宝鸡市PM2.5中水溶性离子组分污染特征及来源分析
2017-03-15曹军骥刘随心
张 婷,曹军骥,,刘随心
1.中国科学院地球环境研究所 中国科学院气溶胶化学与物理重点实验室,西安 710061
2.中国科学院地球环境研究所 黄土与第四纪地质国家重点实验室,西安 710061
3.西安交通大学 环境科学与工程系,西安 710049
宝鸡市PM2.5中水溶性离子组分污染特征及来源分析
张 婷1,2,曹军骥1,2,3,刘随心1,2
1.中国科学院地球环境研究所 中国科学院气溶胶化学与物理重点实验室,西安 710061
2.中国科学院地球环境研究所 黄土与第四纪地质国家重点实验室,西安 710061
3.西安交通大学 环境科学与工程系,西安 710049
本文通过对2012年3月至2013年3月宝鸡市大气PM2.5中各个水溶性无机离子组分的质量浓度进行研究,获得了水溶性离子的时间变化特征,并结合主成分分析方法讨论了不同离子的来源。结果显示,宝鸡市PM2.5中水溶性离子主要由组成,分别占总水溶性离子质量浓度的40.47%、30.75%和15.07%;PM2.5整体偏酸性。比值随API指数的升高而增大,当空气质量较好时PM2.5中硫酸盐居多,而随着空气污染发生硝酸盐逐渐增多并占优势。主成分分析结果表明PM2.5中水溶性离子的主要来源有二次气溶胶、生物质燃烧和土壤尘。
宝鸡;PM2.5;水溶性离子;来源
大气气溶胶是由大气与悬浮在其中的固体和液体微粒共同组成的多相体系,其中空气动力学等效直径<2.5 μm的颗粒物定义为PM2.5。大气PM2.5颗粒由于其粒径小、比表面积大、富含大量有毒有害物质、输送距离远,因而对气候、人体健康、空气质量及能见度均有重要影响(曹军骥,2012)。水溶性无机离子是PM2.5的重要组分之一,其控制颗粒物的酸碱性从而影响气溶胶的形成机制、在大气中的生命周期以及健康效应(Hu et al,2014)。因此研究PM2.5中水溶离子的组成及来源等对于有效控制大气细粒子污染具有重要意义(Chow et al,2012)。
宝鸡是关中城市群的重要城市之一,是西北乃至西部地区的比较优势区域。近年来随着区域经济的快速发展,宝鸡市空气污染事件时有发生,给大气环境、群众健康、交通安全等带来了严重影响,因此大气气溶胶研究及污染治理刻不容缓。然而目前对宝鸡市大气污染状况的研究相对较少且主要集中在颗粒物的质量浓度方面(胡淑圆等,2010;张红芳等,2014;黄战胜和范铠,2015;薛平等,2015),而对颗粒物中主要化学组分污染特征的研究比较匮乏。
本文通过对陕西省宝鸡市2012年3月— 2013年3月大气PM2.5中水溶性离子组分的观测研究,深入分析了不同水溶性离子的浓度水平及污染特征,并对其来源进行探讨,为宝鸡市未来解决日益严重的灰霾污染问题提供了数据支持和治理方向。
1 样品的采集与分析
1.1 PM2.5样品采集
宝鸡(33°35′ — 35°06′ N,106°18′ — 108°03′ E)地处关中盆地的西端,东西长156.6 km,南北宽160.6 km,总面积1.8×104km2,总人口375万(2014年)。宝鸡属于暖温带半湿润气候,全年气候变化受东亚季风控制,年平均气温12—14℃,年平均降水量在590 — 900 mm,是关中盆地降水量较多的地区之一。
2012年3月15日至2013年3月14日,使用便携式气溶胶采样仪(Mini volume portable sampler,Airmetrics,Oregon,USA)每6天采集一个PM2.5样品,采样时间为24小时,流量为5 L · min−1。使用直径为47 mm石英滤纸(QM/A,Whatman Inc.,U.K.),采样前在780℃马弗炉中焙烧3小时,以去除可能的污染物。采样点位于宝鸡市环境监测站内,距地面高度约为15 m。
1.2 水溶性离子分析
截取1/4的采样滤纸加入10 mL去离子水(R>18.2 MΩ),超声、震荡各1小时后采用0.45 μm的水系过滤器过滤待分析。水溶性离子的定量分析采用Dionex-600型离子色谱仪(包括Ion Pac-AS23型分析柱、Ion Pac-AG23型保护柱、AERS自身再生抑制器、Ion Pac-CS12A型分析柱、Ion Pac-CG12A型保护柱、CERS自身再生抑制器、ED50电导检测器和GP40梯度泵)进行检测,用Chromeleon软件进行谱图分析,得到10种水溶性离子组分(F−、Cl−、、 K+、Mg2+、Ca2+)的质量浓度。本方法的详细介绍及质量控制参见Zhang et al(2011)。
1.3 气象数据及API值的获取
观测期内气象数据从宝鸡气象信息网(http:// www.bjqx.gov.cn/)获得。API指数从宝鸡市环境保护局(http://www.baojihb.gov.cn/)获得。
API(Air pollution index)空气污染指数是将常规监测的几种空气污染物(SO2、NO2和PM10)浓度简化成单一的概念性指数数值形式。各污染物污染分指数中的最大值即为该区域或城市的空气污染指数。API指数可以用来反映和评价空气污染程度和空气质量状况。
2 结果与讨论
2.1 PM2.5中水溶性离子浓度分布特征
如表1所示,2012年3月— 2013年3月宝鸡市PM2.5中总水溶性离子的平均质量浓度为51.62 μg · m−3,变化范围在10.54 — 207.00 μg · m−3。其中4月总水溶性离子的平均值最小,为22.1 μg · m−3,2013年2月的平均值最大,为92.33 μg · m−3。总水溶性离子的质量浓度在春季较低,夏季整体浓度值较低但有个别浓度约100 μg · m−3的污染天,秋季总水溶性离子浓度略有升高,冬季显著升高,是全年当中污染最严重的季节(见图1)。宝鸡市PM2.5中总水溶性离子质量浓度的季节变化趋势与我国大多数北方城市相似,冬季最高,夏季次之,春秋季较低。冬季宝鸡市总水溶性离子浓度平均为76.72 μg · m−3,主要是由于冬季居民燃煤取暖造成排放增加以及气象条件不利于污染物的扩散等因素导致。春季易发生沙尘暴、扬沙及浮尘天气,这可能会造成某些水溶性离子发生事件性的升高。夏季由于关中平原秸秆焚烧现象增多,高温高湿的气象条件有利于二次气溶胶的形成,所以气溶胶的浓度可能出现事件性的升高。秋季由于雨水较多,气象状况有利于气溶胶的湿沉降,因此水溶性离子浓度相对夏季略有下降。

表1 宝鸡市PM2.5中水溶性离子的浓度水平(单位:μg · m−3)Tab.1 Concentrations of water-soluble ions in PM2.5 in Baoji (unit:μg · m−3)
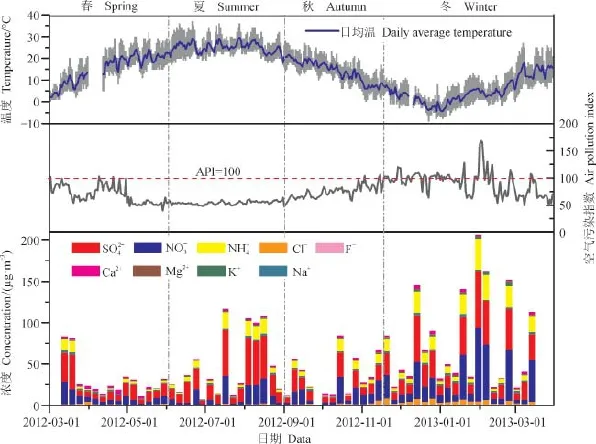
图1 气象参数、API指数及水溶性离子浓度时间序列Fig.1 Temporal variations of temperature,API and water-soluble ions mass concentrations
API指数能粗略地反映空气污染程度,API<100表示空气质量状况在良以上。2012年3月—2013年3月宝鸡市空气污染API指数的年平均值为73.9,变化范围是40 — 169。从API指数看宝鸡市空气污染状况可知,API大部分都小于100,只有冬季一些天的API指数较高,污染较严重(见图1)。
2.2 各水溶性离子组分的时间变化规律
PM2.5中主要水溶性离子组分的时间变化规律如图1所示,可以看到的时间变化规律基本一致,由于冬季人为排放的增加及气象条件不利于颗粒物的清除和扩散,三种主要离子组分均在冬季浓度最高。而夏季浓度仅次于冬季,比春季和秋季高,这主要与夏季温、湿度的升高有利于人为排放的气态污染物(如SO2和NOx)等经过大气化学反应生成二次气溶胶有关。但是温度的升高会促使硝酸盐挥发至气相,所以冬季的浓度相当甚至高于,而夏季的浓度则远高于。
一次来源的气溶胶粒子组分中Cl−、Ca2+和K+的质量浓度在总水溶性无机离子中所占比重相对较小,分别为4.55%、3.73%和1.73%。这些离子的季节变化主要反映了排放源的变化特征,如氯离子(Cl−)与燃烧活动(燃煤以及秸秆焚烧等)密切相关,钾离子(K+)可用于示踪生物质燃烧排放以及烟花燃放活动(Duan et al,2004;徐红梅等,2012),而钙离子(Ca2+)主要来自于地壳源,是土壤尘的指示物。如图1所示,冬季K+的浓度远高于其他季节,表明宝鸡冬季生物质燃烧对气溶胶的贡献较大,并且伴有中国传统节日春节时的燃放烟花爆竹的影响。Ca2+在干旱少雨的冬春季浓度均较高,除了受远源传输的沙尘气溶胶影响之外,本地扬尘也是其主要贡献之一。Cl−表现出冬季显著高于其他季节的时间变化特征,也与排放源的变化规律一致。
2.3 阴阳离子平衡及中和因子分析
根据以下公式(1)和(2),使用主要阴、阳离子组分做离子平衡分析:

如图2,可以看出2012年3月—2013年3月宝鸡市大气中PM2.5整体偏酸性。阴阳离子的相关性很好(R2> 0.95),样品测试数据有效,所分析的离子能够代表PM2.5中主要的水溶性组分。分别对不同季节的样品进行阴阳离子平衡分析,可以看出夏、冬季比春、秋季拟合曲线的斜率更大,指示了夏、冬季宝鸡市大气中PM2.5的酸性更强,这可能与夏、冬季形成更多的二次硫酸盐和硝酸盐有关。而春季拟合曲线的相关性(R2= 0.86)对比其他季节略低,可能是受到了春季沙尘样品中碳酸盐的影响。
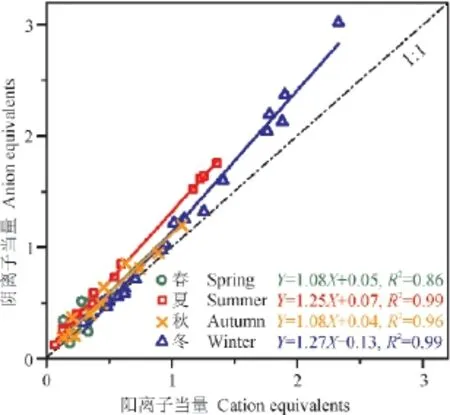
图2 阴阳离子平衡Fig.2 Ion balance in PM2.5
为研究PM2.5中各个阴、阳离子之间的中和反应,使用以下公式(3)、(4)和(5)来计算不同阳离子的中和因子(Neutralization factor,NF)(Kulshrestha et al,1995;Shen et al,2012):


图3 钙离子、铵根离子和镁离子中和因子的三角图Fig.3 Triangular diagrams of NF for Ca2+,and Mg2+



表2 PM2.5中水溶性离子相关系数矩阵Tab.2 The correlation coef fi cients of water-soluble ions in PM2.5
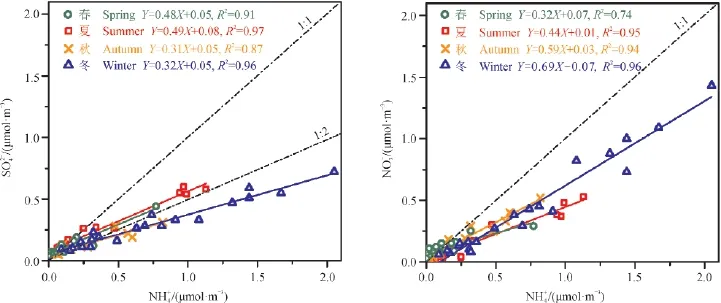
图4 与及摩尔浓度的相关性Fig.4 Relationship betweenmole concentration and amp;mole concentration

2.6 来源分析
本研究使用主成分分析法(PCA,SPSS 17.0, SPSS Inc.1988)识别水溶性无机离子的主要来源,并半定量判别各来源的大致贡献。主成分分析法是一种广泛使用的多元统计方法,可以很好地按照多变量之间的相关性密切程度来分类从而简化和解释数据。使用主成分分析可以从大量的质量浓度数据中提取出少数几个潜在因子(主成分)以便于解释被测变量之间的关系(Han et al,2006)。该组数据KMO统计量>0.5表明适用主成分分析,并且设定特征值>1作为需满足的条件得到两个主成分(Kaiser,1960)。表3为宝鸡市大气PM2.5中主要水溶性离子组分经主成分分析得到的旋转后因子载荷矩阵。如表3所示,主成分1因子载荷较大的离子组分为和K+,代表了二次气溶胶来源以及和它们相关性较好的生物质燃烧来源;主成分2因子载荷较大的离子组分为F−、Cl−、Mg2+和Ca2+,代表了一次土壤尘排放源。主成分1和2的累计贡献率达80.0%。
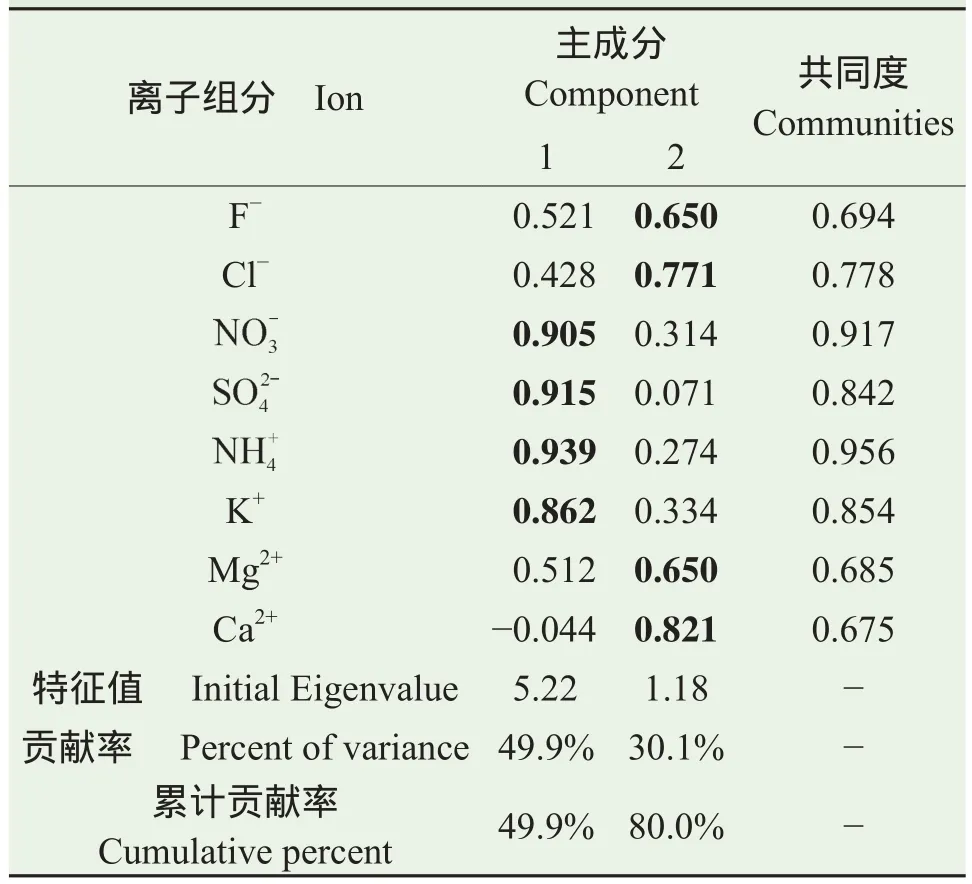
表3 PM2.5中水溶性离子的旋转后因子载荷矩阵Tab.3 Rotated component matrix for water-soluble ions in PM2.5
3 结论
本研究通过对宝鸡市2012年3月至2013年3月PM2.5中水溶性无机离子组分的研究,得到以下结论:
(1)2012年3月— 2013年3月宝鸡市大气PM2.5中的总水溶性离子平均质量浓度为51.62 μg · m−3,其主要组分是,分别占总水溶性离子的40.47%、30.75%和15.07%,它们的年平均质量浓度分别为20.89 μg · m−3、15.88 μg · m−3和7.78 μg · m−3。
(2)研究期间,阴阳离子的相关性很好(R2> 0.95),PM2.5整体偏酸性。宝鸡市大气PM2.5中Ca2+和起主要的中和作用。
(3)研究期间,当宝鸡市空气质量较好时PM2.5中硫酸盐居多,而随着空气污染发生硝酸盐逐渐增多并占优势。
(4)主成分分析结果显示,宝鸡市PM2.5中水溶性离子的主要来源有二次气溶胶,生物质燃烧和土壤尘。
曹军骥.2012.我国PM2.5污染现状与控制对策[J].地球环境学报,3(5):1030 – 1036.[Cao J J.2012.Pollution status and control strategies of PM2.5in China [J].Journal of EarthEnvironment,3(5):1030 – 1036.]
Chow J C,曹军骥,李顺诚,等.2012.PM2.5及其测量与影响研究简史[J].地球环境学报,3(5):1019 – 1029.[Chow J C,Cao J J,Lee S C,et al.2012.A brief history of PM2.5,its measurement and adverse effects [J].Journal of Earth Environment,3(5):1019 – 1029.]
胡淑圆,谢鸣捷,王格慧,等.2010.宝鸡市大气PM10中水溶性物质的组成及特征研究[J].环境污染与防治,32(3):34 – 38.[Hu S Y,Xie M J,Wang G H,et al.2010.Component and characteristics of water-soluble species in PM10aerosol of Baoji [J].Environmental Pollution amp; Control,32(3):34 – 38 ]
黄战胜,范 铠.2015.宝鸡市近年来大气质量变化趋势分析[J].黑龙江环境通报,39(3):45 – 48.[Huang Z S,Fan K.2015.Analysisi of variation tendency of atmospheric environment quality in Baoji city in recent years [J].Heilongjiang Environmental Journal,39(3):45 – 48.]
徐红梅,张 婷,刘随心.2012.春节期间燃放烟花对西安市PM2.5的影响[J].地球环境学报,3(5):1037 – 1042.[Xu H M,Zhang T,Liu S X.2012.Impact of burning fi reworks during Spring Festival to PM2.5of Xi’an [J].Journal of Earth Environment,3(5):1037 – 1042.]
薛 平,刘丽霞,董卫民,等.2015.宝鸡市城区空气细颗粒物(PM2.5)污染特征及源分析[J].宝鸡文理学院学报(自然科学版),35(2):63 – 69.[Xue P,Liu L X,Dong W M,et al.2015.Analysis of pollution characteristics and source of PM2.5in Baoji city [J].Journal of Baoji University of Arts and Sciences (Natural Science),35(2):63 – 69.]
张红芳,范 锴,张 珏.2014.宝鸡市空气中PM10、PM2.5污染状况研究[J].宝鸡文理学院学报(自然科学版),34(3):42 – 47.[Zhang H F,Fan K,Zhang J.2014.Research on PM10and PM2.5particle pollution in the urban air of Baoji [J].Journal of Baoji University of Arts and Sciences (Natural Science),34(3):42 – 47.]
Arimoto R,Duce R A,Savoie D L,et al.1996.Relationships among aerosol constituents from Asia and the North Pacific during Pem-West A [J].Journal of GeophysicalResearch,101:2011 – 2023.
Duan F K,Liu X D,Yu T,et al.2004.Identification and estimate of biomass burning contribution to the urban aerosol organic carbon concentrations in Beijing [J].Atmospheric Environment,38:1275 – 1282.
Han Y M,Du P X,Cao J J,et al.2006.Multivariate analysis of heavy metal contamination in urban dusts of Xi’an,Central China [J].Science of the Total Environment,355:176 – 186.
Hu G Y,Zhang Y M,Sun J Y,et al.2014.Variability,formation and acidity of water-soluble ions in PM2.5in Beijing based on the semi-continuous observations [J].Atmospheric Research,145 – 146.
Kaiser H F.1960.The application of electronic computers to factor analysis [J].Educational and Psychological Measurement,20:141 – 151.
Kulshrestha U C,Sarkar A K,Srivastava S S,et al.1995.Wetonly and bulk deposition studies at New Delhi (India) [J].Water,Air,and Soil Pollution,85:2137 – 2142.
Shen Z X,Zhang L M,Cao J J,et al.2012.Chemical composition,sources,and deposition fluxes of water-soluble inorganic ions obtained from precipitation chemistry measurements collected at an urban site in northwest China [J].Journal of Environmental Monitoring,14:3000 – 3008.
Wang P,Cao J J,Shen Z X,et al.2015.Spatial and seasonal variations of PM2.5mass and species during 2010 in Xi’an,China [J].Science of the Total Environment,508:477 – 487.
Wang Y,Zhuang G S,Tang A,et al.2005.The ion chemistry and the source of PM2.5aerosol in Beijing [J].Atmospheric Environment,39:3771 – 3784.
Yao X H,Chak K C,Fang M,et al.2002.The water-soluble ionic composition of PM2.5in Shanghai and Beijing,China [J].Atmospheric Environment,36:4223 – 4234.
Zhang T,Cao J J,Tie X X,et al.2011.Water-soluble ions in atmospheric aerosols measured in Xi’an,China:Seasonal variations and sources [J].Atmospheric Research,102:110 – 119.
Zhang Y,Seigneur C,Seinfeld J H,et al.2000.A comparative review of inorganic aerosol thermodynamic equilibrium modules:similarities,differences,and their likely causes [J].Atmospheric Environment,34:117 – 137.
Pollution characteristics and sources of water-soluble ions in PM2.5in Baoji
ZHANG Ting1,2,CAO Junji1,2,3,LIU Suixin1,2
1.Key Laboratory of Aerosol Chemistry amp; Physics,Institute of Earth Environment,Chinese Academy of Sciences,Xi’an 710061,China
2.State Key Laboratory of Loess and Quaternary Geology,Institute of Earth Environment,Chinese Academy of Sciences,Xi’an 710061,China
3.Department of Environmental Science and Technology,Xi’an Jiaotong University,Xi’an 710049,China
Background,aim,and scopeFine particulate matter (PM2.5) causes air quality problems in urban areas,especially visibility reduction and health problems,such as asthma and even mortality.Water-soluble inorganic ions are major components of the atmospheric aerosols,especially PM2.5.They can compose up to 60% — 70% of the total mass of suspended particulate matter.Therefore,observations on the chemical composition of watersoluble fine aerosols would be valuable for understanding their physical/chemical characteristics,sources,and behavior and formation mechanism.Baoji (33°35′ — 35°06′N,106°18′—108°03′E) is an inland city situated in the mid-west part of China.Due to the rapid increase of motor vehicles and the growth in energy consumption over the past few decades,Baoji is facing serious air quality problems,mainly due to the high aerosol loadings in the region.However there has been no much attention paid for the problem in these mid-scale cities.In the current study,we collected the water-soluble ions in PM2.5samples at urban site of Baoji and to investigate the temporal variations and possible sources for these species.This study can provide useful information for establishingcontrol strategies of aerosol pollution.Materials and methodsWater-soluble inorganic ions in PM2.5were collected in Baoji from March 2012 to March 2013.The sampling site was located in Baoji Environmental Monitoring Station that is surrounded by a big residential area.Aerosols were collected by mini-volume samplers equipped with pre-baked quartz fi ber fi lters.A total of 10 water-soluble ions (Na+,,K+,Mg2+,Ca2+,F−,Cl−,,,and) were analyzed by Ion Chromatograph in the aqueous extracts of the air filters.ResultsThe 24-hr average mass concentrations of total water-soluble ions in PM2.5varied from 10.54 μg · m−3to 207.00 μg · m−3,with an overall average of 51.62 μg · m−3.Monthly average concentrations of total water-soluble ions were highest during February (92.33 μg · m−3) and lowest during April (22.1 μg · m−3).In anion,the concentrations of the most abundant ionic species followed the order of>>Cl−>F−,while in cation were>Ca2+>Na+>K+>Mg2+.Overall,,andwere dominant ionic species in PM2.5,the annual average concentrations were 20.89 μg · m−3,15.88 μg · m−3and 7.78 μg · m−3,respectively.A strong correlation (R2>0.95) between cation and anion equivalents for all samples indicates that the five cations and five anions were the major ions extracted from the fi lters.The slope (anion/cation) of the linear regression was close to 1.25 in summer and winter,1.08 in spring and autumn.The annual volume-weighted mean values of NF for Ca2+,and Mg2+were 0.23,0.50 and 0.04,respectively,indicating that Ca2+andwere the major neutralizers in PM2.5in Baoji.The seasonal variation of/was shown winter(0.85) > autumn(0.83) > spring(0.66) > summer(0.36).DiscussionThe seasonal variations of secondary components,,and,were similar,i.e.high concentrations in winter and low concentrations in spring and autumn.The seasonal variation trend may be ascribed to (1) the increase of emission sources (i.e.,residential and commercial coal combustion); (2) meteorological conditions during winter were characterized by stagnation with a low inversion layer,which intensify secondary components levels through accumulation of air pollutants.Ion balance calculations are useful for studying the acid base balance of aerosol particles.This result implies that the aerosol particles from summer and winter are more acidic.The buffering of acidity in spring is likely due to the high dust loadings.The neutralization factor (NF) of Ca2+,and Mg2+were calculated using their equivalent concentrations that can be used to describe the interaction between cations and anions.The NF forwas higher in four seasons and NF for Ca2+was higher in spring and autumn,suggesting that Ca2+played a dominant role in the spring and autumn acid neutralization whilehad a stronger buffering ability in the whole year.It showed clearly in correlation coefficients between major ions thatmainly existed as (NH4)2SO4and NH4HSO4,whileas NH4NO3.The mass ratio of/was not suitable for indicator of mobile vs.stationary sources of sulfur and nitrogen in the atmosphere in Baoji./ratio was relatively high with increasing API,it means sulfate decreasing and nitrate increasing while poor air quality.A preliminary source identi fi cation study of the ws-ions was carried out by principal component analysis.The two factor PCA model for the ws-ions in the PM2.5aerosol samples accounted for ~80.0% of the total variance in the concentration data.Factor 1 account for ~49.9% of the total variance,and it was strongly loaded with,,and K+,suggesting likely origins from secondary aerosol and biomass burning.Factor 2 is dominated by F−,Cl−,Mg2+and Ca2+,and it accounted for ~30.1% of the total variance,suggesting their concentrations are affected by primary soil dust.ConclusionsThe annual average concentration of total water-soluble ions in PM2.5was 51.62 μg · m−3in Baoji from March 2012 to March 2013.Water-soluble ions were mainly composed of,and,accounting for 40.47%,30.75% and 15.07% in total water-soluble ions,respectively.PM2.5was generally acidic./ratio was relatively high with increasing API,it means sulfate decreasing and nitrate increasing while poor air quality.The PCA results indicated that secondary aerosol,biomass burning and soil dust were mainly sources of water-soluble ion in PM2.5.Recommendations and perspectivesThe search provide a signi fi cant scienti fi c basis for understanding the pollution characteristics of water-soluble ion in PM2.5at Baoji.To alleviate the PM2.5pollution,reducing coal burning,biomass burning and controlling soil dust should be performed.
National Natural Science Foundation of China (41503123)
ZHANG Ting,E-mail:zhangting@ieecas.cn
Baoji; PM2.5; water soluble ion; source
2016-11-22;录用日期2017-01-09
Received Date:2016-11-22;Accepted Date2017-01-09
国家自然科学基金项目(41503123)
张 婷,E-mail:zhangting@ieecas.cn
张 婷,曹军骥,刘随心.2017.宝鸡市PM2.5中水溶性离子组分污染特征及来源分析 [J].地球环境学报,8(1):46 – 54.
: Zhang T,Cao J J,Liu S X.2017.Pollution characteristics and sources of water-soluble ions in PM2.5in Baoji [J].Journal of Earth Environment,8(1):46 – 54.
10.7515/JEE201701006
