistochemical method
At 1, 2, and 3 weeks after transplantation, rats (n= 5 in each group at different time points) were killed by paraformaldehyde perfusion. Brains were harvested and cryosectioned at 16 μm thickness along the coronal plane. To determine neuronal survival and growth within lesions, immunohistochemistry was performed using neurofilament 200 (NF200) antibody. Immunofluorescence was used to assess microtubule-associated protein 2 (MAP2) expression in host cells and neuron-like cells with concomitant PKH26.
Three sections per rat (n= 5) were immunostained, and three regions per section observed. Sections were washed twice with PBS and preincubated in 10% normal goat serum. Sections were incubated at 4°C overnight with one of the following primary antibodies: mouse monoclonal anti-NF200 (1:500; Sigma-Aldrich) or MAP2 (1:200; Boster, Wuhan, China). After washing three times with PBS, sections were incubated with an appropriate secondary antibody for 1 hour at room temperature: goat anti-mouse IgG (1:1,000; ZSGB-Bio, Beijing, China) for NF200 and avidin-biotin complex (Boster), and fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgG (1:1,000; Sigma-Aldrich) for MAP2. Digital color images were obtained using a Nikon C1 Plus confocal microscope (Nikon, Tokyo, Japan). NF200-positive cells were counted in three random regions/section at 400× magnification, with integrated optical density of NF200 immunoreactivity calculated using Image-Pro Plus 5.1 software (Media Cybernetics, Rockville, MD, USA).
Western blot assay
Western blot assay was performed as previously described (Ma et al., 2012). The remaining rats (n= 5 in each group at different time points) were sacrificed. Brain tissue encompassing the transplant site was immediately collected onto a bed of ice. Samples were lysed and protein quantified using a KeyGen assay (KeyGen biotech, Nanjing, China). Protein (50 μg protein per lane) was boiled and separately loaded onto 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis mini-gels, and then transferred to polyvinylidene fluoride membranes. Membranes were blocked with 5% non-fat dry milk in Tris buffered saline with Tween (TBST) for 2 hours at room temperature, and then incubated overnight at 4°C with rabbit anti-actin antibody (1:1,000; Sigma-Aldrich), mouse monoclonal anti-CaM (1:2,000; Sigma-Aldrich), mouse monoclonal anti-β-catenin (1:1,000; Sigma-Aldrich), or mouse monoclonal anti-GAPDH (1:1,000; ZSGB-Bio). Blots were washed three times for 10 minutes each with TBST, incubated for 1 hour with horseradish peroxidase-conjugated goat anti-rabbit IgG or goat anti-mouse IgG (1:5,000; ZSGB-Bio), washed three times for 10 minutes each, and then bound antibodies detected by chemiluminescence using an ECL western blotting detection system kit (Amersham Biosciences, Piscataway, NJ, USA), before exposure to ChemiDOCTMXRS+ with Image LabTMSoftware (BIO-RAD Laboratories, Inc., Hercules, CA, USA). The relative optical density of each band was standardized to optical density of the GAPDH band.
Statistical analysis
Data are expressed as the mean ± SD, and were analyzed using GraphPad Prism 5.0 software (GraphPad Software, Inc., La Jolla, CA, USA). Statistical analyses were performed using one-way analysis of variance and independent samplest-tests. A value ofP< 0.05 was considered statistically significant.
Results
BDNF/NSCs promoted neuronal survival, neurite growth, and NF200 immunoreactivity in TBI lesions
Immunostaining showed more NF200-positive cells in lesions from the BDNF/NSCs group compared with the NSC group at different time points after transplantation (Figure 1B). Further, the cell bodies of surviving neurons (NF200-positive cells) were larger, and with longer neurites in BDNF/NSCs-transplanted rats than NSCs-transplanted rats, even in the first week after transplantation. NF200 was mainly distributed in the cytoplasm of neurons in NSCs-transplanted rats (Figure 1A). NF200 immunoreactivity was significantly increased (P< 0.05 orP< 0.01;Figure 1C) and extended into neurites in many neurons in BDNF/ NSCs-transplanted rats compared with NSCs-transplanted rats (Figure 1A).
MAP2 expression in host cells and neuron-like cells with concomitant PKH26
To determine the effect of BDNF on differentiation of surviving transplanted cells, microtubule protein expression of MAP2 was determined in neuron-like cells with concomitant PKH26 as well as host cells (MAP2-positive cells except for marked engrafted cells). MAP2 expression was greater in the BDNF/NSCs group compared with the NSCs group at 2 and 3 weeks. Additionally, MAP2 expression in neuron-like cells with concomitant PKH26 dye was also greater in the BDNF/NSCs group compared with the NSCs group, especially at 3 weeks. MAP2-positive cells coupled with PKH26 displayed a star-like morphology with more processes, and MAP2-positive fluorescence was also enhanced in cell bodies and processes in the BDNF/NSCs group at 2 and 3 weeks (Figure 2).
BDNF increased β-catenin, actin, and CaM expression in TBI rats after NSCs transplantation
Western blot assays were performed to examine β-catenin, actin, and CaM expression at different time points after transplantation. Expression levels of β-catenin, actin, and CaM were all significantly increased in the BDNF/NSCs group compared with the NSCs group (P< 0.05 orP< 0.01;Figure 3).
Discussion
Our previous studies have shown that NSCs over-expressing BDNF significantly improve survival and neural growth in transplanted BDNF/NSCs after TBI (Ma et al., 2012). In this study, we further investigated the correlation between increased survival and cytoskeletal protein expression following transplantation of NSCs over-expressing BDNF. We found that after TBI, neuron survival and neurite growth in lesions significantly improved in BDNF/NSCs-transplanted rats compared with naive NSCs-transplanted rats. Correspondingly, cytoskeletal proteins (specifically, NF200, MAP2, and actin) were increased by BDNF over-expression in BDNF/NSCs-transplanted rats. Thus, we predict that improved survival and neural growth are associated with enhanced cytoskeletal protein expression by BDNF. Furthermore, β-catenin, a key factor and Wnt signaling pathway downstream target, was also increased in BDNF/ NSCs-transplanted rats, suggesting that the Wnt/catenin sig-naling pathway might be involved in improved regeneration due to NSCs genetically modified by BDNF.
In the adult central nervous system, neurofilaments are the most abundant cytoskeletal protein in neurons and axons, and are important intermediate filaments involved in regulating cellular function. Neurofilaments contribute to dynamic properties of the axonal cytoskeleton during neuronal differentiation, axon outgrowth, regeneration, and guidance (Perrot et al., 2008). Neurofilaments consist of nestin, three neurofilament subunits (NFL, NFM, and NFH), α-internexin, peripherin, and synemin. Nevertheless, only NF200 is found in axons under normal conditions (Portier et al., 1983-1984; Lendahl et al., 1990; Liu et al., 2011). It was previously suggested that TBI mainly causes loss of cytoskeletal proteins, including NF66, NF200, and MAPs. Loss of cytoskeletal proteins leads to more pronounced neuronal loss, which suggests that decayed NF200 adversely affects survival of injured neurons (Posmantur et al., 1994; Galvin et al., 2000; Huh et al., 2002; Meng et al., 2008). NF200 is commonly used as an axonal marker protein (Portier et al., 1983-1984; Liu et al., 2011). Here, we show significantly increased NF200 in lesions in the BDNF/NSC group compared with the NSC group. In addition, neural growth and neurites were particularly enriched in surviving cells in the BDNF/NSC group compared with the NSC group during the experimental period. These results suggest that BDNF may promote NF200 protein expression, which potentially contributes to growth and differentiation of transplanted NSCs. While alternatively, Wang et al. (2014) demonstrated that BDNF was capable of improving the traumatically injured brain microenvironment and promoting axonal regeneration after TBI. Therefore in this study, increased NF200 expression stimulated by NSCs genetically modified by BDNF might protect host neuronal loss and promote axonal regeneration in lesions (Perrot et al., 2008; Liu et al., 2011). Indeed, it is possible that TBI-induced primary injury and secondary inflammation may suppress regeneration of axons with lower NF200 levels in the early stage of TBI. Although some cells would continue to be lost during the first two weeks, survival will be ongoing (modified by increased levels of important cytoskeletal proteins due to transplantation), leading to neuronal growth and partially repaired or regenerated neurites in lesions, which in turn may lead to further increased NF200 expression, especially in BDNF/NSCs-transplanted rats (Shetty and Turner, 1995; Huh et al., 2002).
To investigate the effect of BDNF on transplanted cell differentiation and microtubules in lesions, PKH26 staining was performed to label transplanted cells, with MAP2 expression detected. MAP2 is an important cytoskeletal element that connects microtubules and actin filaments. Microtubules are polymerized under control of MAP2, which also shapes microtubule networks and confers their distinct functional properties, thereby regulating microtubule stabilityin vitro. Microtubules are required for dendrite and axon formation in neurons (Bonnet et al., 2001; Ikegami et al., 2006), and subsequently regulate neuronal growth and restoration. Our data show that BDNF significantly promotes MAP2 expression not only in neuron-like cells differentiated from transplanted cells, but also in host cells after transplantation. Our previous study showed that BDNF increases expression of β-tubulin III, which is an important microtubule component (Ma et al., 2012). Accordingly, this demonstrates that BDNF increases these cytoskeletal proteins, and may play an important role in improving neuronal growth and differentiation, and axonal regeneration.
Our data also show that BDNF increases CaM and actin expression. Some studies have shown that growth-associated protein 43 is involved in growth and regeneration of neurons and axons, and improves behavioral functional recovery after transplantation of NSCs. The underlying mechanism is that growth-associated protein 43 binds to CaM and actin fibrils (Mosevitsky, 2005; Ma et al., 2011; Chung et al., 2016a). Difato et al. (2011) reported that after axonal lesions, BDNF regulates growth and favors actin wave formation during regeneration. Tus, improved growth and differentiation by BDNF following BDNF/NSCs transplantation may be directly associated with increased CaM and actin protein levels.
β-Catenin is a multifunctional protein extensively involved in neuronal growth, proliferation, and differentiation. Wnt/ β-catenin signaling regulates proliferation and differentiation of neural progenitor cells, and subsequently, timely sequential cortical neurogenesis (Chung et al., 2016b). During axonal remodeling, Wnt affects looped microtubule formation, and acts in the β-catenin pathway, directly resulting in microtubule growth. The Wnt signal also promotes actin cytoskeleton growth (Hall et al., 2000; Purro et al., 2008), and stimulates NSCs growth and proliferation by shortening cell cycle length (Piccin and Morshead, 2011; Ortega et al., 2013). Yang et al. (2015) demonstrated that BDNF promotes neuronal growthin vitrothrough crosstalk with the Wnt/ β-catenin signaling pathway. Tey showed that Wnt signaling factors are upregulated by BDNF in human neurons. Our data show significantly increased β-catenin protein expression in the BDNF/NSCs group compared with the NSCs group. Terefore, we suggest that a possible mechanism underlying BDNF accelerated growth, regeneration, and stability of cytoskeletal proteins might involve the Wnt/β-catenin signaling pathway. Indeed, the underlying molecular mechanisms are still worthy of further study.
In conclusion, NSCs transplantation modified by the BDNF gene significantly promotes neuronal survival and growth in lesions, and differentiation of transplanted NSCs. This is associated with increased cytoskeletal protein expression by BDNF. We predict that the mechanism underlying correlation between neuronal growth and increased cytoskeletal proteins modified by BDNF might involve the Wnt/ β-catenin signaling pathway.
Acknowledgments:We thank Professor Shui Guan and Xue-hu Ma from the Stem Cell and Tissue Engineering Laboratory of the Dalian University of Technology of China for their support in culturing the NSCs.
Author contributions:TC and YY conceived and designed the study, analyzed data and wrote the paper. LJT and HYC participated in cell culture, immunofluorescence and western blot assay. LK provided reference data and technical support. CHZ participated in cell culture and TBImodel establishment. LWY participated in transplantation, ensured the integrity of the data and obtained the funding. HYM served as a principle investigator, obtained the funding, conceived and designed the study. All authors approved the final version of the paper.
Conflicts of interest:None declared.
Plagiarism check:This paper was screened twice using CrossCheck to verify originality before publication.
Peer review:This paper was double-blinded and stringently reviewed by international expert reviewers.

Figure 3 Effect of BDNF/NSCs on actin, CaM, and β-catenin expression in lesions at 1, 2, and 3 weeks after BDNF/NSCs or NSCs transplantation (western blot assay).
Bonnet C, Boucher D, Lazereg S, Pedrotti B, Islam K, Denoulet P, Larcher JC (2001) Differential binding regulation of microtubule-associated proteins MAP1A, MAP1B, and MAP2 by tubulin polyglutamylation. J Biol Chem 276:12839-12848.
Castorina A, Szychlinska MA, Marzagalli R, Musumeci G (2015) Mesenchymal stem cells-based therapy as a potential treatment in neurodegenerative disorders: is the escape from senescence an answer? Neural Regen Res 10:850-858.
Chodobski A, Zink BJ, Szmydynger-Chodobska J (2011) Blood-brain barrier pathophysiology in traumatic brain injury. Transl Stroke Res 2:492-516.
Chung S, Rho S, Kim G, Kim SR, Baek KH, Kang M, Lew H (2016a) Human umbilical cord blood mononuclear cells and chorionic plate-derived mesenchymal stem cells promote axon survival in a rat model of optic nerve crush injury. Int J Mol Med 37:1170-1180.
Chung S, Rho S, Kim G, Kim S-R, Baek K-H, Kang M, Lew H (2016b) Human umbilical cord blood mononuclear cells and chorionic plate-derived mesenchymal stem cells promote axon survival in a rat model of optic nerve crush injury. Int J Mol Med 37:1170-1180.
Darsalia V, Kallur T, Kokaia Z (2007) Survival, migration and neuronal differentiation of human fetal striatal and cortical neural stem cells grafted in stroke-damaged rat striatum. Eur J Neurosci 26:605-614.
Davies AM (1994) The role of neurotrophins in the developing nervous system. J Neurobiol 25:1334-1348.
Difato F, Tsushima H, Pesce M, Benfenati F, Blau A, Chieregatti E (2011) The formation of actin waves during regeneration after axonal lesion is enhanced by BDNF. Sci Rep 1:183.
Fauza DO, Jennings RW, Teng YD, Snyder EY (2008) Neural stem cell delivery to the spinal cord in an ovine model of fetal surgery for spina bifida. Surgery 144:367-373.
Galvin JE, Nakamura M, McIntosh TK, Saatman KE, Sampathu D, Raghupathi R, Lee VMY, Trojanowski JQ (2000) Neurofilament-rich intraneuronal inclusions exacerbate neurodegenerative sequelae of brain trauma in NFH/LacZ transgenic mice. Exp Neurol 165:77-89.
Hall AC, Lucas FR, Salinas PC (2000) Axonal remodeling and synaptic differentiation in the cerebellum is regulated by WNT-7a signaling. Cell 100:525-535.
Huh JW, Laurer HL, Raghupathi R, Helfaer MA, Saatman KE (2002) Rapid loss and partial recovery of neurofilament immunostaining following focal brain injury in mice. Exp Neurol 175:198-208.
Ikegami K, Mukai M, Tsuchida J, Heier RL, MacGregor GR, Setou M (2006) TTLL7 is a mammalian beta-tubulin polyglutamylase required for growth of MAP2-positive neurites. The Journal of biological chemistry 281:30707-30716.
Kallur T, Darsalia V, Lindvall O, Kokaia Z (2006) Human fetal cortical and striatal neural stem cells generate region-specific neurons in vitro and differentiate extensively to neurons after intrastriatal transplantation in neonatal rats. J Neurosci Res 84:1630-1644.
Kang H, Schuman E (1995) Long-lasting neurotrophin-induced enhancement of synaptic transmission in the adult hippocampus. Science 267:1658-1662.
Kitamura Y, Arima T, Imaizumi R, Sato T, Nomura Y (1995) Inhibition of constitutive nitric oxide synthase in the brain by pentamidine, a calmodulin antagonist. Eur J Pharmacol 289:299-304.
Larsson C (2006) Protein kinase C and the regulation of the actin cytoskeleton. Cell Signal 18:276-284.
Lee IS, Jung K, Kim IS, Lee H, Kim M, Yun S, Hwang K, Shin JE, Park KI (2015) Human neural stem cells alleviate Alzheimer-like pathology in a mouse model. Mol Neurodegener 10:38.
Lendahl U, Zimmerman LB, McKay RD (1990) CNS stem cells express a new class of intermediate filament protein. Cell 60:585-595.
Levinson H, Moyer KE, Saggers GC, Paul Ehrlich H (2004) Calmodulin-myosin light chain kinase inhibition changes fibroblast-populated collagen lattice contraction, cell migration, focal adhesion formation, and wound contraction. Wound Repair Regen 12:505-511.
Lin W, Yang LK, Cai S, Zhu J, Feng Y, Yang LX, Feng ZZ, Li PP, Chen JH, Wang YH (2016) Cognitive function and biomarkers after traumatic brain injury: protocol for a prospective inception cohort study. Asia Pac J Clin Trials Nerv Syst Dis 1:170-176.
Liu WG, Wang ZY, Huang ZS (2011) Bone marrow-derived mesenchymal stem cells expressing the bFGF transgene promote axon regeneration and functional recovery after spinal cord injury in rats. Neurol Res 33:686-694.
Lou YL, Chen P, Jiang Y, Zhang H, Min YH (2016) Neural stem cell transplantation for sequela of traumatic brain injury: the best timing for treatment. Zhongguo Zuzhi Gongcheng Yanjiu 20:1474-1480.
Lv XM, Liu Y, Wu F, Yuan Y, Luo M (2016) Human umbilical cord blood-derived stem cells and brain-derived neurotrophic factor protect injured optic nerve: viscoelasticity characterization. Neural Regen Res 11:652-656.
Ma H, Yu B, Kong L, Zhang Y, Shi Y (2011) Transplantation of neural stem cells enhances expression of synaptic protein and promotes functional recovery in a rat model of traumatic brain injury. Mol Med Rep 4:849-856.
Ma H, Yu B, Kong L, Zhang Y, Shi Y (2012) Neural stem cells over-expressing brain-derived neurotrophic factor (BDNF) stimulate synaptic protein expression and promote functional recovery following transplantation in rat model of traumatic brain injury. Neurochem Res 37:69-83.
Ma XH, Shi Y, Hou Y, Liu Y, Zhang L, Fan WX, Ge D, Liu TQ, Cui ZF (2010) Slow-freezing cryopreservation of neural stem cell spheres with different diameters. Cryobiology 60:184-191.
McKay R (1997) Stem cells in the central nervous system. Science 276:66-71.
Mendes Arent A, de Souza LF, Walz R, Dafre AL (2014) Perspectives on molecular biomarkers of oxidative stress and antioxidant strategies in traumatic brain injury. Biomed Res Int 2014:723060.
Meng XT, Li C, Dong ZY, Liu JM, Li W, Liu Y, Xue H, Chen D (2008) Co-transplantation of bFGF-expressing amniotic epithelial cells and neural stem cells promotes functional recovery in spinal cord-injured rats. Cell Biol Int 32:1546-1558.
Mosevitsky MI (2005) Nerve ending “signal” proteins GAP-43, MARCKS, and BASP1. Int Rev Cytol 245:245-325.
Ortega F, Gascón S, Masserdotti G, Deshpande A, Simon C, Fischer J, Dimou L, Chichung Lie D, Schroeder T, Berninger B (2013) Oligodendrogliogenic and neurogenic adult subependymal zone neural stem cells constitute distinct lineages and exhibit differential responsiveness to Wnt signalling. Nat Cell Biol 15:602-613.
Perrot R, Berges R, Bocquet A, Eyer J (2008) Review of the multiple aspects of neurofilament functions, and their possible contribution to neurodegeneration. Mol Neurobiol 38:27-65.
Piccin D, Morshead CM (2011) Wnt signaling regulates symmetry of division of neural stem cells in the adult brain and in response to injury. Stem Cells 29:528-538.
Portier MM, de Néchaud B, Gros F (1983-1984) Peripherin, a new member of the intermediate filament protein family. Dev Neurosci 6:335-344.
Posmantur R, Hayes RL, Dixon CE, Taft WC (1994) Neurofilament 68 and neurofilament 200 protein levels decrease after traumatic brain injury. J Neurotrauma 11:533-545.
Purro SA, Ciani L, Hoyos-Flight M, Stamatakou E, Siomou E, Salinas PC (2008) Wnt regulates axon behavior through changes in microtubule growth directionality: a new role for Adenomatous Polyposis Coli. J Neurosci 28:8644-8654.
Santamaria S, Garcia-Sanz JA (2015) Insights of the brain damage response using antibodies identifying surface antigens on neural stem cells and neuroblasts. Neural Regen Res 10:1574-1575.
Schaloske RH, Lusche DF, Bezares-Roder K, Happle K, Malchow D, Schlatterer C (2005) Ca2+regulation in the absence of the iplA gene product in Dictyostelium discoideum. BMC Cell Biol 6:13.
Shear DA, Tate CC, Tate MC, Archer DR, LaPlaca MC, Stein DG, Dunbar GL (2011) Stem cell survival and functional outcome after traumatic brain injury is dependent on transplant timing and location. Restor Neurol Neurosci 29:215-225.
Shetty AK, Hattiangady B (2013) Postnatal age governs the extent of differentiation of hippocampal CA1 and CA3 subfield neural stem/ progenitor cells into neurons and oligodendrocytes. Int J Dev Neurosci 31:646-656.
Shetty AK, Turner DA (1995) Intracerebroventricular kainic acid administration in adult rat alters hippocampal calbindin and non-phosphorylated neurofilament expression. J Comp Neurol 363:581-599.
Wang LJ, Zhang RP, Li JD (2014) Transplantation of neurotrophin-3-expressing bone mesenchymal stem cells improves recovery in a rat model of spinal cord injury. Acta Neurochir (Wien) 156:1409-1418.
Yang JW, Ru J, Ma W, Gao Y, Liang Z, Liu J, Guo JH, Li LY (2015) BDNF promotes the growth of human neurons through crosstalk with the Wnt/β-catenin signaling pathway via GSK-3β. Neuropeptides 54:35-46.
Yao Y, Zheng XR, Zhang SS, Wang X, Yu XH, Tan JL, Yang YJ (2016) Transplantation of vascular endothelial growth factor-modified neural stem/progenitor cells promotes the recovery of neurological function following hypoxic-ischemic brain damage. Neural Regen Res 11:1456-1463.
Ye LJ, Bian H, Fan YD, Wang ZB, Yu HL, Ma YY, Chen F (2016) Rhesus monkey neural stem cell transplantation promotes neural regeneration in rats with hippocampal lesions. Neural Regen Res 11:1464-1470.
Yu B, Ma H, Du Z, Hong Y, Sang M, Liu Y, Shi Y (2011) Involvement of calmodulin and actin in directed differentiation of rat cortical neural stem cells into neurons. Int J Mol Med 28:739-744.
Yuan LL, Guan YJ, Ma DD, Du HM (2015) Optimal concentration and time window for proliferation and differentiation of neural stem cells from embryonic cerebral cortex: 5% oxygen preconditioning for 72 hours. Neural Regen Res 10:1516-1522.
Copyedited by James R, Norman C, Yu J, Li CH, Qiu Y, Song LP, Zhao M
*Correspondence to: Hai-ying Ma, Ph.D. or Liang-wei Yin, M.D., hyma20060602@aliyun.com or Lwyin2008@163.com.
orcid: 0000-0002-1314-7816 (Hai-ying Ma) 0000-0001-8893-3929 (Liang-wei Yin)
10.4103/1673-5374.202947
Accepted: 2017-01-13
杂志排行
中国神经再生研究(英文版)的其它文章
- Telomerase and mTOR in the brain: the mitochondria connection
- Impacts of the retinal environment and photoreceptor type on functional regeneration
- Tissue-type plasminogen activator is a homeostatic regulator of synaptic function in the central nervous system
- Novel insights into the role of NF-κB p50 in astrocytemediated fate specification of adult neural progenitor cells
- Anesthetic considerations for patients with acute cervical spinal cord injury
- Neuromelanin, one of the most overlooked molecules in modern medicine, is not a spectator
