Electroacupuncture reduces apoptotic index and inhibits p38 mitogen-activated protein kinase signaling pathway in the hippocampus of rats with cerebral ischemia/ reperfusion injury
2017-04-07ChunLiXiuhongXu
Chun Li Xiu-hong Xu
1 School of Traditional Chinese Medicine, Southern Medical University, Guangzhou, Guangdong Province, China
2 College of Acupuncture, Moxibustion and Tuina, Hunan University of Chinese Medicine, Changsha, Hunan Province, China
RESEARCH ARTICLE
Electroacupuncture reduces apoptotic index and inhibits p38 mitogen-activated protein kinase signaling pathway in the hippocampus of rats with cerebral ischemia/ reperfusion injury
Xiao Lan1,#, Xin Zhang2,#, Guo-ping Zhou1,*, Chun-xiao Wu1, Chun Li2, Xiu-hong Xu1
1 School of Traditional Chinese Medicine, Southern Medical University, Guangzhou, Guangdong Province, China
2 College of Acupuncture, Moxibustion and Tuina, Hunan University of Chinese Medicine, Changsha, Hunan Province, China
How to cite this article:Lan X, Zhang X, Zhou GP, Wu CX, Li C, Xu XH (2017) Electroacupuncture reduces apoptotic index and inhibits p38 mitogen-activated protein kinase signaling pathway in the hippocampus of rats with cerebral ischemia/reperfusion injury. Neural Regen Res 12(3):409-416.
Open access statement:This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Funding:This work was supported by the National Natural Science Foundation of China, No. 81173355.
Graphical Abstract

Electroacupuncture attenuates cerebral hypoxia and neuronal apoptosis induced by cerebral ischemia/reperfusion injury. To further identify the involved mechanisms, we assumed that electroacupuncture used to treat cerebral ischemia/reperfusion injury was associated with the p38 mitogen-activated protein kinase (MAPK) signaling pathway. We established rat models of cerebral ischemia/reperfusion injury using the modified Zea-Longa’s method. At 30 minutes before model establishment, p38 MAPK blocker SB20358 was injected into the leftlateral ventricles. At 1.5 hours after model establishment, electroacupuncture was administered at acupoints ofChize(LU5),Hegu(LI4),Zusanli(ST36), andSanyinjiao(SP6) for 20 minutes in the affected side. Results showed that the combination of EA and SB20358 injection significantly decreased neurologic impairment scores, but no significant differences were determined among different interventional groups. Hematoxylin-eosin staining also showed reduced brain tissue injuries. Compared with the SB20358 group, the cells were regularly arranged, the structures were complete, and the number of viable neurons was higher in the SB20358 + electroacupuncture group. Terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick-end labeling assay showed a decreased apoptotic index in each group, with a significant decrease in the SB20358 + electroacupuncture group. Immunohistochemistry revealed reduced phosphorylated p38 expression at 3 days in the electroacupuncture group and SB20358 + electroacupuncture group compared with the ischemia/reperfusion group. There was no significant difference in phosphorylated p38 expression between the ischemia/reperfusion group and SB20358 group. These findings confirmed that the electroacupuncture effects on mitigating cerebral ischemia/reperfusion injury are possibly associated with the p38 MAPK signaling pathway. A time period of 3 days could promote the repair of ischemic cerebral nerves.
nerve regeneration; brain injury; electroacupuncture; cell apoptosis; cerebral ischemia/reperfusion injury; neurological impairment score; morphological changes; immunohistochemical assay; p38 mitogen-activated protein kinases; phosphorylated p38; hippocampus; neural regeneration
Introduction
Currently, cerebrovascular disease is one of the most common diseases causing disability and mortality in humans. Ischemic stroke is the most common type of cerebrovascular disease, accounting for approximately 80% of all cerebrovascular diseases (Yates et al., 2012). The sudden interruption of cerebral blood flow triggers inflammatory responses and leads to irreversible brain damage (Moskowitz et al., 2010; Chen et al., 2016; Cheng et al., 2016). The delayed restoration of intracerebral blood and oxygen supply further induces more severe inflammation, aggravates ischemic damage, and results in cerebral ischemia/reperfusion (I/R) injuries (Pundik et al., 2012; Chen et al., 2016), which involve a series of continuous complex pathological processes. Previous studies have shown that the mechanisms of I/R injuries are associated with free radical chain reactions (Cao et al., 2001), intracellular calcium overload (Matsuda et al., 2001), toxic effects of excitatory amino acids (Wei et al., 1998), and high aggregation of neutrophils (Wang, 2006). Conversely, I/R injuries activate multiple enzymes and initiate several apoptosis pathways that induce cell death. Previous studies have shown that ischemic cerebral injuries induce neuronal death, which could be divided into two different types: cells in the central area of ischemia experience necrotic death, while cells in surrounding ischemic penumbra experience apoptotic death (Schmidt-Kastner et al., 1997; Kumaran et al., 2008).
Mitogen-activated protein kinases (MAPKs), a group of conservative serine-threonine kinases, are important signal transduction enzymes in a variety of cells. Previous studies have shown that the MAPK signaling pathway participates in processing and transducing extracellular signals into cells (Huang et al., 2016; Wang et al., 2016a). It is a common pathway for intracellular signal transduction, which can affect downstream gene transcription and regulation, thereby influencing multiple physiological functions and processes such as apoptosis (Ma et al., 1999). The most typical MAPK signal transduction pathways include the extracellular signal-regulated kinase pathway, which inhibits cell apoptosis (Gao et al., 2005), and c-Jun N-terminal kinase and p38 pathways, which promote cell apoptosis (Kim et al., 2006; Dhanasekaran et al., 2008). The p38 MAPK pathway, one of the most important members of the MAPK family, was discovered by Brewster et al. (1993) while investigating changes in yeast in a high-osmotic environment. Extracellular stimuli (such as stress, activation of G-protein-coupled receptors, and cytokines) initiate a series of intracellular responses, induce p38 MAPK phosphorylation, and thus activate the p38 MAPK pathway (Zhang et al., 2006). p38 is the most important MAPK family member that regulates inflammatory responses, and inflammatory responses post-cerebral ischemia play important roles in the development and progression of I/R injuries (Nogawa et al., 1997). Terefore, the associations between the p38 MAPK pathway and ischemic cerebral injuries have gained a lot of attention in recent years.
Acupuncture, as a traditional therapy, is widely used to relieve cerebral I/R injury. The curative effect has been proven in the treatment and rehabilitation of ischemic stroke (Xia et al., 2012; Feng et al., 2016). The acupoints ofChize(LU5),Hegu(LI4),Zusanli(ST36), andSanyinjiao(SP6) are commonly used in electroacupuncture (EA) to treat ischemic stroke (Yang et al., 2015; Wang et al., 2016b), which can improve neurological deficit symptoms. Based on the aforementioned studies, we observed apoptosis and phosphorylated p38 MAPK-positive cells in the CA1 area of the hippocampus at 2 hours, 6 hours, 1 day, 3 days, and 1 week. EA treatment was administered at 3 days and 1 week, once per day, which has been previously used in clinical treatment. The once-daily treatment was a different experimental design compared with conventional treatment timepoints. We explored the effects of different time points to determine the best time window for EA treatment.
In this study, rat models of cerebral I/R were established, and then the effects of EA acupoint combination ofChize,Hegu,Zusanli, andSanyinjiaoon the p38 MAPK signaling pathway were explored.
Additionally, the effects of the acupoint combination on neurological impairment score, cell morphological changes, apoptotic index, and expression of phosphorylated p38 MAPK were also analyzed to explore the possible mechanisms underlying the effect of EA on cerebral I/R injuries.
Materials and Methods
Animals
Two hundred and fifty specific pathogen-free, male, Sprague-Dawley rats weighing 260-300 g were obtained from the Laboratory Animal Center, Southern Medical University, China (Certification No. SCXK (Yue) 2011-2015). The study was approved by the Institutional Animal Care and Use Committee of Southern Medical University of China (Ethics No. 2013-032). The rats went through an acclimation period of 1 week, with free access to food and water. The temperature of the laboratory was maintained at 20-25°C, with a relative humidity of 60-70%. The rats were maintained in a quiet environment for at least 24 hours, and were fasted on water for 12 hours prior to surgery.
Model induction
A total of 250 Sprague-Dawley rats were equally and randomly divided into the sham-operated group (sham group), cerebral I/R model group (I/R group), EA group, p38 MAPK blocking group (SB group), and p38 MAPK blocking + EA group (SB + EA group). According to the time points, the rats in each group were further divided into five subgroups and received 2-hour, 6-hour, 1-day, 3-day, and 1-week intervention after reperfusion respectively (n= 10).
The rats were fasted on water for 12 hours, and then the middle cerebral artery of the rat was blocked according to the modified Zea-Longa’s method (Longa et al., 1989) to induce cerebral I/R model. The rats were anesthetized by injecting 10% chloral hydrate (0.35 mL/100 g) (Guangtuo Chemical, Shanghai, China) intraperitoneally. A 1.0- to 1.5-cm longitudinal incision was made 0.3 mm to the anterior midline of the neck to expose the left common carotid artery, internal carotid artery, external carotid artery, and va-gus nerve. Ten, a thread was placed on the common carotid artery, internal carotid artery, and external carotid artery. The common carotid artery and external carotid artery were ligated at the proximal ends, and a small bulldog clamp was used to temporarily clip the distal end of internal carotid artery. A small incision was made 3 mm to the point where the common carotid artery divided into internal carotid artery and external carotid artery, and a Nylon line bolt was inserted into the internal carotid artery toward the brain. After reaching the bulldog clamp site, the clamp was removed and the line knot was rapidly inserted until the distance to the arterial divergence was about 19 mm. The line knot was fixed to induce occlusion of the left middle artery. Penicillin was injected to prevent inflammation of the incisions, after which the incisions were sutured, layer by layer. The line knot was withdrawn to about 10 mm after 30 minutes of ischemia to restore middle cerebral artery patency and thus induce I/R. For the remaining rats, the same procedures were performed. However, only the carotid triangle was exposed, and no line knot was applied. The rats were singly caged after surgery, with free access to food and water. Catheterized feeding was performed if necessary. After vital signs stabilized, the 5-point Zea-Longa’s (Longa et al., 1989) criteria were used for evaluation: 1-3 points indicated successful model establishment, and the corresponding rats were included in the study; while rats that died after surgery scored 0 or 4 points, or were found with evident subarachnoid hemorrhage and were excluded from the study.
Treatment
The acupoint combination ofChize,Hegu,Zusanli, andSanyinjiaoon the affected side (right side) was selected, according to methods of acupoint selection and acupuncture described inExperimental Acupunctureedited by Li (2003). The detailed locations and acupuncture method of the acupoints were as follows:Heguwas located between the first and second metacarpals of the forelimb, and acupunctured straight for 1 mm;Chizewas located at a depression on the lateral side of the cubital crease of the forelimb, and was acupunctured straight for 3 mm;Zusanliwas located on the posterolateral side of the knee joint of the hind limb, about 5 mm lower from the capitulum of the fibula, and acupunctured straight for 7 mm; andSanyinjiaowas located 10 mm above the prominence of the lateral malleolus of the hind limb, and acupunctured straight for 4 mm. Once vital signs were stabilized after model induction, treatments were administered. In the sham and I/R groups, the rats were bound to the plate for 20 minutes, once per day, and no other treatments were administered.
In the EA group, the rats received the first acupuncture at 1.5 hours after I/R model establishment. Each subgroup received treatment once per day. The rats were bound to the plate. The four acupoints were stimulated using an electroacupuncture apparatus (Tianxie Acupuncture Instruments Co., Ltd., Suzhou, China), and the needles were retained in the body for 20 minutes. In the EA group, a dilatational wave with the frequency of 2 Hz/50 Hz was applied for acupoints on the right side (affected side, until the surrounding tissues shook slightly) for 20 minutes.
In the SB group, the p38 MAPK blocker SB20358 (Sigma, St. Louis, MO, USA) was injected into the lateral ventricles 30 minutes before I/R model establishment. All rats received only one injection, and rats in each subgroup were sacrificed at 2 hours, 6 hours, 1 day, 3 days, and 1 week after reperfusion; the same procedures were performed as in the I/R group. In the SB group, the skin of the head was anesthetized and incised, and the skull was exposed and maintained in a horizontal position. H2O2was applied to the anterior fontanel, and then a microsyringe was used to inject 10 μL of SB203580 (concentration 100 μM, dissolved in dimethyl sulfoxide; Sigma) (Shi et al., 2012) into the left lateral ventricle (1.0 mm posterior and 1.5 mm lateral to the anterior fontanel) (Yang et al., 2011).
In the SB + EA group, the p38 MAPK blocker SB20358 was injected 30 minutes before model establishment similar to the SB group; the same procedures were performed as in the EA group. Each subgroup received acupuncture once per day; the 2-hour, 6-hour, and 1-day subgroups received only one treatment.
Specimen extraction
After the rats were sacrificed, brain tissues were sliced into 1-4-mm coronary sections behind the optic chiasma and were subsequently fixed in 4% paraformaldehyde at 4°C. The sections were then dehydrated in a dehydration box. Melted paraffin was poured into the embedding box, followed by the brain sections. When the paraffin was completely hardened, the paraffin block was removed from the embedding box and stored at 4°C. For slicing, the paraffin block was placed on a paraffin slicing machine and sectioned from the front to the back to make 4-μm-thick coronal sections. The slices, which floated on the surface, were flattened and lifted onto the slides. The sections were then placed in a 60-°C incubator overnight.
Neurological behavior scores
The 5-point Zea-Longa’s method was used to assess neurological impairment (Longa et al., 1989). Rats in all groups underwent the first treatment at 1.5 hours after I/R model establishment. Rats in the 2-hour, 6-hour, and 1-day subgroups were sacrificed at 2 hours, 6 hours, and 1 day after reperfusion, and only received one treatment; the 3-day and 1-week subgroups received treatment once per day. All rats were sacrificed according to the time points after reperfusion (2 hours, 6 hours, 1 day, 3 days, and 1 week). Neurological impairment scores were evaluated prior to sacrifice. “0” indicated no neurological impairment; “1” indicated that the rat could not fully extend the contralateral forelimb; “2” indicated that the rat circled to the contralateral side; “3” indicated that the rat fell to the contralateral side; and “4” indicated that the rat could not spontaneously walk, and lost consciousness.
Hematoxylin and eosin staining
The sections were baked for 2 hours and then dewaxed twicein dimethylbenzene for 20 minutes each time. The sections were exposed to a gradient ethanol dehydration, and then hematoxylin (Yuanmu Biotechnology Co., Ltd., Shanghai, China) staining was performed for 5 minutes after the sections were washed in distilled water. The sections were then washed, immersed in 1% hydrochloric acid-alcohol solution, washed in water, treated with 0.6% ammonium hydroxide, washed again, and then stained with eosin (Huihong Reagent Co., Ltd., Hunan, China) for 1-3 minutes. Following gradient ethanol dehydration, the sections were dehydrated and cleared in dimethylbenzene. Gradient ethanol dehydration was performed again, and the sections were mounted in neutral balsam. Subsequently, morphological changes in the cerebral tissues were observed under a light microscope and photographed using a BX-70 microscopic imaging system (Olympus, Tokyo, Japan).
Detecting apoptosis using terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick-end labeling (TUNEL) assay
The rats were sacrificed according to time points after reperfusion (2 hours, 6 hours, 1 day, 3 days, and 1 week). The apoptotic index was measured using TUNEL assay after sacrifice. Paraffin sections from the hippocampus were obtained, routinely dewaxed, dehydrated, and hydrated. Proteinase K working solution was added. The sections were incubated at 37°C for 30 minutes and washed in phosphate-buffered saline (PBS) (Ruigu Biotechnology Co., Ltd., Shanghai, China) solution three times (5 minutes each). Membrane lysis solution was added, and the sections were incubated at room temperature for 20 minutes and washed in PBS three times (5 minutes each). Reagent 1 (TdT) and 2 (dUTP) from the TUNEL kit (Kaiji Biotechnology Co., Ltd., Nanjing, China) were obtained, mixed at a ratio of 1:9, added to cover the tissues, and then incubated in a 37°C water bath (Kaiwei Biotechnology Co., Ltd., Guangzhou, China) for 60 minutes. The slices were washed in PBS three times (5 minutes each), placed in 3% H2O2(Jielong Chemical Industry Co., Ltd., Guangzhou, China) solution (prepared with methyl alcohol), and incubated in the dark for 20 minutes. The sections were washed in PBS three times (5 minutes each) again. An appropriate volume of reagent 3 (converter POD) was added to cover the tissues. The sections were then incubated in a 37°C water bath for 30 minutes and washed in PBS three times (5 minutes each). Counterstaining with 4,6-diamidino-2-phenylindole dihydrochloride was performed, and then the slices were washed, dehydrated, cleared, and mounted in neutral balsam. Morphological changes in the cerebral tissues were observed under a microscope (Jiangnan Optical Instrument Group, Nanjing, China), and the images were collected for analysis (Leica Microsystem Co., Ltd., Shanghai, China). For each sample, two sections were randomly selected, and four CA1 areas from the hippocampus were chosen from each section for analysis. The numbers of apoptotic cells and total cells were quantified in each visual field, and the mean numbers were calculated (apoptotic index = number of apoptotic cells/ number of total cells × 100%).
Immunohistochemical assay
All rats were sacrificed according to time points after reperfusion (2 hours, 6 hours, 1 day, 3 days, and 1 week). After the last neurological impairment score evaluation, expression of phosphorylated p38 MAPK was assessed by immunohistochemical assay after sacrifice. Paraffin sections from the hippocampus were dewaxed in dimethylbenzene (Fanhong Trade Co., Ltd., Guangzhou, China) twice (20 minutes each), dehydrated in gradient ethanol, washed in distilled water, and heated to boiling in ethylenediaminetetraacetic acid antigen retrieval buffer (2.5 minutes). The sections were cooled, washed in PBS three times (5 minutes each), and then incubated in 3% H2O2at room temperature for 20 minutes in the dark and washed again in PBS three times (5 minutes each). The primary p-p38 specific-antibody was diluted to 1:150 (Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing, China), added to the sections, and incubated at 4°C overnight, followed by three PBS washes (5 minutes each). Afterwards, secondary antibody rabbit anti-goat horseradish peroxidase (Zhongshan Golden Bridge Biotechnology Co., Ltd.) was added. The sections were incubated at 37°C for 30 minutes and washed in PBS three times (5 minutes each), then 3,3′-diaminobenzidine was added, and the sections were washed in water 5 minutes later, counterstained with hematoxylin for 3 minutes, washed, treated with 1% hydrochloric acid-alcohol solution for few seconds, washed again, treated with ammonia for few seconds, washed in running water, dehydrated and cleared with gradient ethanol, and mounted in neutral balsam. The images were obtained under a microscope (Jiangnan Optical Instrument Group, Nanjing, China) and processed using IPP analysis software (Media Cybernetics, Inc., Bethesda, MD, USA). Gray scale values were used to measure phosphorylated p38 positive cells: a higher gray scale value indicated a lower number of phosphorylated p38 MAPK-positive cells, and vice versa.
Statistical analysis
SPSS 20.0 software (IBM, Armonk, NY, USA) was used for statistical analysis. Quantitative data are expressed as the mean ± SD. The normality test and homogeneity test of variances were initially performed. One-way analysis of variance and least-significant difference test was used to compare data. Statistical significance was set toα= 0.05.
Results
EA reduced neurological impairment scores of rats with I/R injuries
Neurological impairment scores were highest at 2 hours after successful I/R model establishment in each group. Neurological impairment also gradually improved with time of I/R. Compared with the I/R group, neurological impairment scores were lower in the EA group and were significantly reduced after 3 days of continuous treatment (P< 0.05). The neurological impairment scores in the SB and SB + EA groups were better than in the I/R group, and the effects were alsosignificantly better after 3 days of continuous treatment (P<0.05). No significant differences were found between the EA, SB, and SB + EA groups (Table 1).
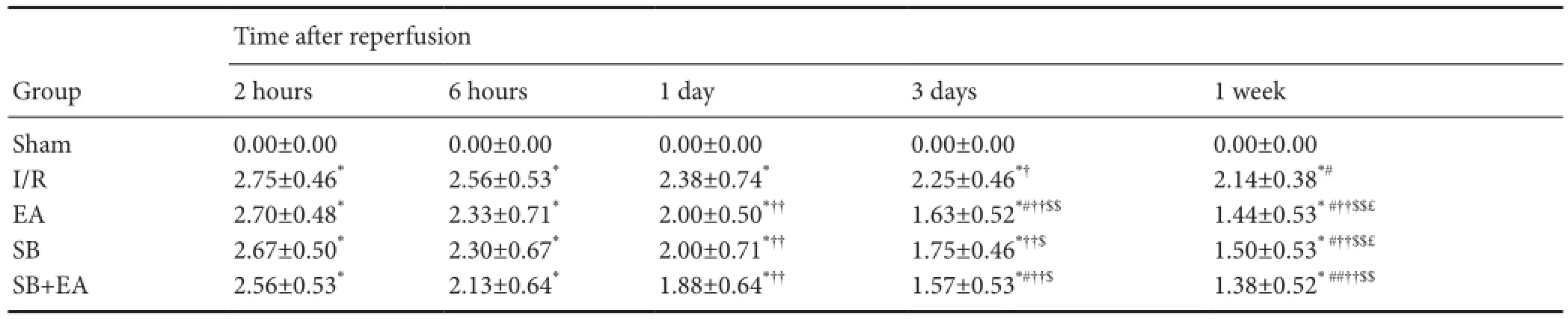
Table 1 Neurological impairment scores of rats at different time points
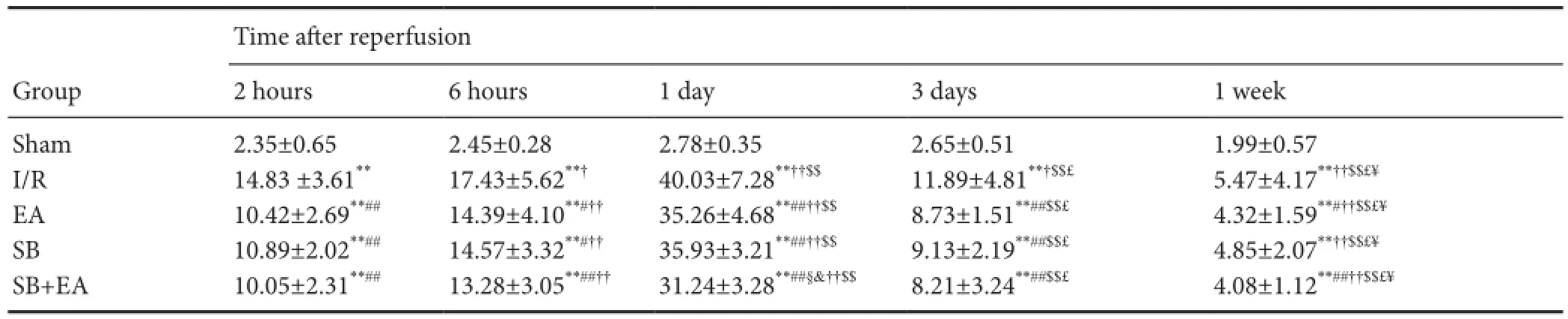
Table 2 Apoptosis rates of (%) neural cells in ischemic areas in rats of each group

Table 3 Gray scale value of phosphorylated p38 MAPK-positive cells in the hippocampal CA1 area in different groups
EA improved morphological changes in cells in the CA1 area of the hippocampus
In the sham group, microscopy showed evenly stained brain tissues in the CA1 area. Cell morphologies were complete, and structures were clearly displayed. The cell nuclei were blue and round or oval; the nuclear membrane was complete; the nucleolus was clear; and no pathological changes, including evident cell swelling, pyknosis, or neutrophil infiltration, were found. In the I/R group, cell morphologies gradually changed with time of I/R. Some necroticand mildly swollen cells were visible; the intercellular space increased, and the number of neurons decreased. Neurons were also irregularly arranged with an unclear nucleolus and nucleolus pyknosis. Further necrosis of cells was observed at 3 days after reperfusion. The cells were loosely arranged; intercellular edema was noted; the number of viable neurons significantly decreased; the cell nucleus disappeared; and significant infiltration of neutrophils was observed. In the EA group, cell damage at each time point was significantly less severe than damage in the I/R group. At 3 days after model induction, neuronal structures were slightly unclear; cell bodies were moderately swollen; pyknosis and lysis of cell nuclei were observed in some cells; and infiltration of a few neutrophils was seen. The cells were regularly arranged; the structures were complete; and the number of viable neurons was higher in the SB group than in the SB + EA group (Figure 1A).

Figure 1 Apoptosis and phosphorylated p38 MAPK-immunoreactive positive cells in the CA1 area of the hippocampus at 3 days after surgery in all groups (light microscope, × 200).
EA reduced apoptosis in the CA1 area of the hippocampus in rats with I/R injuries
After successful model establishment, the number of TUNEL-immunoreactive cells significantly increased. The positive rate also further increased with time and peaked at 1 day post-reperfusion, after which the apoptotic index rapidly decreased. Compared with the I/R group, positive rates were significantly less than in the EA group at all time points (P< 0.05). The positive rates were significantly less at different time points to different degrees in the EA, SB, and SB + EA groups (P< 0.05). The effects were more pronounced at 1 day after reperfusion, and the number of apoptotic cells was significantly less in all groups at 3 days after reperfusion. When p38 MAPK blocker and EA were applied in combination, the apoptotic index was more significantly decreased compared with p38 MAPK blocker alone, and the difference was statistically significant at 1 day post-reperfusion (P<0.05;Table 2andFigure 1B).
EA inhibited expression of phosphorylated p38 MAPK in the CA1 area of the hippocampus
After successful model establishment, expression of phosphorylated p38-immunoreactive cells in each group gradually increased with time of reperfusion, which peaked at 3 days, and then began to decrease again. However, the number of phosphorylated p38-immunoreactive cells at 1 week after reperfusion was higher than in the sham group. Compared with the I/R group, the positive rate in the treatment groups at each time point decreased. At 3 days after reperfusion, the decrease in phosphorylated p38 expression was statistically significant in the EA group (P< 0.05), and the decrease in the positive rate was also statistically significant in the SB + EA group (P< 0.01). However, at the same time point, there was no significant difference in the SB group compared with the I/R group. The decreases in phosphorylated p38 expression were statistically significant at 6 hours, 1 day, and 1 week between the I/R group and the SB + EA group (P< 0.05). These results showed that application of EA inhibited phosphorylated p38 expression, and the combination of acupuncture with p38 MAPK blocker effectively inhibited phosphorylated p38 expression at different time points. Tree days of continuous acupuncture resulted in themost beneficial effects (Table 3andFigure 1C).
Discussion
To verify the hypothesis that EA initiates p38 MAPK signal regulation, thereby inhibiting apoptosis, rat models of middle cerebral artery occlusion were established and the effects of EA acupoint combination ofChize,Hegu,Zusanli, andSanyinjiaoon cerebral I/R injuries were analyzed at 2 hours, 6 hours, 1 day, 3 days, and 1 week. TUNEL assay was used to evaluate the effects of EA on cell apoptosis in rats with I/R injuries. Immunohistochemistry was used to assess phosphorylation of p38 MAPK in the infarction area, suggesting that electroacupuncture at the four acupoints could inhibit expression of phosphorylated p38 MAPK following cerebral I/R injuries. We also attempted to clarify the influence of EA on cell apoptosis and p38 MAPK signaling pathway expression to provide experimental evidence for treating ischemic stroke with EA.
Neurological impairment scores indirectly reflect the severity of brain injuries, and are was considered a reliable indicator for evaluating I/R injuries. Results from this study suggested that EA atChize,Hegu,Zusanli, andSanyinjiaoeffectively improved neurological impairment symptoms in rats with cerebral I/R injuries, and the effects were most evident after treatment for three consecutive days. The tolerance to ischemia and anoxia varies among different cerebral regions. For instance, the hippocampus, corpus striatum, and cortical neurons are the most sensitive to ischemia and anoxia, and delayed neuronal death can occur in these regions from several hours to several days after reperfusion (Abe et al., 1995). In this study, the decrease in apoptotic index was more significant when p38 MAPK blocker and EA were applied in combination, with a significant difference at 1 day, suggesting that EA atChize,Hegu,Zusanli, andSanyinjiaoprovided protective effects against cerebral ischemia.
Cell apoptosis has been shown to occur in the hippocampal CA1 area, with high expression of phosphorylated p38, 3 days after focal I/R injuries (Takagi et al., 2000). p38 MAPK activation induces neuronal death in the hippocampal CA1 area in rats with I/R injury, while pretreatment with a p38 MAPK blocker protects against neuronal damage (Seger and Krebs, 1995). In this study, immunohistochemical analyses showed that EA atChize,Hegu,Zusanli, andSanyinjiaoinhibited expression of phosphorylated p38 MAPK, indicating that the protective effects of EA on cerebral ischemic injuries took placeviainhibition of the p38 MAPK signaling pathway.
We included subgroups, according to different time points, to observe apoptotic changes following EA treatment. After treatment, apoptosis and phosphorylated p38 expression decreased according to the different time points in the EA group, after which the levels were stable. These findings suggested that EA intervention reduced I/R injury during the acute phase. However, EA intervention was used once per day. The findings from this study showed lower neurological impairment scores in the treatment groups than in the I/R group, with statistically significant differences at 3 days. Morphological changes in cells from the hippocampal CA1 area were less severe at 3 days after reperfusion in the EA group than in the I/R group. However, in the SB + EA group, the cells were more regularly arranged, the structures were integrated, and the number of viable cells was higher compared with the SB group. The number of apoptotic cells was significantly reduced in all groups at 3 days after reperfusion, at which time self-repair functions were initiated (Takagi et al., 2000). Additionally, EA and/or SB effectively inhibited phosphorylated p38 expression, and these effects were most notable at 3 days after reperfusion. These findings showed that continuous EA intervention for at least 3 days resulted in better treatment of I/R injury, suggested that 3 days could be a good time window to promote repair of cerebral ischemic damage.
In summary, combined EA atChize,Hegu,Zusanli, andSanyinjiaosignificantly improved neurological impairment symptoms and morphological changes in brain cells, reduced the apoptotic index in the ischemic area, and provided protective effects against ischemic injuries in cerebral tissues in rats with cerebral I/R injuries. This treatment also inhibited expression of phosphorylated p38 MAPK, suggesting that the protective effects could be exerted through the p38 MAPK signaling pathway. The protective effects on neurological impairment, pathological morphological changes in brain cells, apoptotic index in ischemic areas, and improvement in phosphorylated p38 MAPK expression were more pronounced at 3 days after reperfusion, indicating that 3 days could be a good time window to promote repair of ischemic cerebral neurons. The hypothesis that “EA initiates p38 MAPK signal regulation, and thus inhibits cell apoptosis” could be one of the possible mechanisms involved in the effects of EA on ischemic cerebral injuries.
Acknowledgments:The authors thank all the researchers involved in animal experiments, and are grateful to all staffs from Experimental Animal Center of Southern Medical University in China.
Author contributions:GPZ obtained funding. GPZ, XL and XZ provided conception. XL, XZ and CXW designed the study. CL and XHX purchased animals and materials. CL and XL analyzed data. XZ and XL wrote the paper. CXW performed the acupuncture. XHX and CXW ensured the integrity of the data. All authors approved the final version of the paper.
Conflicts of interest:None declared.
Plagiarism check:This paper was screened twice using CrossCheck to verify originality before publication.
Peer review:This paper was double-blinded and stringently reviewed by international expert reviewers.
Abe K, Aoki M, Kawagoe J, Yoshida T, Hattori A, Kogure K, Itoyama Y (1995) Ischemic delayed neuronal death. A mitochondrial hypothesis. Stroke 26:1478-1489.
Brewster JL, de Valoir T, Dwyer ND, Winter E, Gustin MC (1993) An osmosensing signal transduction pathway in yeast. Science 259:1760-1763.
Cao G, Minami M, Pei W, Yan C, Chen D, O’Horo C, Graham SH, Chen J (2001) Intracellular Bax translocation after transient cerebral ischemia: implications for a role of the mitochondrial apoptotic signaling pathway in ischemic neuronal death. J Cereb Blood Flow Metab 21:321-333.
Chen Y, Zhang L, Ni J, Wang X, Cheng J, Li Y, Zhen X, Cao T, Jia J (2016) LLDT-8 protects against cerebral ischemia/reperfusion injury by suppressing post-stroke inflammation. J Pharmacol Sci 131:131-137.
Cheng LF (2016) Bone marrow mesenchymal stem cell transplantation for rat cerebral infarction: recovery of neurological function and expression of synaptophysin. Zhongguo Zuzhi Gongcheng Yanjiu 20:4182-4188.
Dhanasekaran DN, Reddy EP (2008) JNK signaling in apoptosis. Oncogene 27:6245-6251.
Feng YH, Zhu ZH, Wu CX, Zhou GP (2016) Effects of electroacupuncture at points selected by orthogonal experiment on the extracellular signal regulated kinase signal pathway in a rat model of cerebral ischenia-reperfusion injury. Zhongguo Zuzhi Gongcheng Yanjiu 20:5953-5958.
Gao J, Duan B, Wang DG, Deng XH, Zhang GY, Xu L, Xu TL (2005) Coupling between NMDA receptor and acid-sensing ion channel contributes to ischemic neuronal death. Neuron 48:635-646.
Huang L, Liu LF, Liu J, Dou L, Wang GY, Liu XQ, Yuan QL (2016) Ginsenoside Rg1 protects against neurodegeneration by inducing neurite outgrowth in cultured hippocampal neurons. Neural Regen Res 11:319-325.
Kim BJ, Ryu SW, Song BJ (2006) JNK- and p38 kinase-mediated phosphorylation of Bax leads to its activation and mitochondrial translocation and to apoptosis of human hepatoma HepG2 cells. J Biol Chem 281:21256-21265.
Kumaran D, Udayabanu M, Nair RU, RA, Katyal A (2008) Benzamide protects delayed neuronal death and behavioural impairment in a mouse model of global cerebral ischemia. Behav Brain Res 192:178-184.
Li ZR (2003) Experimental acupuncture science. Beijing: The Medicine Science and Technology Press of China: 425-431.
Longa EZ, Weinstein PR, Carlson S, Cummins R (1989) Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 20:84-91.
Ma XL, Kumar S, Gao F, Louden CS, Lopez BL, Christopher TA, Wang C, Lee JC, Feuerstein GZ, Yue TL (1999) Inhibition of p38 mitogen-activated protein kinase decreases cardiomyocyte apoptosis and improves cardiac function after myocardial ischemia and reperfusion. Circulation 99:1685-1691.
Matsuda T, Arakawa N, Takuma K, Kishida Y, Kawasaki Y, Sakaue M, Takahashi K, Takahashi T, Suzuki T, Ota T, Hamano-Takahashi A, Onishi M, Tanaka Y, Kameo K, Baba A (2001) SEA0400, a novel and selective inhibitor of the Na+-Ca2+exchanger, attenuates reperfusion injury in the in vitro and in vivo cerebral ischemic models. J Pharmacol Exp Ter 298:249-256.
Moskowitz MA, Lo EH, Iadecola C (2010) The science of stroke: mechanisms in search of treatments. Neuron 67:181-198.
Nogawa S, Zhang F, Ross ME, Iadecola C (1997) Cyclo-oxygenase-2 gene expression in neurons contributes to ischemic brain damage. J Neurosci 17:2746-2755.
Pundik S, Xu K, Sundararajan S (2012) Reperfusion brain injury: focus on cellular bioenergetics. Neurology 79:S44-51.
Schmidt-Kastner R, Fliss H, Hakim AM (1997) Subtle neuronal death in striatum after short forebrain ischemia in rats detected by in situ end-labeling for DNA damage. Stroke 28:163-170.
Seger R, Krebs EG (1995) The MAPK signaling cascade. FASEB J 9:726-735.
Shi QJ, Xiao L, Zhao B, Zhang XY, Wang XR, Xu DM, Yu SY, Fang SH, Lu YB, Zhang WP, Sa XY, Wei EQ (2012) Intracerebroventricular injection of HAMI 3379, a selective cysteinyl leukotriene receptor 2 antagonist, protects against acute brain injury after focal cerebral ischemia in rats. Brain Res 1484:57-67.
Takagi Y, Nozaki K, Sugino T, Hattori I, Hashimoto N (2000) Phosphorylation of c-Jun NH(2)-terminal kinase and p38 mitogen-activated protein kinase after transient forebrain ischemia in mice. Neurosci Lett 294:117-120.
Wang ZF, Tang LL, Yan H, Wang YJ, Tang XC (2006) Effects of huperzine A on memory deficits and neurotrophic factors production after transient cerebral ischemia and reperfusion in mice. Pharmacol Biochem Behav 83:603-611.
Wang ZK, Liu FF, Wang Y, Jiang XM, Yu XF (2016a) Let-7a gene knockdown protects against cerebral ischemia/reperfusion injury. Neural Regen Res 11:262-269.
Wang Y, Shen Y, Lin HP, Li Z, Chen YY, Wang S (2016b) Large-conductance Ca(2+)-activated K (+) channel involvement in suppression of cerebral ischemia/reperfusion injury after electroacupuncture at Shuigou (GV26) acupoint in rats. Neural Regen Res 11:957-962.
Wei J, Quast MJ (1998) Effect of nitric oxide synthase inhibitor on a hyperglycemic rat model of reversible focal ischemia: detection of excitatory amino acids release and hydroxyl radical formation. Brain Res 791:146-156.
Xia Y, Ding GH, Wu GC (2012) Current Research in Acupuncture. Springer Press, NY, USA.
Yang Y, Bai X, Dong H, Lu Y, Xiong L (2011) Intracerebroventricular injection of human prostatic acid phosphatase has potent neuroprotective effects against transient focal cerebral ischemia in rats. Neurosci Lett 504:321-324.
Yang ZX, Xie JH, Liu YP, Miao GX, Wang YH, Wu SM, Li Y (2015) Systematic review of long-term Xingnao Kaiqiao needling effcacy in ischemic stroke treatment. Neural Regen Res 10:583-588.
Yates HL, McCullough S, Harrison C, Gill AB (2012) Hypoxic ischaemic encephalopathy: accuracy of the reported incidence. Arch Dis Child Fetal Neonatal Ed 97:F77-78.
Zhang JZ, Jing L, Ma AL, Wang F, Yu X, Wang YL (2006) Hyperglycemia increased brain ischemia injury through extracellular signal-regulated protein Kinase. Pathol Res Pract 202:31-36.
Copyedited by Cooper C, Yajima W, Wang J, Li CH, Qiu Y, Song LP, Zhao M
*Correspondence to: Guo-ping Zhou, M.D., doctorzgp@sina.com.
#These authors contributed equally to this study.
orcid: 0000-0002-4613-4384 (Guo-ping Zhou)
10.4103/1673-5374.202944
Accepted: 2016-12-25
杂志排行
中国神经再生研究(英文版)的其它文章
- Telomerase and mTOR in the brain: the mitochondria connection
- Impacts of the retinal environment and photoreceptor type on functional regeneration
- Tissue-type plasminogen activator is a homeostatic regulator of synaptic function in the central nervous system
- Novel insights into the role of NF-κB p50 in astrocytemediated fate specification of adult neural progenitor cells
- Anesthetic considerations for patients with acute cervical spinal cord injury
- Neuromelanin, one of the most overlooked molecules in modern medicine, is not a spectator
