武汉东湖水体异味物质及其与水环境因子相互关系
2017-01-20胡宇飞余得昭过龙根
胡宇飞,余得昭,过龙根,谢 平
(1:中国科学院水生生物研究所东湖湖泊生态系统试验站,武汉 430072)(2:中国科学院大学,北京 100049)
武汉东湖水体异味物质及其与水环境因子相互关系
胡宇飞1,2,余得昭1,2,过龙根1,谢 平1
(1:中国科学院水生生物研究所东湖湖泊生态系统试验站,武汉 430072)(2:中国科学院大学,北京 100049)
根据2014年1-12月东湖3个湖区(水果湖、郭郑湖及汤菱湖)中9种溶解态异味物质(DMS、DMDS、DMTS、β-cyclocitral、β-ionone、MIB、GEO、IBMP和IPMP)的月间采样结果,对异味化合物浓度之间的相关性及其与东湖水体中主要环境因子的相关关系进行了分析. 研究发现9种异味物质浓度整体水平在夏、秋季相对冬、春季较高,其中DMS、DMTS、β-cyclocitral、β-ionone的月平均浓度较高,且在夏季均超出嗅味阈值,其他几种异味物质浓度检出较低,对东湖的异味强度影响较小. 低浓度β-cyclocitral及β-ionone具有烟草或芳香味,DMS与DMTS具有腐臭味,因此DMS及DMTS为东湖异味的主要致嗅物质. 从异味物质空间分布来看,郭郑湖区的DMS、DMDS、β-cyclocitral及β-ionone年平均浓度均低于其他两个湖区,综合该湖区中相对较低的年平均总氮(TN)和叶绿素a(Chl.a)浓度以及受人类活动影响程度较低的情况,该湖区的异味问题要轻于水果湖及汤菱湖区. 此外,研究发现DMTS、β-cyclocitral和β-ionone浓度与Chl.a浓度均呈显著正相关,DMDS及DMTS浓度与TN浓度呈显著正相关,DMS及β-cyclocitral浓度与溶解氧浓度呈显著负相关,表明由于大量藻类快速腐败导致的水体含氧量下降可能会对水体异味产生重要影响. 为防止东湖水体恶臭的发生,对藻类进行控制尤为重要.
东湖;异味物质;环境因子;相互关系
湖泊是重要的饮用水及水产品源地. 近年来的湖泊水体富营养化引起的蓝藻水华或形成的优势种群的藻类导致的水体异味污染对湖泊生态及人类生活有严重的危害. 水体中高含量异味物质的存在不仅严重影响水质,而且还可进一步积累于水生生物体内,带来一系列水产品异味问题. 如在1970s末,北欧挪威Mjosa湖中大量颤藻“水华”引起的难闻霉味影响了当地约20万人的正常供水[1];1969年在芬兰Oulu海域,由于鱼肉中难闻的霉味致使当地渔民失去主要经济收入[2];类似事件在其他国家也经常发生. 在众多水体异味的报道中,GEO(geosmin,土臭素)和MIB(2-methylisoborneol,二甲基异莰醇)两种土霉味化合物在藻类次生代谢产物中最为常见. 近年来,对β-cyclocitral(β-环柠檬醛)、β-ionone(β-紫罗兰酮)、IPMP(2-isopropyl-3-methoxy pyrazine,2-异丙基-3-甲氧基吡嗪)及IBMP(2-isobutyl-3-methoxy pyrazine,2-异丁基-3-甲氧基吡嗪)的研究也逐渐增多[3-5]. 挥发性有机硫化物也会导致水体产生异味,Bechard等[6]在调查湖泊的富营养化时发现在绿藻水华腐烂过程中产生了大量的DMS(甲硫醚)、DMDS(二甲基二硫醚)和DMTS(二甲基三硫醚). 以上9种主要异味物质与水环境因子之间有着密切的联系,且现在经常被用来判断水体异味的情况. 因此找出这9种物质与水环境因子间的关系对于湖泊水体中异味物质的防控具有重要意义.
东湖(30°31′~30°36′N,114°21′~114°28′E)位于长江中游,是一个典型的中型浅水内陆湖泊,面积约32 km2,平均水深2.2 m,最大水深4.8 m. 它是武汉市生活、工业与农业灌溉用水的水源地,全国闻名的风景区和水上休闲运动场,还具有防汛调蓄、水产养殖、调节小气候等多种功能[7]. 由于东湖受周边地区工农业迅速发展及人口迅速增加的影响,水体富营养化程度较重,严重破坏了生态平衡,水体中藻源性次生代谢产物产生的异味物质对饮用水安全及水产品质量具有重要影响,因此对东湖水体中异味物质的研究十分必要. 本研究以东湖为研究对象,分析了2014年东湖水体中溶解态异味物质的含量,并分析了各异味物质之间以及其水环境因子之间的相关关系,旨在找出影响东湖水体中异味化合物的主要环境因素.
1 材料与方法
1.1 试剂与仪器
DMS、DMDS、DMTS、MIB、GEO、β-Cyclocitral、β-ionone、IBMP及IPMP的标准品购自Sigma公司,并用色谱级甲醇(Merck公司)配置成1 mg/L的溶液用于标准曲线溶液的配置. 用于分离溶解态及结合态异味物质的玻璃纤维素薄膜GF/C膜购自Brentford公司. 水体中的溶解态异味物质采用吹扫捕集(Eclipse4660,OI分析仪器公司,美国)-气相色谱-质谱联用(P&T-GCMS,岛津GCMS-QP2010Plus,岛津公司,日本)法进行分析[8]. 色谱柱为HP-5MSUI石英毛细管柱(30 m×0.25 mm×0.25 μm).
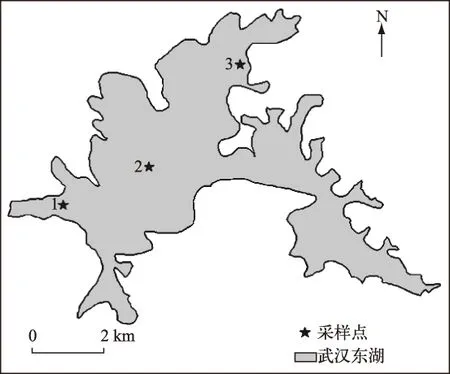
图1 武汉东湖采样点分布Fig.1 Schematic diagram of the sampling sites in Lake Donghu
1.2 样品采集与水环境因子的测定

1.3 水中溶解态异味物质的测定
参考Deng等[8]的方法对经Whatman GF/C膜过滤后的水样中的异味物质进行测定.
1.4 数据分析
采用SPSS 22.0软件对各样点的水环境因子与异味物质的空间分布数据进行分析.
2 结果与分析
2.1 水环境理化指标的变化
表1 研究期间东湖水环境因子范围及均值
Tab.1 Annual means and ranges of water quality variables in Lake Donghu during the study period

参数范围年平均值Tw/℃5.51~31.2019.97DO/(mg/L)4.73~13.738.94pH5.39~10.208.75电导率/(μS/cm)307~439398.20Chl.a/(μg/L)5.58~68.6328.33TN/(mg/L)0.49~1.951.16NH+4⁃N/(mg/L)0.02~0.620.25TP/(mg/L)0.03~0.420.12

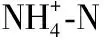
表2 研究期间东湖各采样点环境因子的年均值
Tab.2 Annual means of water quality variables at each sampling site of Lake Donghu during the study period
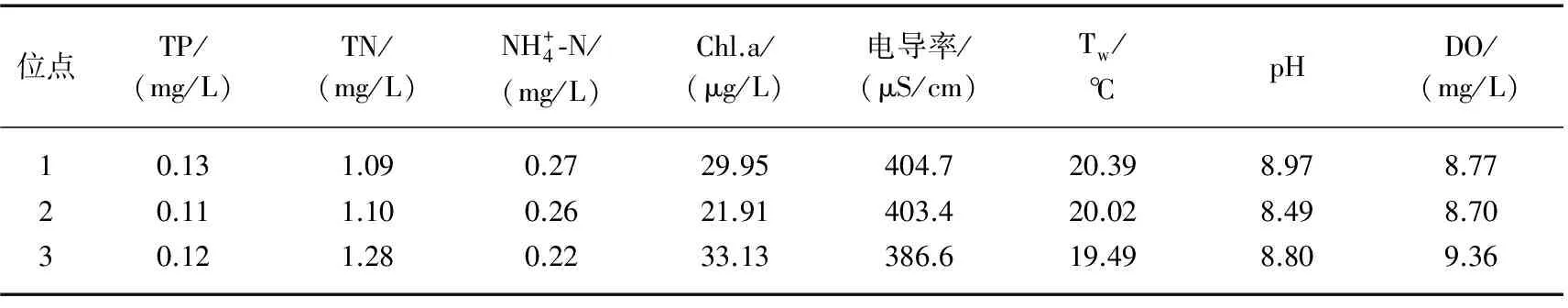
位点TP/(mg/L)TN/(mg/L)NH+4⁃N/(mg/L)Chl.a/(μg/L)电导率/(μS/cm)Tw/℃pHDO/(mg/L)10.131.090.2729.95404.720.398.978.7720.111.100.2621.91403.420.028.498.7030.121.280.2233.13386.619.498.809.36
2.2 溶解态异味物质浓度变化
在对东湖水体周年采样过程中,可以感知到6-9月期间东湖水体整体散发出轻微腥臭味,夏、秋之交时较轻,秋、冬季时明显减轻,甚至没有感知到异味. 从湖区分布来看,水果湖及汤菱湖区异味程度相近,在夏季时均散发轻微腥臭味,而郭郑湖区夏季散发的腥臭味相比另外两个湖区要弱很多.
9种溶解态异味物质中除IBMP在6、7、8、9、10及12月均未被检测到,其余异味物质在3个采样点中均被检出. 除DMDS及IPMP外,其他7种异味物质均有月平均浓度超出异味阈值(OTC)的情况. 超出OTC的异味物质中以DMS、β-cyclocitral及β-ionone最为显著,其中DMS及β-cyclocitral浓度全年均超出异味阈值,β-ionone浓度仅1月低于7 ng/L,其余11个月均超出. DMS、DMTS、β-cyclocitral及β-ionone浓度在夏季时普遍偏高. 由于β-ionone及β-cyclocitral嗅味主要为烟叶或花香味,而DMS及DMTS主要为腐臭味,因此夏季东湖散发出的微腥臭味主要由DMS及DMTS贡献(表3).
从异味物质空间分布(表4)来看,水果湖区的DMS年平均浓度为54.09 ng/L,高于郭郑湖区及汤菱湖区(49.17及49.61 ng/L). DMDS的年平均浓度中,郭郑湖区最低,为4.96 ng/L,郭郑湖及汤菱湖区稍高,分别为8.04及9.23 ng/L. 3个位点的β-cyclocitral年平均浓度分别为14.27、12.39 和16.12 ng/L. 水果湖区及汤菱湖区的β-ionone年平均浓度(24.43及32.53 ng/L)均高于郭郑湖区(19.94 ng/L). 其他几种异味物质在3个采样位点的差异不大. 由此可见,郭郑湖区的异味问题要轻于其他两个位点.
表3 研究期间东湖3个位点水样异味物质月平均浓度(ng/L)变化*
Tab.3 Seasonal changes of taste and odor compounds concentrations at the 3 sampling sites of Lake Donghu during the study period
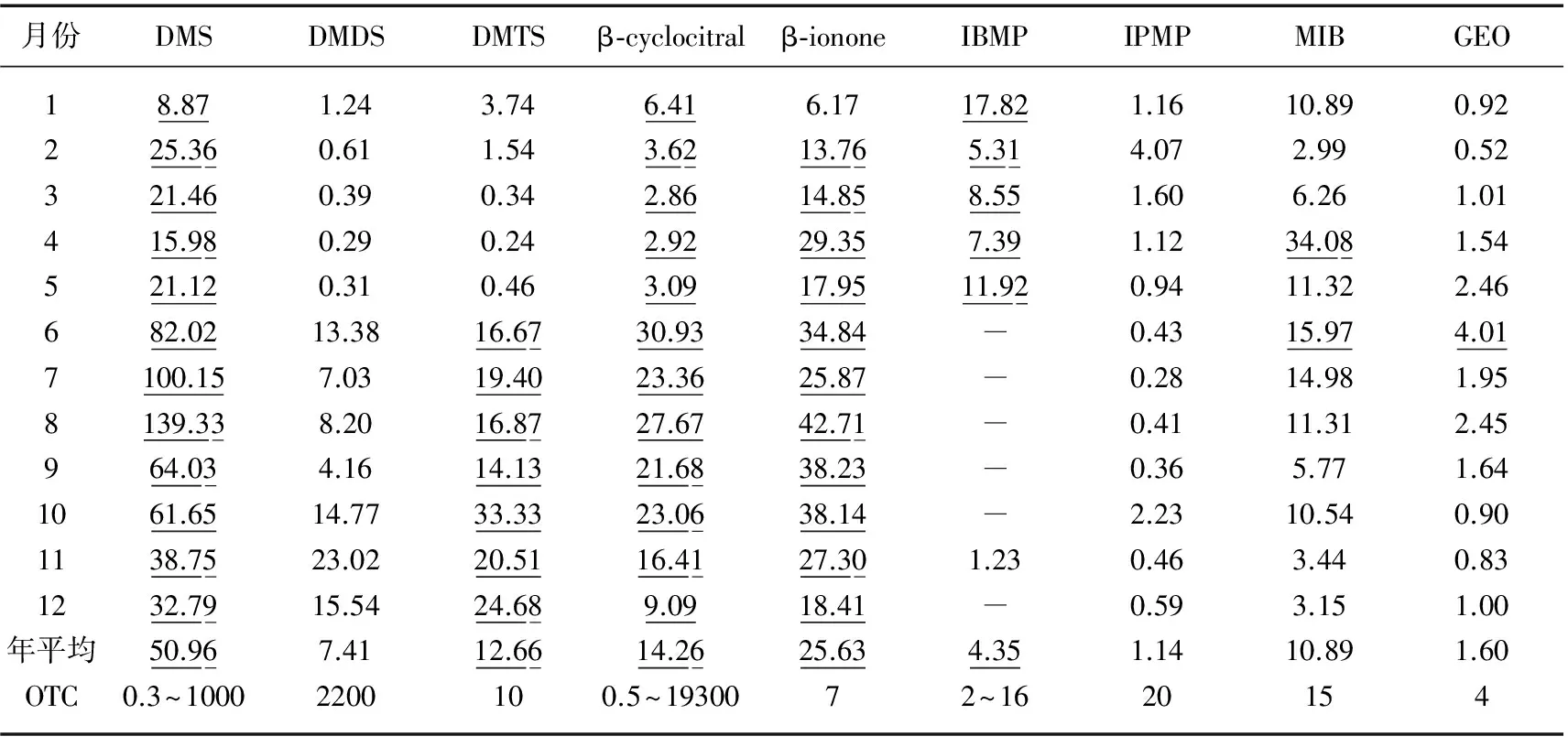
月份DMSDMDSDMTSβ⁃cyclocitralβ⁃iononeIBMPIPMPMIBGEO18.871.243.746.416.1717.821.1610.890.92225.360.611.543.6213.765.314.072.990.52321.460.390.342.8614.858.551.606.261.01415.980.290.242.9229.357.391.1234.081.54521.120.310.463.0917.9511.920.9411.322.46682.0213.3816.6730.9334.840.4315.974.017100.157.0319.4023.3625.870.2814.981.958139.338.2016.8727.6742.710.4111.312.45964.034.1614.1321.6838.230.365.771.641061.6514.7733.3323.0638.142.2310.540.901138.7523.0220.5116.4127.301.230.463.440.831232.7915.5424.689.0918.410.593.151.00年平均50.967.4112.6614.2625.634.351.1410.891.60OTC0.3~10002200100.5~1930072~1620154
*各异味物质OTC值引自文献[10-13];月平均值为3个位点的月平均值,超出OTC的用下划线标出.
表4 研究期间东湖各采样点异味物质年浓度(ng/L)
Tab.4 Annual concentrations of taste and odor compounds at each sampling site of Lake Donghu during the study period
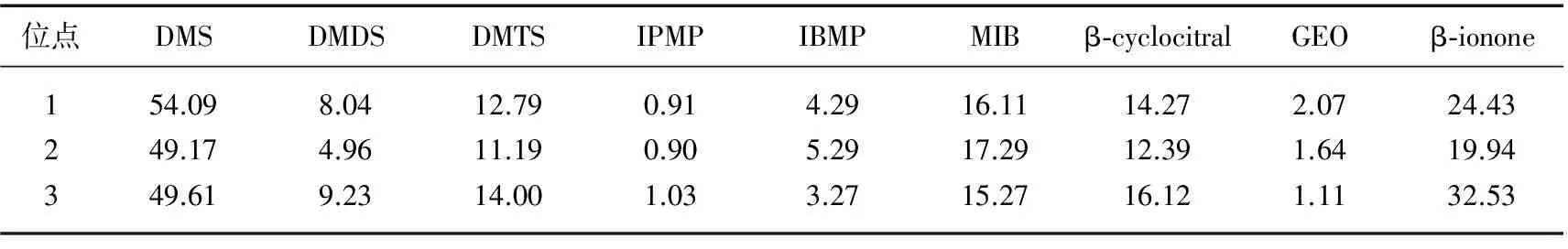
位点DMSDMDSDMTSIPMPIBMPMIBβ⁃cyclocitralGEOβ⁃ionone154.098.0412.790.914.2916.1114.272.0724.43249.174.9611.190.905.2917.2912.391.6419.94349.619.2314.001.033.2715.2716.121.1132.53
2.3 水环境因子与异味物质及异味物质之间的相关关系
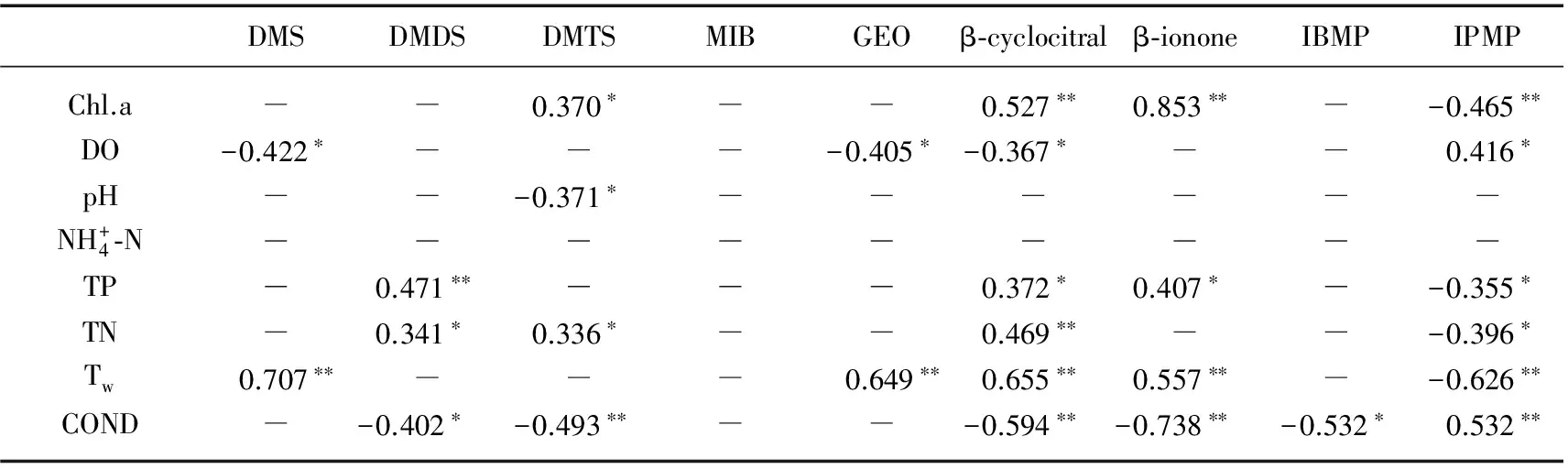
相关分析结果(表5)表明,东湖水体中DMS浓度与水温呈极显著正相关(P<0.01),与DO浓度呈显著负相关(P<0.05). DMDS浓度与TP浓度呈极显著正相关(P<0.01),与TN浓度呈显著正相关(P<0.05). DMTS浓度与Chl.a和TN浓度呈显著正相关(P<0.05),与pH呈显著负相关(P<0.05). β-cyclocitral及β-ionone浓度与Chl.a浓度和水温分别呈极显著正相关(P<0.01),分别与TP浓度显著正相关(P<0.05),并与电导率呈极显著负相关(P<0.01). β-cyclocitral浓度还与DO浓度呈显著负相关(P<0.05),与TN浓度呈极显著正相关(P<0.01). 同时分析了9种异味物质浓度之间的相关关系(表6),发现DMTS浓度与DMS及DMDS浓度均呈极显著正相关(P<0.01),β-cyclocitral浓度与β-ionone浓度呈极显著正相关(P<0.01),同时与3种硫醚类异味物质也分别呈极显著正相关(P<0.01).
表5 东湖异味物质和水质变量之间的相关关系
Tab.5 Correlations between water quality variables and taste and odor compounds in Lake Donghu
DMSDMDSDMTSMIBGEOβ⁃cyclocitralβ⁃iononeIBMPIPMPChl.a0.370∗0.527∗∗0.853∗∗-0.465∗∗DO-0.422∗-0.405∗-0.367∗0.416∗pH-0.371∗NH+4⁃NTP0.471∗∗0.372∗0.407∗-0.355∗TN0.341∗0.336∗0.469∗∗-0.396∗Tw0.707∗∗0.649∗∗0.655∗∗0.557∗∗-0.626∗∗COND-0.402∗-0.493∗∗-0.594∗∗-0.738∗∗-0.532∗0.532∗∗
*表示P<0.05,**表示P<0.01,“-”表示相关性不显著,下同.
表6 东湖异味物质之间的相关关系
Tab.6 Correlations among taste and odor compounds in Lake Donghu

DMSDMDSDMTSMIBGEOβ⁃cyclocitralβ⁃iononeIBMPIPMPDMS1DMDS1DMTS0.433∗∗0.764∗∗1MIB1GEO0.432∗∗1β⁃cyclocitral0.778∗∗0.479∗∗0.701∗∗0.400∗1β⁃ionone0.544∗∗0.419∗0.698∗∗1IBMP0.677∗∗0.712∗∗0.783∗∗-0.489∗1IPMP-0.433∗-0.355∗-0.514∗∗-0.527∗∗-0.396∗1
3 讨论
3.1 异味物质来源
除了工农业废水及生活污水污染外,水体富营养化导致的藻类水华也是引起水体异味的重要原因. 在富营养化水体中,由于营养物质过剩,使得淡水生态系统的平衡遭到破坏,一些藻、菌微生物群落过剩生长,这些藻、菌等能在生长繁殖或死亡分解时不断分泌和产生出各种具有异味的次生代谢产物. 东湖自1980s的观测数据就显示富营养化程度比较严重[14-15],近年来虽然水体中个别因子(如浮游植物等)变化较大,但总体上富营养化程度没有显著变化.
在以往水体中检测到的硫化物中,最常见的为DMS、DMDS和DMTS[16-17],而本研究也表明在东湖水体中广泛存在硫化物类异味物质,尤其是DMS(表3). 研究表明,DMS、DMDS和DMTS可由藻类或水生植物在生长过程中及死亡腐烂后的有机质中分解产生[18-19]. 在湖泊等富营养化水体中,藻体细胞死亡分解可放出二甲基磺基丙酯(DMSP),DMSP是一种可以通过调节细胞渗透压及抗凝作用来对细胞本身提供保护的化学物质. 经过一些微生物(如淡水SAR11 细菌)以及物理化学降解过程,DMSP可以转化成DMS并进一步转化成DMDS和DMTS[20]. 其次,微生物降解含硫有机物也是这类异味物质产生的重要途径之一[21]. 含有蛋白质的工业废水和生活污水等排入水体后,在厌氧条件下也可以生成甲硫醇,甲硫醇可以在好氧条件下转化成DMDS,DMDS可以进一步转化为DMS[22]. 因此,本研究中DMTS与DMS及DMDS分别表现出显著相关的关系可能是由于它们的产生途径类似. 2#采样点郭郑湖区夏季时散发的腥臭味相比另外两个湖区要弱很多,该湖区的DMS及DMTS年平均浓度要低于另外两个湖区,且该湖区年平均TN及Chl.a浓度均为3个湖区中最低的,表明可能是水体中相对较低的营养盐浓度没有造成藻类的大量产生,从而由藻类生长及死亡腐烂后的有机质中产生的DMS及DMTS浓度相对较低;且相比其他两个采样点,该湖区采样点距离岸边较远,可能受人类活动(工业废水及生活污水的排放等)干扰较小,污染物来源主要是工业尘埃,生活污水及工业废水所占比例低于28%,湖中心具有较强的自净能力[23],在厌氧条件下产生了相对较少的硫化物类异味物质. 综合以上原因,郭郑湖区的异味问题相比其他两个湖区较轻. β-cyclocitral和β-ionone主要是由微囊藻属体内的β-胡萝卜素氧化得来,在藻体生长过程中伴随少量产生,当藻体衰败死亡时,经酶催化作用会大量产生[24-25]. 此外β-cyclocitral和β-ionone能够加速蓝藻的腐败,从而增加DMS和DMTS的释放量,这也解释了本实验中β-cyclocitral和β-ionone与DMS、DMTS紧密相关. MIB和GEO是放线菌、黏细菌和一些浮游藻类如颤藻属、束丝藻属及鱼腥藻的代谢产物[10,26-27]. IBMP和IPMP检出量较低,通常在水的贮存过程中产生,是蓝藻在微生物分解过程中的产物[3,28],已有研究报道IBMP及IPMP两种物质的检出都很低[29-30]. DMS、DMDS及DMTS 3种硫化物和β-cyclocitral、β-ionone的浓度较高,分别为50.96、7.41、12.66、14.26和25.63 ng/L,其中DMS、DMTS、β-cyclocitral和β-ionone超出它们的异味阈值. β-cyclocitral和β-ionone是具有烟叶或花香味的异味化合物,虽然能引起人的嗅觉感知,但对东湖水体异味贡献不大,为非主要致嗅物质. 而DMS及DMTS具有腐臭味,夏季浓度较高时可以使得东湖水体呈现出轻微腥臭味,对湖泊生态系统及人类生活具有较大的潜在危害.
3.2 东湖水体中与异味物质密切相关的水环境因子
异味物质不仅受到其来源的影响,也受到众多非生物因子的影响. Qi等[31]通过对太湖中常见异味物质与环境因子之间的分析表明:TN、总溶解氮、DO、化学需氧量、pH等均与水体中的异味物质有重要的关系. 这些非生物环境因子不仅能够影响藻类产异味的能力,还能够直接导致异味物质的分解与转化.
致谢:感谢中国科学院水生生物研究所陈隽研究员的指导,感谢于佳帮助制作采样位点示意图.
[1] Holtan H. The Lake Mjøsa story.ArchHydrobiolBeihErgebnLimnol, 1979, 13: 242-258.
[2] Presson PE. Off-flavours in aquatic ecosystem—an introduction.WaterScienceandTechnology,1983,15(6/7): 1-11.
[3] Peter A, Köster O, Schildknecht Aetal. Occurrence of dissolved and particle-bound taste and odor compounds in Swiss lake waters.WaterResearch, 2009, 43(8): 2191-2200.
[4] Li L, Wan N, Gan Netal. Annual dynamics and origins of the odorous compounds in the pilot experimental area of Lake Dianchi, China.WaterScienceandTechnology, 2007, 55(5): 43-50.
[5] Xu LP, Xiong BX, Pan Yetal. Relationship between concentrations of odorous compounds and biomass of phytoplankton and actinomycetes in freshwater ponds of Beijing, China.AquacultureInternational, 2010, 18(3): 245-254.
[6] Bechard RG, Rayburn WR. Volatile organic sulfides from freshwater algae.JournalofPhycology, 1979, 15(4): 379-383.
[7] Shen Xiaoli. Ecological environment of East Lake: Vicissitude and recovery.EnvironmentalScienceandTechnology, 2003, 26(4): 24-26. [沈晓鲤. 武汉东湖的生态环境变迁与恢复问题. 环境科学与技术, 2003, 26(4): 24-26.]
[8] Deng XW, Liang G, Chen Jetal. Simultaneous determination of eight common odors in natural water body using automatic purge and trap coupled to gas chromatography with mass spectrometry.JournalofChromatographyA, 2011, 1218(24): 3791-3798.
[9] Jin Xiangcan, Tu Qingying eds. The standard methods in lake eutrophication investigation. Beijing: China Environmental Science Press, 1990.[金相灿, 屠清瑛. 湖泊富营养化调查规范. 北京: 中国环境科学出版社, 1990: 229-230.]
[10] Mallevialle J, Suffet IH. Identification and treatment of tastes and odors in drinking water.RegionalAnesthesiaandPainMedicine, 1981, 33(5): e99.
[11] Young W,Horth H, Crane Retal. Taste and odour threshold concentrations of potential potable water contaminants.WaterResearch, 1996, 30: 331-340.
[12] Waston SB, Ridal J. Periphyton: A primary source of widespread and severe taste and odour.WaterScienceandTechnology, 2000, 49: 33-39.
[13] Juttner F. Characterization ofMicrocystisstrains by alkyl sulfides and beta-cyclocitral. Zeit Schrift fur Anturforschung C-A.JournalofBiosciences, 1984, 39: 867-871.
[14] Liu Jiankang ed. Studies on the ecology of Lake Donghu. Beijing: Science Press, 1990: 1-407.[刘建康. 东湖生态学研究. 北京: 科学出版社, 1990: 1-407.]
[15] Ruan Jingrong, Cai Qinghua, Liu Jiankang. A phosphorus-phytoplankton dynamics model for lake Donghu in Wuhan.ActaHydrobiologicaSinica, 1988, 12(4): 289-307.[阮景荣, 蔡庆华, 刘健康. 武汉东湖的磷-浮游植物动态模型. 水生生物学报, 1988, 12(4): 289-307.]
[16] Richards SR, Kelly CA, Rudd JWM. Organic volatile sulfur in lakes of the Canadian Shield and its loss to the atmosphere.LimnologyandOceanography, 1991, 36(3): 468-482.
[17] Hu H, Mylon SE, Benoit G. Volatile organic sulfur compounds in a stratified lake.Chemosphere, 2007, 67(5): 911-919.
[18] Lomans BP, Smolders A, Intven LMetal. Formation of dimethyl sulfide and methanethiol in anoxic freshwater sediments.AppliedandEnvironmentalMicrobiology, 1997, 63(12): 4741-4747.
[19] Ginzburg B, Dor I, Chalifa Ietal. Formation of dimethyloligosulfides in Lake Kinneret: Biogenic formation of inorganic oligosulfide intermediates under oxic conditions.EnvironmentalScienceandTechnology, 1999, 33(4): 571-579.
[20] Jiang Lin, Hu Min, Ren Changjiu. Biogenic production and consumption of dimethylsulfide in ocean.ActaScientiarumNaturaliumUniversitatisPekinensis, 1997, 33(2): 240-245. DOI: 10.13209/j.0479-8023.1997.034.[蒋林, 胡敏, 任长久. 海洋二甲基硫的生物生产与降解. 北京大学学报: 自然科学版, 1997, 33(2): 240-245.]
[21] Lu X, Fan C, He Wetal. Sulfur-containing amino acid methionine as the precursor of volatile organic sulfur compounds in algea-induced black bloom.JournalofEnvironmentalSciences, 2013, 25(1): 33-43.
[22] Zhang Xiaojian, Zhang Yue, Wang Huanetal. Emergent drinking water treatment for taste and odor control in Wuxi City water pollution incident.WaterAndWastewaterEngineering, 2007, 33(9): 7-12.[张晓健, 张悦, 王欢等. 无锡自来水事件的城市供水应急除臭处理技术. 给水排水, 2007, 33(9): 7-12.]
[23] Xiong Zhiping. Study on water environment pollution factors and renovation measurements of the East Lake.WaterSavingIrrigation, 2007, (4): 13-16.[熊志平. 东湖水环境污染因素与整治措施. 节水灌溉, 2007, (4): 13-16.]
[24] Watson SB, Ridal J, Boyer GL. Taste and odour and cyanobacterial toxins: Impairment, prediction, and management in the Great Lakes.CanadianJournalofFisheriesandAquaticSciences, 2008, 65(8): 1779-1796.
[25] Smith JL, Boyer GL, Zimba PV. A review of cyanobacterial odorous and bioactive metabolites: Impacts and management alternatives in aquaculture.Aquaculture, 2008, 280(1): 5-20.
[26] Wnorowski AU. Tastes and odours in the aquatic environment: A review.WaterSA, 1992, 18(3): 203-214.
[27] Xu Ying, Li Wen, Wu Wenzhongetal. Study on aquatic off-flavors in eutrophic Donghu Lake.ActaEcologicaSinica, 1999, 19(2): 212-216.[徐盈, 黎雯, 吴文忠等. 东湖富营养水体中藻菌异味性次生代谢产物的研究. 生态学报, 1999, 19(2): 212-216.]
[28] Khiari D, Barrett SE, Suffet IH. Sensory GC analysis of decaying vegetation and septic odors.AmericanWaterWorksAssociationJournal, 1997, 89(4): 150.
[29] Ma ZM, Xie P, Chen Jetal. Microcystis blooms influencing volatile organic compounds concentrations in Lake Taihu.FreseniusEnvironmentalBulletin, 2013, 22(1): 95-102.
[30] Chen J, Xie P, Ma ZMetal. A systematic study on spatial and seasonal patterns of eight taste and odor compounds with relation to various biotic and abiotic parameters in Gonghu Bay of Lake Taihu, China.ScienceoftheTotalEnvironment, 2010, 409(2): 314-325.
[31] Qi M, Chen J, Sun Xetal. Development of models for predicting the predominant taste and odor compounds in Taihu Lake, China.PLoSOne, 2012, 7(12): e51976.
[32] Barnard WR, Andreae MO, Watkins WEetal. The flux of dimethylsulfide from the oceans to the atmosphere.JournalofGeophysicalResearch:Oceans, 1982, 87(C11): 8787-8793.
[33] Smith VH. Eutrophication of freshwater and coastal marine ecosystems a global problem.EnvironmentalScienceandPollutionResearch, 2003, 10(2): 126-139.
[34] Watson SB. Cyanobacterial and eukaryotic algal odour compounds: Signals or by-products? A review of their biological activity.Phycologia, 2003, 42(4): 332-350.
[35] Tanaka A, Oritani T, Uehara Fetal. Biodegradation of a musty odour component, 2-methylisoborneol.WaterResearch, 1996, 30(3): 759-761.
[36] Li Lin, Song Lirong, Chen Weietal. Degradation of algae production odorous compounds in freshwater by photolysis and TO2photocatalysis.ChinaWater&Wastewater, 2007, 23(13): 102-105.[李林, 宋立荣, 陈伟等. 淡水藻源异味化合物的光降解和光催化降解研究. 中国给水排水, 2007, 23(13): 102-105.]
[37] Selli S, Prost C, Serot T. Odour-active and off-odour components in rainbow trout (Oncorhynchusmykiss) extracts obtained by microwave assisted distillation-solvent extraction.FoodChemistry, 2009, 114(1): 317-322.
[38] Robin J, Cravedi JP, Hillenweck Aetal. Off flavor characterization and origin in French trout farming.Aquaculture, 2006, 260(1): 128-138.
[39] Chorus I ed. Cyanotoxins: Occurrence, causes, consequences. Berlin: Springer, 2012. DOI: 10.1007/9/978-3-642-59514-1.
Relationships of water taste and odor compounds and their related environmental factors in Lake Donghu, Wuhan
HU Yufei1,2, YU Dezhao1,2, GUO Longgen1& XIE Ping1**
(1:DonghuExperimentalStationofLakeEcosystems,InstituteofHydrobiology,ChineseAcademyofSciences,Wuhan430072,P.R.China)(2:UniversityofChineseAcademyofSciences,Beijing100049,P.R.China)
Nine dissolved taste and odor compounds (T & O), namely DMS, DMDS, DMTS, β-cyclocitral, β-ionone, MIB, GEO, IBMP and IPMP were investigated in three water areas (Lake Shuiguo, Lake Guozheng and Lake Tangling) of Lake Donghu monthly in 2014. According to the changes of the nine T & O compounds and related environmental factors of each month, relationships of these compounds as well as the relationships with environmental factors were analyzed in this study. It was found that the concentrations of the nine T & O compounds were higher in the summer and fall than in the winter and spring. The seasonal concentrations of DMS, DMDS, DMTS, β-cyclocitral and β-ionone were high and their concentrations all surpassed OTC (Over The Count) in the summer, while other T & O compounds made little contributions to the odor problems of Lake Donghu because of their relative low concentrations. Low concentrations of β-cyclocitral and β-ionone can make water smell flavoury while DMS and DMTS contribute fusty smelling, making the main odors that led Lake Donghu smelly. As for the distributions of odors, the yearly concentrations of DMS, DMDS, β-cyclocitral and β-ionone in Lake Guozheng were all lower than that of the other two areas in Lake Donghu. Based on the relative low concentrations of total nitrogen and chlorophyll-a(Chl.a) and the relative low influence of human activities on Lake Guozheng, odor problem in this area is lighter than that of Lake Shuiguo and Lake Tangling. Meanwhile, there were significantly positive correlations between concentrations of Chl.a and the three taste and odor compounds (DMTS, β-cyclocitral and β-ionone), as well as between concentrations of total nitrogen and the two odors (DMDS and DMTS) in the study. Besides, negative correlations were presented in concentration of dissolved oxygen (DO) with DMS and β-cyclocitral, respectively, indicating that low DO concentration induced by decayed algae could aggravate odor problems. To manage the occurrences of taste and odor events in Lake Donghu, it is critical to control the growth of algae.
Lake Donghu; taste and odor compounds; environmental factors; correlation
*淡水生态与生物技术国家重点实验室项目(2015FB13,2014FBZ02)资助. 2016-03-03收稿; 2016-05-03收修改稿. 胡宇飞(1991~),女,硕士研究生; E-mail: yufeihu@ihb.ac.cn.
*通信作者; E-mail: xieping@ihb.ac.cn.
J.LakeSci.(湖泊科学), 2017, 29(1): 87-94
DOI 10.18307/2017.0110
©2017 byJournalofLakeSciences
