JAK2-STAT3信号通路在肝细胞癌中的研究进展
2017-03-29廖明媚王成志杨满意潘一峰综述赵劲风审校
廖明媚,王成志,杨满意,潘一峰 综述 赵劲风 审校
(中南大学湘雅医院 卫生部纳米生物技术重点实验室,湖南 长沙 410008)
肝癌是世界排名第五的恶性肿瘤,也是第三大致死率肿瘤,同时还是中国第二大导致死亡的癌症。肝细胞癌(hepatocellular carcinoma,HCC)是最常见的一种肝癌,肿瘤的发生发展与原癌基因的过度表达和抑癌基因的沉默相关,在多种恶性肿瘤中,JAK2-STAT3持续性激活,使下游原癌基因过度表达。
Janus激酶(JAK)-信号转导子与转录激活子(STAT)信号通路早在90年代就被发现了[1],该通路能快速将膜外信号转导进入细胞核内,调控下游相关基因survivin、c-Myc、JunB、clAP2、cyclin D1、MMP-2、ICAM-1、VEGF和Bcl-2基因家族的表达。随着分子生物学的发展,JAK2-STAT3通路成为研究热点,大量研究表明JAK2-STAT3通路的持续性激活与肿瘤的发生发展密切相关,JAK2-STAT3通路的持续性激活也与肿瘤的耐药相关。索拉非尼作为分子靶向药物现已经用于晚期HCC的治疗,可以改善晚期HCC患者的生存期,针对JAK2-STAT3和其下游基因设计小分子药物,可以精确的靶向肿瘤细胞,减少对正常细胞的毒副作用。本文就JAK2-STAT3在HCC中的研究做一综述,为HCC的靶向治疗提供新思路。
1 JAK2-STAT3信号通路的组成与调控
1.1 JAK蛋白酪氨酸激酶
JAK家族现在已经发现4个成员,包括JAK1、JAK2、JAK3、TYK2。它们的蛋白质都超过1 000个氨基酸,分子量在120~140 kDa左右,都有着相似的同源结构域,包括FERM结构域、SH2结构域、JAK假激酶区和蛋白质酪氨酸激酶(PTK)结构域[2]。JAK1、JAK2、TYK2几乎在所有细胞中表达,而JAK3则主要在造血细胞中表达[3]。在急性骨髓性白血病中,p-JAK2过表达,与总体生存率相关,AZ960能抑制JAK2磷酸化,有一定疗效[4]。在骨髓增生性疾病中,JAK2在V617F位点发生突变,G6[5-6]、AG490能选择性抑制JAK2[7],Ruxolitinib、Fedratinib[8]、BMS-911543[9]等能选择性抑制JAK2,对骨髓瘤都有一定的疗效。TG101348也能抑制JAK2,能克服非小细胞肺癌对埃罗替尼耐药[10]。miR-135a抑制JAK2蛋白的表达[11],阻止下游STAT3的磷酸化。
1.2 STATs家族
STAT信号转导子和转录激活子是JAKs的下游底物,它能将信号带到细胞核,调节基因的表达。STATs家族有7个成员STAT1、STAT2、STAT3、STAT4、STAT5a、STAT5b、STAT6。STAT蛋白质约800个氨基酸,分子量在89~97 kDa左右。根据功能STATs蛋白家族可分为两类[12],STAT2,STAT4,STAT6这一类主要被一小部分细胞因子激活,在IFN信号和T细胞的发育中起作用。另一类由STAT1、STAT3、STAT5组成,它们由一系列配体激活,在不同的组织中均发挥作用,如乳腺的发育,生长激素的反应,胚胎发育等等。STATs家族有着相似的6个同源结构域,从氨基酸到羧基端依次为:氨基末端结构域、coiled-coiled结构域(CCD)、DNA结合结构域(DBD)、linker结构域、SH2结构域、羧基末端转录活化结构域(TAD)。STAT3可发生赖氨酸乙酰化[13](Lys685),发生丝氨酸(Ser727)磷酸化[14],还可以发生酪氨酸磷酸化(Tyr705),在Tyr705磷酸化促进其核转移,提高转录活性。SHP-1是SH2同源结构域酪氨酸磷酸酶,可以抑制STAT3(T705)磷酸化,阻止STAT3的激活[15]。葫芦素(JSI-124)[16]、LLL12[17]、SC-2001[18]、Niclosamide等能抑制STAT3,抑制下游基因的表达 。 MiR-125a[19], MiR-143[20]靶 向 STAT3 mRNA的3'-UTR区抑制STAT3的蛋白表达水平,能抑制肿瘤细胞的增殖、侵袭与转移。
1.3 JAK2-STAT3信号通路的过程及调控
细胞外信号或细胞因子通过特异性与细胞膜上受体结合使受体构像变化,使JAK向膜受体移动,JAK发生酪氨酸磷酸化而被激活,JAK招募STATs,使STATs磷酸化,形成同源或异源化磷酸二聚体,磷酸化的二聚体迅速进入细胞核调控基因表达或与核内的其他转录因子相互作用调控基因表达。
JAK2-STAT3信号通路的调节有多种机制共同参与,通常发生在JAK激活阶段、STAT进人细胞核过程以及负反馈途径等多个环节进行。STAT的翻译后的修饰极大的影响了其转录活性,当配体和受体相结合并使其活化后,其邻近的受体可发生交互磷酸化或下调非特异抑制因子的作用,而使通路得以加强。JAK2-STAT3信号通路还被细胞因子信号抑制物(SOCSs)、含SH2的磷酸酶(SHPs)以及STATs蛋白抑制子(PIASs)3个蛋白家族迅速终止。SOCSs通过结合并抑制JAKs或与STAT竞争细胞因子受体的磷酸结合位点而终止JAK2-STAT3信号转导,SHPs通过抑制STAT3的磷酸化而阻断STAT3进入细胞核。高水平的cAMP能抑制JAK1-STAT3信号通路[21]。多种抑制剂可以抑制JAK2-STAT3信号通路的进程,调控基因的表达,调节细胞生长,增殖,分化等。
2 JAK2-STAT3信号通路与HCC
JAK2-STAT3信号通路是细胞内的一条重要信号通路,与细胞的生长、分化、增殖、凋亡有密切关系,也与很多疾病的发生发展密切相关。有研究[22]表明JAK蛋白和STAT蛋白在HCC细胞中过表达,JAK1蛋白和STAT3蛋白与HCC患者的预后相关,预示着它可能与HCC的发生发展有关。LLL12能抑制STAT3的T705位点磷酸化,阻止其核转移,抑制抗凋亡蛋白的表达,引起HCC细胞的凋亡[17]。B7-H3(免疫球蛋白)能促进JAK2和STAT3的磷酸化,能促进HCC细胞转移的侵袭[23],而对细胞的生长和增殖无影响。Ruxolitinib(INCB018424)是JAK2(V617F突变)抑制剂,初步临床效果表明对骨髓瘤有较好的疗效[24-25],目前被FDA批准用于治疗该病的晚期治疗。ruxolitinib对JAK有非常高的特异性,而对非JAK信号不敏感,在体外HCC实验[26]中,ruxolitinib有效抑制JAK,显著减少其下游产物p-STAT1和p-STAT3,显著减少HCC的增殖和克隆形成。ruxolitinib还能减轻CCL4引起的肝损伤和坏死性炎症[27]。Ruxolitinib特异性的抑制JAK1、JAK2酪氨酸磷酸化,可能成为靶向治疗HCC有较药物。
在HCC中STAT3主要在SH2结构域发生突变[28],在T705位点磷酸化,影响着STAT3的二聚化作用和向核转移运动。索拉非尼能抑制STAT3在T705位点和S727位点磷酸化而抑制STAT3的活性[29],而在索拉非尼耐药的HCC细胞中,p-STAT3和p-JAK1和p-JAK2显著高表达,SC-2001能增强SHP-1的活性,抑制STAT3的磷酸化,SC-2001与索拉菲尼联合治疗能显著抑制对索拉菲尼耐药的HCC细胞的克隆形成,抑制肿瘤的生长[18],索拉菲尼与mapatumumab,lexatumumab(TRAIL受体激动剂)联合治疗抑制JAK2-STAT3通路,下调mcl-1表达,在体内外均能引起HCC细胞其他实体瘤细胞凋亡[30]。NSC74859(S3I-201)能抑制p-STAT3,单独作用对HCC细胞的活性影响不大,但它与cetuximab(表皮生长因子受体抑制剂)联合治疗对HCC细胞的治疗效果比cetuximab单独作用效果更显著[31]。
乙型肝炎病毒(HBV)和丙型肝炎病毒(HCV)的感染是HCC发生发展的一个重要因素[32]。在HBV与HCV相关的HCC中,STAT3与己糖激酶II高表达,且STAT3与己糖激酶II的表达具有相关性[33]。HBV可以激活STAT3信号通路[34],促进HBV病毒的复制,阻断STAT3信号可以抑制HBV阳性的HCC细胞的生长[35],抑制STAT3可以减少HBV的表达[36]。活化的STAT3上调LncRNA(长链非编码RNA Lethe、lncIGF2AS、lnc7SK)的表达[37-38],促进HCV的复制。Stat3调整微管动力学影响HCV的复制,抑制STAT3可以抑制HCV的复制[39]。HCV核心蛋白通过STAT3通路调节NANOG的表达,促进细胞生长和细胞周期进程[40]。
瘦素(leptin)通过JAK2-STAT3信号通路引起ERK和AKT磷酸化,促进HCC细胞迁移。通过JAK2-STAT3抑制剂或ERK和PI3K抑制剂能阻断瘦素引起的HCC细胞侵袭[41],对肥胖的HCC患者的治疗具有潜在的临床意义。有研究[42]表明CIMO能抑制JAK1、JAK2和STAT3 T705磷酸化,阻碍STAT3向核转移,影响其与DNA结合,下调STAT3下游的靶基因(Bcl-2、Bxl-xL、cyclin D、survivin、ICAM-1、Bid)表达,使癌细胞停留在G1期,抑制HCC细胞的增殖、生存、侵袭与转移,促进细胞凋亡。
SOCS3是信号转导器gp130生理上的抑制剂,能够竞争性的抑制JAK2-STAT3信号通路,有研究[43]表明,相对临近的非肿瘤组织,HCC组织中SOCS3表现出高甲基化,甲基化程度与SOCS3的表达呈负相关,而SOCS3的甲基化预示着JAK2-STAT3信号通路的过度活化。甲基化沉默SOCS3,增强JAK2-STAT3和FAK信号通路活性,因此促进HCC细胞的生长和转移[44],在伊马替尼耐药的细胞中,SOCS3基因甲基化程度升高,SOCS3蛋白表达下调,STAT3过度活化,细胞过度增殖[45]。在HCC细胞中,信号转导器gp130在SOCS3连接位点(Y186/Y759F)上发生双突变[46],通过特异性激活JAK1使STAT3过度活化。使用Ras和JAK2-STAT3抑制剂或SOCS3去甲基化药物zebularine引起HCC细胞的强烈凋亡[47]。miRNA-155能下调SOCS1的表达[48],使STAT3过度活化。
全基因组测序[49-50]显示,在HCC中,Wnt/β-catenin和JAK2-STAT3信号通路是两个主要的致癌通路。许多植物化学物质[51](白藜芦醇、葫芦素、姜黄色素等)对JAK2-STAT3信号通路表现出较好的抑制性,从而抑制肿瘤的发生和发展。
JAK2-STAT3信号通路在HCC的发生发展中起重要作用,明确JAK2-STAT3的上游物质和下游底物,找到其激活剂或抑制剂,从而调节JAK2-STAT3信号通路和它的底物,从而调控基因表达,靶向治疗HCC。
3 JAK2-STAT3信号通路和其他信号通路的联系
细胞内的信号通路是极其复杂的,纵横交错(图1)。细胞内的信号通路不是单独作用,而是与其他信号通路共同协调,调控细胞的各种生命活动。细胞内各信号通路协调作用与肿瘤的发生发展密切相关。Wnt/beta-catenin和JAK2-STAT3信号通路在HCC发生突变最高[49-50],可能是主要的致癌通路。活化的JAK1也可以激活PI3K信号通路,PI3K信号通路也可以作用于STAT3,调控基因表达。在许多疾病中PI3K信号通路和JAK2-STAT3信号通路都存在相互协调作用[52-54]。STATs还可以直接被受体酪氨酸激酶(RTK)激活,如表皮生长因子受体(EGFR)可以通过Src而使STAT3/5酪氨酸磷酸化[55]。环腺苷酸(cAMP)高水平表达能够抑制JAK2-STAT3信号通路,从而在转录水平上下调MCL-1的表达[21],可导致多发性骨髓瘤细胞凋亡。细胞内的信号通路不是单独作用,细胞内的信号通路如一张纵横交错的网,它们相互联系,相互影响,形成复杂的网络体系,控制着细胞的各种生命活动。
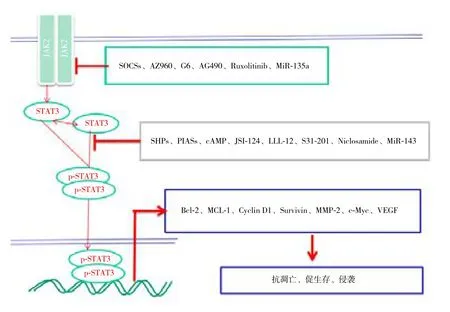
图1 JAK2-STAT3信号通路与调控Figure 1 JAK2-STAT3 signaling pathway and its regulation
4 展 望
晚期HCC的手术治疗并无太大收益,分子靶向治疗将是未来治疗HCC的主要手段,而JAK2-STAT3信号通路在HCC的发生发展中扮演者重要角色,针对JAK2-STAT3信号通路的靶向治疗将具有巨大的应用潜力。明确JAK2-STAT3信号通路的组成及其调控机制,将更有利于理解HCC的发病的分子机理。找到JAK2-STAT3信号通路上游及其下游分子,可以找到更多的靶位点调节细胞的生命活动。JAK2-STAT3信号通路持续性激活通过调节下游靶基因包括c-Myc、JunB、clAP2、Mcl-1、survivin、Bcl-2、Cyclin D1、MMP-2、Bcl-xl、ICAM-1、Bid和VEGF(血管内皮生长因子)等的表达[17,42],来促进细胞增殖和生存,抑制凋亡,促进肿瘤侵袭与转移,促进血管生成的。找到IL-6受体、信号转导器gp130、JAK家族、STAT家族、SOCS3、PIAS、CIS及其它相关分子的激活剂或抑制剂,有效的调节JAK2-STAT3信号通路过程,JAK2-STAT3信号通路与Wnt/beta-catenin通路,Ras信号通路,PI3K信号通路,cAMP介导的第二信使通路等也存在着联系。对JAK2-STAT3信号通路的调控可能成为调控细胞生命活动的关键点,可能成为治疗HCC的靶向位点。分子靶向药物联合起来抑制癌细胞的各种存活通路,抑制HCC细胞的增殖和侵袭,促进HCC细胞凋亡,达到有效的靶向治疗HCC。
[1] Darnell JJ, Kerr IM, Stark GR. JAK-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins[J]. Science, 1994, 264(5164):1415–1421.
[2] Cai B, Cai JP, Luo YL, et al. The Specific Roles of JAK/STAT Signaling Pathway in Sepsis[J]. Inflammation, 2015, 38(4):1599–1608. doi: 10.1007/s10753–015-0135-z.
[3] Musso T, Johnston JA, Linnekin D, et al. Regulation of JAK3 expression in human monocytes: phosphorylation in response to interleukins 2, 4, and 7[J]. J Exp Med, 1995, 181(4):1425–1431.
[4] Ikezoe T, Kojima S, Furihata M, et al. Expression of p-JAK2 predicts clinical outcome and is a potential molecular target of acute myelogenous leukemia[J]. Int J Cancer, 2011, 129(10):2512–2521.doi: 10.1002/ijc.25910.
[5] Kirabo A, Park SO, Wamsley HL, et al. The small molecule inhibitor G6 significantly reduces bone marrow fibrosis and the mutant burden in a mouse model of JAK2-mediated myelofibrosis[J]. Am J Pathol, 2012, 181(3):858–865. doi: 10.1016/j.ajpath.2012.05.033.
[6] Kirabo A, Park SO, Majumder A, et al. The JAK2 inhibitor, G6,alleviates JAK2-V617F-mediated myeloproliferative neoplasia by providing significant therapeutic efficacy to the bone marrow[J].Neoplasia, 2011, 13(11):1058–1068.
[7] Yoshikawa H, Matsubara K, Qian GS, et al. SOCS-1, a negative regulator of the JAK/STAT pathway, is silenced by methylation in human hepatocellular carcinoma and shows growth-suppression activity[J]. Nat Genet, 2001, 28(1):29–35.
[8] Debeurme F, Lacout C, Moratal C, et al. JAK2 inhibition has different therapeutic effects according to myeloproliferative neoplasm development in mice[J]. J Cell Mol Med, 2015,19(11):2564–2574. doi: 10.1111/jcmm.12608.
[9] Wan H, Schroeder GM, Hart AC, et al. Discovery of a Highly Selective JAK2 Inhibitor, BMS-911543, for the Treatment of Myeloproliferative Neoplasms[J]. ACS Med Chem Lett, 2015,6(8):850–855. doi: 10.1021/acsmedchemlett.5b00226.
[10] Zhang FQ, Yang WT, Duan SZ, et al. JAK2 inhibitor TG101348 overcomes erlotinib-resistance in non- small cell lung carcinoma cells with mutated EGF receptor[J]. Oncotarget, 2015, 6(16):14329–14343.
[11] Wu H, Huang M, Cao P, et al. MiR-135a targets JAK2 and inhibits gastric cancer cell proliferation[J]. Cancer Biol Ther, 2012,13(5):281–288. doi: 10.4161/cbt.18943.
[12] Subramaniam A, Shanmugam MK, Perumal E, et al. Potential role of signal transducer and activator of transcription (STAT)3 signaling pathway in inflammation, survival, proliferation and invasion of hepatocellular carcinoma[J]. Biochim Biophys Acta, 2013,1835(1):46–60. doi: 10.1016/j.bbcan.2012.10.002.
[13] Hung MH, Tai WT, Shiau CW, et al. Downregulation of signal transducer and activator of transcription 3 by sorafenib: A novel mechanism for hepatocellular carcinoma therapy[J]. World J Gastroenterol, 2014, 20(41):15269–15274. doi: 10.3748/wjg.v20.i41.15269.
[14] Yang E, Henriksen MA, Schaefer O, et al. Dissociation time from DNA determines transcriptional function in a STAT1 linker mutant[J]. J Biol Chem, 2002, 277(16):13455–13462.
[15] Kim DJ, Tremblay ML, Digiovanni J. Protein tyrosine phosphatases,TC-PTP, SHP1, and SHP2, cooperate in rapid dephosphorylation of Stat3 in keratinocytes following UVB irradiation[J]. PLoS One,2010, 5(4):e10290. doi: 10.1371/journal.pone.0010290.
[16] Qi J, Xia G, Huang C R, et al. JSI-124 (Cucurbitacin I) Inhibits Tumor Angiogenesis of Human Breast Cancer Through Reduction of STAT3 Phosphorylation[J]. Am J Chin Med, 2015, 43(2):337–347. doi: 10.1142/S0192415X15500226.
[17] Zuo M, Li C, Lin J, et al. LLL12, a novel small inhibitor targeting STAT3 for hepatocellular carcinoma therapy[J]. Oncotarget, 2015,6(13):10940–10949.
[18] Su JC, Tseng PH, Wu SH1, et al. SC-2001 overcomes STAT3-mediated sorafenib resistance through RFX-1/SHP-1 activation in hepatocellular carcinoma[J]. Neoplasia, 2014, 16(7):595–605. doi:10.1016/j.neo.2014.06.005.
[19] Fan Z, Cui H, Xu X, et al. MiR-125a suppresses tumor growth, invasion and metastasis in cervical cancer by targeting STAT3[J]. Oncotarget, 2015, 6(28):25266–25280. doi: 10.18632/oncotarget.4457.
[20] Liu J, Mao Y, Zhang D, et al. MiR-143 inhibits tumor cell proliferation and invasion by targeting STAT3 in esophageal squamous cell carcinoma[J]. Cancer Letters, 2016, 373(1):97–108.doi: 10.1016/j.canlet.2016.01.023.
[21] Follin-Arbelet V, Torgersen ML, Naderi EH, et al. Death of multiple myeloma cells induced by cAMP-signaling involves downregulation of Mcl-1 via the JAK/STAT pathway[J]. Cancer Lett, 2013, 335(2):323–331. doi: 10.1016/j.canlet.2013.02.042.
[22] 张斌, 钟德玝, 王群伟, 等. JAK/STAT信号通路与肝细胞性肝癌的肿瘤进展和预后的相关性研究[J]. 细胞与分子免疫学杂志,2010, 26(4):368–370.Zhang B, Zhong DW, Wang QW, et al. Study on correlation of JAK/STAT signal pathway with progression and prognosis in hepatocellular carcinoma[J]. Chinese Journal of Cellular and Molecular Immunology, 2010, 26(4):368–370.
[23] Kang F, Wang L, Jia H, et al. B7-H3 promotes aggression and invasion of hepatocellular carcinoma by targeting epithelialto-mesenchymal transition via JAK2/STAT3/Slug signaling pathway[J]. Cancer Cell Int, 2015, 15:45. doi: 10.1186/s12935–015-0195-z.
[24] Quintas-Cardama A, Vaddi K, Liu P, et al. Preclinical characterization of the selective JAK1/2 inhibitor INCB018424:therapeutic implications for the treatment of myeloproliferative neoplasms[J]. Blood, 2010, 115(15):3109–3117. doi: 10.1182/blood-2009–04-214957.
[25] Verstovsek S, Kantarjian H, Mesa RA, et al. Safety and efficacy of INCB018424, a JAK1 and JAK2 inhibitor, in myelofibrosis[J].N Engl J Med, 2010, 363(12):1117–1127. doi: 10.1056/NEJMoa1002028.
[26] Wilson GS, Tian A, Hebbard L, et al. Tumoricidal effects of the JAK inhibitor Ruxolitinib (INC424) on hepatocellular carcinoma in vitro[J]. Cancer Lett, 2013, 341(2):224–230. doi: 10.1016/j.canlet.2013.08.009.
[27] Hazem SH, Shaker ME, Ashamallah SA, et al. The novel Janus kinase inhibitor ruxolitinib confers protection against carbon tetrachloride-induced hepatotoxicity via multiple mechanisms[J].Chem Biol Interact, 2014, 220:116–127. doi: 10.1016/j.cbi.2014.06.017.
[28] Raft MB, Jørgensen EN, Vainer B. Gene mutations in hepatocellular adenomas[J]. Histopathology, 2015, 66(7):910–921. doi: 10.1111/his.12539.
[29] Zhai B, Sun XY. Mechanisms of resistance to sorafenib and the corresponding strategies in hepatocellular carcinoma[J]. World J Hepatol, 2013, 5(7):345–352. doi: 10.4254/wjh.v5.i7.345.
[30] Abdulghani J, Allen JE, Dicker DT, et al. Sorafenib sensitizes solid tumors to Apo2L/TRAIL and Apo2L/TRAIL receptor agonist antibodies by the JAK2-Stat3-Mcl1 axis[J]. PLoS One, 2013,8(9):e75414. doi: 10.1371/journal.pone.0075414.
[31] Chen W, Shen X, Xia X, et al. NSC 74859-mediated inhibition of STAT3 enhances the anti-proliferative activity of cetuximab in hepatocellular carcinoma[J]. Liver Int, 2012, 32(1):70–77. doi:10.1111/j.1478–3231.2011.02631.x.
[32] Zhu Q, Li N, Zeng X, et al. Hepatocellular carcinoma in a large medical center of China over a 10-year period: evolving therapeutic option and improving survival[J]. Oncotarget, 2015, 6(6):4440–4450.
[33] Li M, Wang W, Jin R, et al. Differential association of STAT3 and HK-II expression in hepatitis B virus- and hepatitis C virus-related hepatocellular carcinoma[J]. J Med Virol, 2016, 88(9):1552–1559.doi: 10.1002/jmv.24498.
[34] Choudhari SR, Khan MA, Harris G, et al. Deactivation of Akt and STAT3 signaling promotes apoptosis, inhibits proliferation, and enhances the sensitivity of hepatocellular carcinoma cells to an anticancer agent, Atiprimod[J]. Mol Cancer Ther, 2007, 6(1):112–121.
[35] Yang Y, Zheng B, Han Q, et al. Targeting blockage of STAT3 inhibits hepatitis B virus-related hepatocellular carcinoma[J]. Cancer Biol Ther, 2016, 17(4):449–456. doi: 10.1080/15384047.2016.1156257.
[36] Hong Y, Zhou L, Xie H, et al. Differences in antiproliferative effect of STAT3 inhibition in HCC cells with versus without HBV expression[J]. Biochem Biophys Res Commun, 2015, 461(3):513–518. doi: 10.1016/j.bbrc.2015.04.058.
[37] Xiong Y, Yuan J, Zhang C, et al. The STAT3-regulated long non-coding RNA Lethe promote the HCV replication[J].Biomed Pharmacother, 2015, 72:165–171. doi: 10.1016/j.biopha.2015.04.019.
[38] Xiong Y, Jia M, Yuan J, et al. STAT3-regulated long non-coding RNAs lnc-7SK and lnc-IGF2-AS promote hepatitis C virus replication[J]. Mol Med Rep, 2015, 12(5):6738–6744. doi: 10.3892/mmr.2015.4278.
[39] McCartney EM, Helbig KJ, Narayana SK, et al. Signal transducer and activator of transcription 3 is a proviral host factor for hepatitis C virus[J]. Hepatology, 2013, 58(5):1558–1568. doi: 10.1002/hep.26496.
[40] Zhou JJ, Chen RF, Deng XG, et al. Hepatitis C virus core protein regulates NANOG expression via the STAT3 pathway[J]. FEBS Lett, 2014, 588(4):566–573. doi: 10.1016/j.febslet.2013.11.041.
[41] Saxena NK, Sharma D, Ding X, et al. Concomitant activation of the JAK/STAT, PI3K/AKT, and ERK signaling is involved in leptinmediated promotion of invasion and migration of hepatocellular carcinoma cells[J]. Cancer Res, 2007, 67(6):2497–2507.
[42] Mohan CD, Bharathkumar H, Bulusu KC, et al. Development of a novel azaspirane that targets the Janus kinase-signal transducer and activator of transcription (STAT) pathway in hepatocellular carcinoma in vitro and in vivo[J]. J Biol Chem, 2014,289(49):34296–34307. doi: 10.1074/jbc.M114.601104.
[43] Zhang X, You Q, Zhang X, et al. SOCS3 Methylation Predicts a Poor Prognosis in HBV Infection-Related Hepatocellular Carcinoma[J]. Int J Mol Sci, 2015, 16(9):22662–22675. doi:10.3390/ijms160922662.
[44] Niwa Y, Kanda H, Shikauchi Y, et al. Methylation silencing of SOCS-3 promotes cell growth and migration by enhancing JAK/STAT and FAK signalings in human hepatocellular carcinoma[J].Oncogene, 2005, 24(42):6406–6417.
[45] Al-Jamal HA, Jusoh SA, Yong AC, et al. Silencing of suppressor of cytokine signaling-3 due to methylation results in phosphorylation of STAT3 in imatinib resistant BCR-ABL positive chronic myeloid leukemia cells[J]. Asian Pac J Cancer Prev, 2014, 15(11):4555–4561.
[46] Poussin K, Pilati C, Couchy G, et al. Biochemical and functional analyses of gp130 mutants unveil JAK1 as a novel therapeutic target in human inflammatory hepatocellular adenoma[J].Oncoimmunology, 2013, 2(12):e27090.
[47] Calvisi DF, Ladu S, Gorden A, et al. Ubiquitous activation of Ras and JAK/Stat pathways in human HCC[J]. Gastroenterology, 2006,130(4):1117–1128.
[48] Yan XL, Jia YL, Chen L, et al. Hepatocellular carcinoma-associated mesenchymal stem cells promote hepatocarcinoma progression: role of the S100A4-miR155-SOCS1-MMP9 axis[J]. Hepatology, 2013,57(6):2274–2286. doi: 10.1002/hep.26257.
[49] Tong HV, Bock CT, Velavan TP. Genetic insights on host and hepatitis B virus in liver diseases[J]. Mutat Res Rev Mutat Res,2014, 762:65–75. doi: 10.1016/j.mrrev.2014.06.001.
[50] Kan Z, Zheng H, Liu X, et al. Whole-genome sequencing identifies recurrent mutations in hepatocellular carcinoma[J]. Genome Res,2013, 23(9):1422–1433. doi: 10.1101/gr.154492.113.
[51] Arumuggam N, Bhowmick NA, Rupasinghe HP. A Review:Phytochemicals Targeting JAK/STAT Signaling and IDO Expression in Cancer[J]. Phytother Res, 2015, 29(6):805–817. doi:10.1002/ptr.5327.
[52] Cokic VP, Bhattacharya B, Beleslin-Cokic BB, et al. JAK-STAT and AKT pathway-coupled genes in erythroid progenitor cells through ontogeny[J]. J Transl Med, 2012, 10:116. doi: 10.1186/1479–5876-10–116.
[53] Luo JM, Cen LP, Zhang XM, et al. PI3K/akt, JAK/STAT and MEK/ERK pathway inhibition protects retinal ganglion cells via different mechanisms after optic nerve injury[J]. Eur J Neurosci, 2007,26(4):828–842.
[54] Jung IH, Choi JH, Chung Y, et al. Predominant Activation of JAK/STAT3 Pathway by Interleukin-6 Is Implicated in Hepatocarcinogenesis[J]. Neoplasia, 2015, 17(7):586–597. doi:10.1016/j.neo.2015.07.005.
[55] Coskun M, Salem M, Pedersen J, et al. Involvement of JAK/STAT signaling in the pathogenesis of inflammatory bowel disease[J].Pharmacol Res, 2013, 76:1–8. doi: 10.1016/j.phrs.2013.06.007.
