黄芪甲苷对糖尿病KKAy小鼠肾组织TGF-β1、SMAD2/3及α-SMA表达的影响
2017-03-01王雅宁刘云启高金祥刘乃国
王雅宁,刘云启,高金祥,蔺 超,刘乃国
(滨州医学院附属医院:1.肾内科;2.骨关节外科;3.临床医学实验中心,山东滨州 256603)
黄芪甲苷对糖尿病KKAy小鼠肾组织TGF-β1、SMAD2/3及α-SMA表达的影响
王雅宁1,刘云启1,高金祥1,蔺 超2,刘乃国3
(滨州医学院附属医院:1.肾内科;2.骨关节外科;3.临床医学实验中心,山东滨州 256603)
目的 观察黄芪甲苷对糖尿病KKAy小鼠肾组织转化生长因子β1(TGF-β1)、SMAD2/3、α-平滑肌肌动蛋白(α-SMA)表达的影响,探讨其延缓肾脏纤维化的可能机制。方法 雄性C57BL/6J小鼠10只作为对照组,20只2型糖尿病模型KKAy小鼠予以高脂饮食至14周,随机数字法分为模型组和黄芪甲苷组,每组10只,黄芪甲苷组给予黄芪甲苷40 mg·kg-1·d-1,模型组与对照组给予等量生理盐水。实验期间,各组动物自由饮食、饮水。血糖仪测量16、20、24周龄时各组小鼠的血糖水平。24周时处死,观察各组小鼠肾的病理学变化,免疫组织化学法测定TGF-β1、SMAD2/3、α-SMA的表达。结果 (1)血糖:与对照组相比,14周龄的KKAy小鼠血糖明显升高,模型组血糖在16、20、24周时血糖明显升高(P<0.05),黄芪甲苷组与其相比,血糖下降(P<0.05)。(2)肾组织形态学:对照组肾小球及肾小管结构清晰,未出现肾间质纤维化,模型组肾小球系膜基质增宽,系膜细胞增多,肾小管上皮细胞细胞质空泡样变性,肾间质炎性细胞增多,黄芪甲苷组肾小管上皮细胞细胞质较少,未呈现明显的纤维化。(3)肾组织TGF-β1、SMAD2/3、α-SMA的表达:对照组TGF-β1表达微弱,而模型组TGF-β1显著表达于肾小管上皮细胞细胞质(P<0.01);与模型组相比,黄芪甲苷组TGF-β1、α-SMA表达明显下调(P<0.05);对照组肾小管与肾小球细胞核有少量磷酸化的SMAD2/3表达,模型组表达增加(P<0.01),与模型组相比,黄芪甲苷组表达减少(P<0.05)。结论 黄芪甲苷通过影响TGF-β/SMADS信号通路,下调TGF-β1、α-SMA表达,改善糖尿病小鼠肾脏纤维化。
黄芪甲苷;糖尿病肾病;转化生长因子;肌动蛋白-α;Smads信号蛋白
糖尿病肾病是糖尿病微血管病变中的重要并发症之一,是导致终末期肾病和糖尿病患者死亡的主要原因。肾小管及肾小球的炎症、纤维化是糖尿病肾病进展为终末期肾病的主要病理基础,并且肾小管的损伤先于肾小球损伤[1-2]。因此,阻断肾小管纤维化在延缓肾功能恶化过程中起着重要的作用。包括转化生长因子β1(TGF-β1)在内的许多细胞因子参与了肾脏纤维化的过程。TGF-β1可诱导肾小管上皮细胞向纤维母细胞转分化,且在糖尿病肾病的发展过程中,TGF-β1表达增加[3-4]。作为TGF-β家族的下游信号转导蛋白,Smads信号蛋白(SMAD)蛋白同肾脏纤维化密切相关。有研究发现,TGF-β刺激肾小管上皮细胞时,SMAD2发生磷酸化并且转入核内,促使肾小管上皮细胞表达胶原蛋白及α-平滑肌肌动蛋白(α-SMA)增加[5-6]。黄芪甲苷是从豆科植物黄芪中提取出来的具有抗氧化、抗炎的物质,可通过多种途径保护肾脏。本研究通过观察黄芪甲苷对糖尿病KKAy小鼠肾组织TGF-β1、SMAD2/3及α-SMA表达的影响,探讨其对肾间质纤维化的保护作用,为临床应用黄芪甲苷保护肾脏提供实验依据。
1 材料与方法
1.1 材料
1.1.1 实验动物 雄性C57BL/6J小鼠10只及KKAy小鼠20只,体质量22~24 g,购自中国医学科学院动物中心,饲养于无特定病原体(SPF)级动物实验室;
1.1.2 主要试剂 黄芪甲苷(美国Sigma公司);DAB试剂盒、TGF-β1和α-SMA多克隆抗体(北京中山生物科技有限公司);磷酸化SMAD2/3多克隆抗体(武汉博士德公司)。
1.2 方法
1.2.1 动物分组及给药 高脂饮食饲养至14周龄的KKAy小鼠当随机血糖大于13.9 mmol/L时提示造模成功[7],随机数字法分成模型组和黄芪甲苷组(每日灌胃,剂量为40 mg·kg-1·d-1),同龄雄性C57BL/6J小鼠作为对照组,实验期间,各组动物自由饮食、饮水。
1.2.2 标本采集 分别于16、20、24周时尾静脉取血测量血糖水平;24周龄时处死各组小鼠,以4%多聚甲醛固定肾组织。
1.2.3 指标检测 小鼠禁食6 h后罗氏血糖仪监测血糖。
1.2.4 肾组织病理形态学观察 石蜡包埋肾组织后切片,进行常规苏木精-伊红(HE)染色及Masson染色,在光镜下观察;免疫组织化学:据SABC试剂盒说明书测定TGF-β1、SMAD2/3及α-SMA表达情况,随机数字法选取10个以上肾小球测定上述蛋白表达。
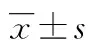
2 结 果
2.1 一般状态比较 对照组老鼠对外界反应灵敏,毛色顺滑。模型组小鼠精神状态差,步履缓慢,烦渴多尿,反应差,毛发枯燥。随着周龄的增加,上述症状更加明显。黄芪甲苷组小鼠的精神状态及反应能力介于两组之间。
2.2 黄芪甲苷对KKAy糖尿病小鼠血糖的影响 与对照组相比,14周龄的KKAy小鼠血糖明显升高,模型组血糖在16、20、24周时血糖明显升高(P<0.05),黄芪甲苷组与其相比,血糖下降(P<0.05)。见表1。
2.3 黄芪甲苷对KKAy糖尿病小鼠组织形态学的影响 对照组小鼠肾小球及肾小管结构清晰,没有肾间质纤维化。模型组肾小球系膜基质增宽,系膜细胞增多,肾小管上皮细胞细胞质空泡样变性,肾间质炎性细胞增多。黄芪甲苷组肾小管上皮细胞细胞质较少,未呈现明显的纤维化。见图1。
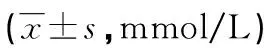
表1 各组小鼠末梢血糖水平
a:P<0.05,与对照组比较;b:P<0.05,与模型组比较。
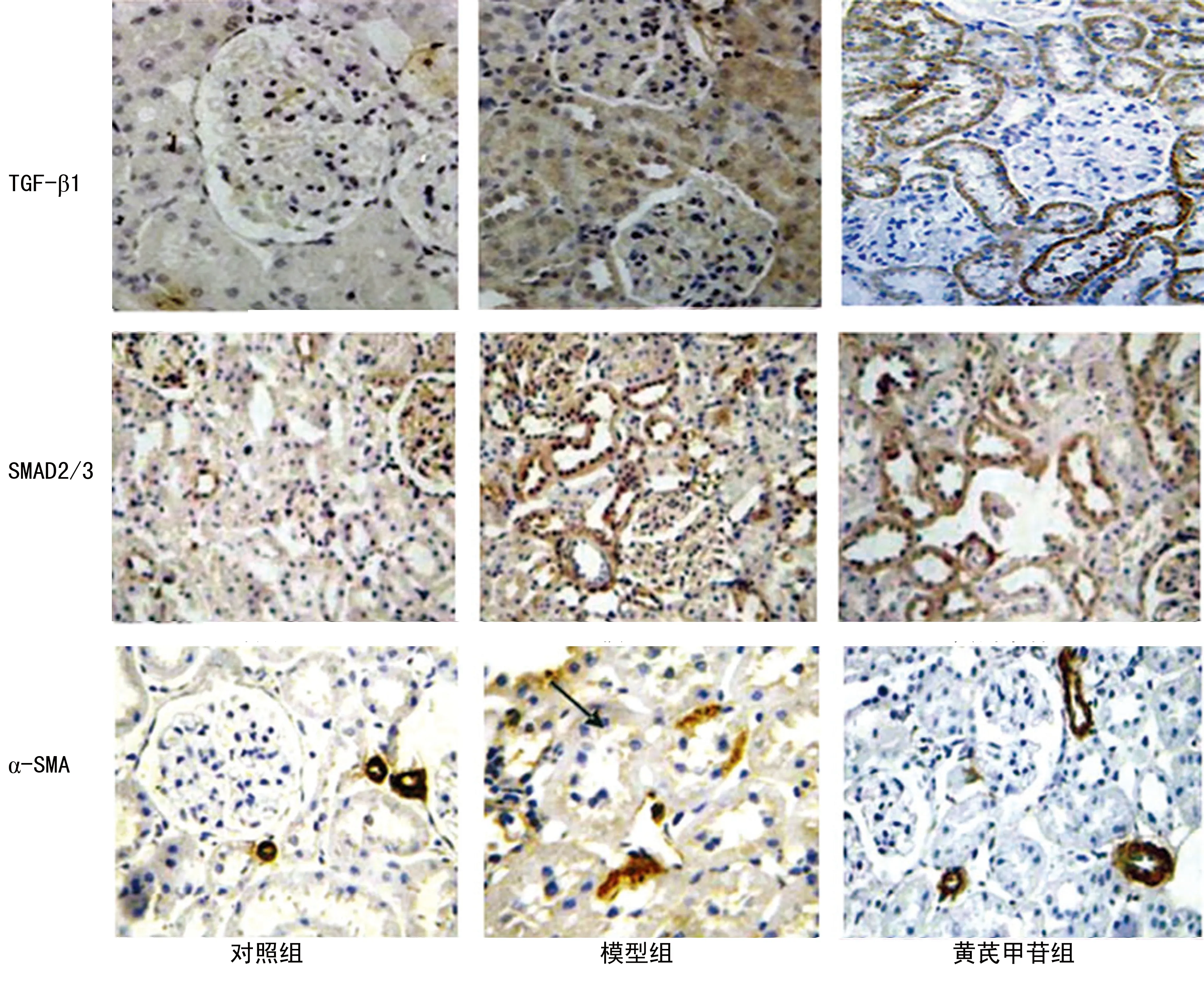
图2 黄芪甲苷对KKAy糖尿病小鼠肾组织TGF-β1、SMAD2/3及α-SMA表达的影响(免疫组织化学×200)
2.4 黄芪甲苷对KKAy糖尿病小鼠肾组织TGF-β1、SMAD2/3及α-SMA表达的影响 对照组TGF-β1表达微弱,而模型组TGF-β1显著表达于肾小管上皮细胞细胞质(P<0.01);与模型组相比,黄芪甲苷组TGF-β1、α-SMA表达明显下调(P<0.05);对照组肾小管与肾小球细胞核有少量磷酸化的SMAD2/3表达,模型组表达增加(P<0.01),同模型组相比,黄芪甲苷组表达减少(P<0.05)。见表2、图2。
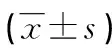
表2 各组小鼠肾组织TGF-β、SMAD2/3、α-SMA平均吸光度值
a:P<0.01,与对照组比较;b:P<0.05,与模型组比较。
3 讨 论
黄芪甲苷是豆科植物膜荚黄芪的干燥根,是从黄芪总苷中分离的一类单体化合物,是豆科植物黄芪的主要成分,具有抗应激、降低血糖、调节免疫、利水消肿、保护肾脏等多种药理学作用[8]。膜荚黄芪具有延缓糖尿病肾病发展及延缓肾小球系膜基质增生的作用。然而,有关黄芪甲苷的肾脏保护机制尚未完全阐明。有研究表明,在高糖或链脲霉素诱导的1型糖尿病大鼠模型中,黄芪甲苷通过增加足细胞α3β1整合素的表达,下调整合素的表达,发挥对足细胞的保护作用[9]。此外,黄芪甲苷通过Caspase-3调节Bax/Bcl-2以抑制足细胞凋亡[10]。有研究者发现,在急性肾损伤时,黄芪甲苷通过抑制核因子-κB(NF-κB)表达及阻断p38MAPK通路来减少炎性因子的释放,修复损伤的肾小管[11-12]。有研究证实,黄芪甲苷可通过下调TGF-β1及阻断p38MAPK通路,抑制高糖诱导的人肾小管上皮细胞发生凋亡[13-14]。最近研究证实,黄芪甲苷通过抑制肾小管上皮细胞产生活性氧从而减轻氧化应激,阻断上皮细胞转分化的发生[15-16]。因此,假设黄芪甲苷通过TGF-β1介导的细胞通路影响炎症及纤维化,此项研究旨在观察黄芪甲苷对糖尿病KKAy小鼠TGF-β1、SMAD2/3及α-SMA表达的影响,探讨其对肾间质纤维化的作用。
肾间质纤维化及肾小管损伤在肾脏疾病发展过程中起了重要的作用,且与肾功能下降密切相关。肾小管上皮细胞转分化是糖尿病肾病进程中的关键一环,且在此进程中肾功能恶化的重要机制之一就是肾小管间质纤维化。在肾小管间质纤维化发展过程中,承担细胞外基质合成的细胞主要为肌成纤维母细胞,而近曲小管上皮细胞可转分化为肌成纤维母细胞。因此,延缓慢性肾脏病发展的关键就是抑制肾小管间质纤维化[17-18]。肾小管上皮细胞在受到刺激后转分化为成纤维母细胞,进而分泌某些细胞因子,细胞极性消失,大量细胞外基质积聚,最终导致肾间质纤维化。在肾小管上皮细胞转分化过程中,TGF-β1扮演了重要角色,而作为TGF-β家族的下游信号转导蛋白,SMAD蛋白同肾脏纤维化密切相关。在本研究中,探讨了黄芪甲苷对糖尿病KKAy小鼠血糖及肾脏病理形态学的影响。实验结果显示,与对照组相比,模型组肾组织纤维化及血糖明显升高;同模型组相比,黄芪甲苷组肾间质纤维化减轻,血糖水平下降,细胞增生减少,这提示黄芪甲苷可减少糖尿病KKAy小鼠血糖水平,延缓肾脏纤维化的发展。此外,与对照组相比,模型组TGF-β1、SMAD2/3及α-SMA表达明显增加,与模型组相比,黄芪甲苷组上述指标表达下降。α-SMA是肾小管上皮细胞转分化的标志,其水平高低可反映肾脏纤维化的程度。先前的体外研究发现,SMAD2/3表达于人系膜细胞,且在TGF-β1导致胶原Ⅰ分泌增加的过程中也有SMAD信号通路的参与[19-20]。肾小管上皮细胞转分化分为4个过程:肾小管上皮细胞间紧密连接的破坏;α-SMA的表达及肌动蛋白细胞骨架的重排; 肾小管基底膜的破坏;肾小管上皮细胞发生迁移,侵袭能力增加。TGF-β1作为关键的细胞因子通过调节细胞增殖和分化参与了上述过程,该因子可抑制基质降解酶的表达,促进肾小管上皮细胞转分化,抑制肾小管细胞增殖,最终导致肾脏炎症及纤维化[20-21]。体外实验证实,在肾小球系膜细胞及肾小管上皮细胞,高糖刺激可激活SMAD信号通路,引起TGF-β1过表达。本实验证实TGF-β1、SMAD2/3及α-SMA在糖尿病小鼠肾组织中高表达,同上述研究一致。此外,TGF-β/SMADS信号通路的激活可使α-SMA表达增加[22-24]。本研究发现,黄芪甲苷可使糖尿病KKAy小鼠TGF-β1、SMAD2/3及α-SMA表达减弱,说明黄芪甲苷可通过下调TGF-β1、SMAD2/3及α-SMA的表达,抑制TGF-β/SMADS信号通路,最终减轻肾脏纤维化。
膜荚黄芪以往被认为有利水消肿的功效。本研究发现,黄芪甲苷可通过调节TGF-β/SMADS信号通路并且下调TGF-β1、SMAD2/3及α-SMA的表达,改善糖尿病小鼠肾脏纤维化,从而通过抑制肾小管间质纤维化及肾小管上皮细胞转分化,发挥肾脏保护作用。
[1]Wang JY,Gao YB,Zhang N,et al.miR-21 overexpression enhances TGF-β1-induced epithelial-to-mesenchymal transition by target smad7 and aggravates renal damage in diabetic nephropathy[J].Mol Cell Endocrinol,2014,392(1/2):163-172.
[2]Araoka T,Abe H,Tominaga T,et al.Transcription factor 7-like 2 (TCF7L2)regulates activin receptor-like kinase 1 (ALK1)/Smad1 pathway for development of diabetic nephropathy[J].Mol Cells,2010,30(3):209-218.
[3]Ding Z,Chen Z,Chen X,et al.Adenovirus-mediated anti-sense ERK2 gene therapy inhibits tubular epithelial-mesenchymal transition and ameliorates renal allograft fibrosis[J].Transpl Immunol,2011,25(1):34-41.
[4]何凤,喻陆,童俊容,等.整合素连接激酶在梗阻性肾病肾小管上皮细胞转分化中的作用及尿激酶对其表达调节的研究[J].南方医科大学学报,2009,29(5):909-913.
[5]Yin Y,Qi F,Song Z,et al.Ferulic acid combined with astragaloside IV protects against vascular endothelial dysfunction in diabetic rats[J].Biosci Trends,2014,8(4):217-226.
[6]Chen J,Chen Y,Luo Y,et al.Astragaloside IV ameliorates diabetic nephropathy involving protection of podocytes in streptozotocin induced diabetic rats[J].Eur J Pharmacol,2014,736(736):86-94.
[7]Yamada Y,Muraki A,Oie M,et al.Soymorphin-5,a soy-derived μ-opioid peptide,decreases glucose and triglyceride levels through activating adiponectin and PPARα systems in diabetic KKAy mice[J].Am J Physiol Endocrinol Metab,2012,302(4):E433-E440.
[8]Li M,Yu L,She T,et al.Astragaloside IV attenuates Toll-like receptor 4 expression via NF-κB pathway under high glucose condition in mesenchymal stem cells[J].Eur J Pharmacol,2012,696(1/3):203-209.
[9]Qi W,Chen X,Poronnik P,et al.The renal cortical fibroblast in renal tubulointerstitial fibrosis[J].Int J Biochem Cell Biol,2006,38(1):1-5.
[10]Weil EJ,Lemley KV,Mason CC,et al.Podocyte detachment and reduced glomerular capillary endothelial fenestration promote kidney disease in type 2 diabetic nephropathy[J].Kidney Int,2012,82(9):1010-1017.
[11]Toyoda M,Najafian B,Kim Y,et al.Podocyte detachment and reduced glomerular capillary endothelial fenestration in human type 1 diabetic nephropathy[J].Diabetes,2007,56(8):2155-2160.
[12]Reidy K,Susztak K.Epithelial-mesenchymal transition and podocyte loss in diabetic kidney disease[J].Am J Kidney Dis,2009,54(4):590-593.
[13]Fukuda A,Chowdhury MA,Venkatareddy MP,et al.Growth-dependent podocyte failure causes glomerulosclerosis[J].J Am Soc Nephrol,2012,23(8):1351-1363.
[14]Zou MS,Yu J,Nie GM,et al.1,25-dihydroxyvitamin D3 decreases adriamycin-induced podocyte apoptosis and loss[J].Int J Med Sci,2010,7(5):290-299.
[15]Lv L,Wu SY,Wang GF,et al.Effect of astragaloside IV on hepatic glucose-regulating enzymes in diabetic mice induced by a high-fat diet and streptozotocin[J].Phytother Res,2010,24(2):219-224.
[16]Yuan W,Zhang Y,Ge Y,et al.Astragaloside IV inhibits proliferation and promotes apoptosis in rat vascular smooth muscle cells under high glucose concentration in vitro[J].Planta Med,2008,74(10):1259-1264.
[17]Salmon AH,Neal CR,Harper SJ.New aspects of glomerular filtration barrier structure and function:five layers (at least)not three[J].Curr Opin Nephrol Hypertens,2009,18(3):197-205.
[18]Xavier S,Niranjan T,Krick S,et al.TbetaRI independently activates Smad- and CD2AP-dependent pathways in podocytes[J].J Am Soc Nephrol,2009,20(10):2127-2137.
[19]Piwkowska A,Rogacka D,Audzeyenka I,et al.High glucose concentration affects the oxidant-antioxidant balance in cultured mouse podocytes[J].J Cell Biochem,2011,112(6):1661-1672.
[20]Tan S,Wang G,Guo Y,et al.Preventive effects of a natural Anti-Inflammatory agent,astragaloside Ⅳ,on ischemic acute kidney injury in rats[J].Evid Based Complement Alternat Med,2013,2013:284025.
[21]Zheng R,Deng Y,Chen Y,et al.Astragaloside Ⅳ attenuates complement membranous attack complex induced podocyte injury through the MAPK pathway[J].Phytother Res,2012,26(6):892-898.
[22]Qi W,Niu J,Qin Q,et al.Astragaloside Ⅳ attenuates glycated albumin-induced epithelial-to-mesenchymal transition by inhibiting oxidative stress in renal proximal tubular cells[J].Cell Stress Chaperones,2014,19(1):105-114.
[23]Meng LQ,Tang JW,Wang Y,et al.Astragaloside Ⅳ synergizes with ferulic acid to inhibit renal tubulointerstitial fibrosis in rats with obstructive nephropathy[J].Br J Pharmacol,2011,162(8):1805-1818.
[24]Gui DK,Huang JH,Guo YP,et al.Astragaloside Ⅳ ameliorates renal injury in streptozotocin-induced diabetic rats through inhibiting NF-kappa B-mediated inflammatory genes expression[J].Cytokine,2013,61(3):970-977.
The effect of astragaloside on TGF-β1,SMAD2/3,and α-SMA expression in the kidney tissues of diabetic KKAy mice
WangYaning1,LiuYunqi1,GaoJinxiang1,LinChao2,LiuNaiguo3
(1.DepartmentofNephrology;2.DepartmentofBoneandJointSurgery;3.DepartmentofClinicalandMedicineLab,theAffiliatedHospitalofBinzhouMedicalUniversity,Binzhou,Shandong256603,China)
Objective To study the effect of astragaloside on TGF-β1,SMAD2/3,and α-SMA expression in the kidney tissue of diabetic KKAy mice,and evaluate its potential role in renal interstitial fibrosis.Methods 20 type 2 diabetic KKAy mice were randomly divided into model group and astragaloside group,while 10 male C57BL/6J mice were selected as the control.Astragaloside at 40 mg·kg-1·d-1was given when the KKAy mice fed with high-fat diet to 14 weeks old.The mice in the control and model group received normal saline at 40 mg·kg-1·d-1.Blood glucose meter was used to detect the blood glucose value of each mice at 16th,20th and 24th week.The mice were killed at 24 weeks old and the kidney tissue samples were collected.Pathology morphological changes were observed.Results (1)blood glucose value:cmpared with the control group,the blood glucose value of KKAy mice at 14 week increased significantly,and that of model group also increased significantly at 16th,20th and 24th week (P<0.05);the blood glucose value of astragaloside group decreased compared with control group (P<0.05).(2)Morphology of kidney:in the control group,the glomerular and tubular had clear structure,there was no renal interstitial fibrosis;in the model group,the renal glomerular mesangial matrix had broaden,mesangial cell had increased,renal tubular epithelial cell cytoplasm showed vacuole degeneration,renal interstitial inflammatory cell had increased.In astragaloside group,there were few renal tubular epithelial cell cytoplasm,and there was no obvious fibrosis.(3)TGF-β1,SMAD2/3,and α-SMA expression levels of the kidney issuse:compared with control group,mice in model group up-regulated TGF-β1,SMAD2/3 and α-SMA expression (P<0.05).TGF-β1,SMAD2/3,and α-SMA expression levels in astragaloside group were significantly lower than those in the model group (P<0.05).There was few phosphorylated SMAD2/3 expression in renal tubular and glomerular nuclei,while that of model group increased (P<0.01),and compared with model group,that of the astragaloside group decreased (P<0.05).Conclusion Astragaloside can delay the renal fibrosis process in diabetic mice by influencing the TGF-β/SMADS signaling pathway and down-regulating TGF-β1 and α-SMA expression,thus to relieve renal fibrosis in diabetic mice.
astragaloside;diabetic nephropathy;TGF-β1;α-SMA;SMAD
王雅宁(1983-),主治医师,硕士,主要从事慢性肾脏病的基础与临床研究。
��·基础研究
10.3969/j.issn.1671-8348.2017.05.007
R692.6
A
1671-8348(2017)05-0596-04
2016-07-08
2016-09-06)
